Non-Invasive Brain Stimulation (NIBS) techniques, such as transcranial Direct Current Stimulation (tDCS) and repetitive Magnetic Transcranial Stimulation (rTMS), are well-known non-pharmacological approaches to improve both motor and non-motor symptoms in patients with neurodegenerative disorders. Their use is of particular interest especially for the treatment of cognitive impairment in Alzheimer’s Disease (AD), as well as axial disturbances in Parkinson’s (PD), where conventional pharmacological therapies show very mild and short-lasting effects. However, their ability to interfere with disease progression over time is not well understood; recent evidence suggests that NIBS may have a neuroprotective effect, thus slowing disease progression and modulating the aggregation state of pathological proteins.
- non-invasive brain stimulation
- tDCS
- rTMS
- neuroprotection
- Parkinson’s Disease
- Alzheimer’s Disease
- neurodegenerative disorders
- Deep Brain Stimulation
- stroke
- disease modifying treatment
1. Introduction
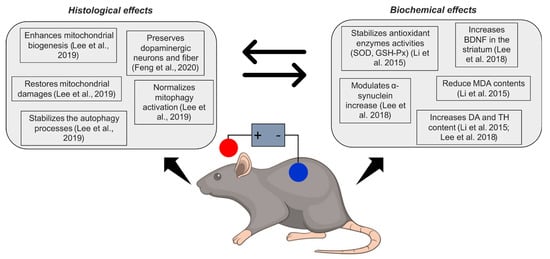
2. NIBS and Neuroprotection in Parkinson’s Disease
2.1. tDCS
| Study | Sample/Animals | Polarity | Configuration | Parameters | Biological Outcomes | Biological Results |
|---|---|---|---|---|---|---|
| Li et al., 2015 [19] | 36 C57Bl mice (n = 9 in control group; n = 9 in sham tDCS group; n = 9 in tDCS groups; n = 9 in drug group) | Anodal/Sham | AE: left frontal cortex; R: between the shoulders |
0.2 mA, 10 min/day, 21 consecutive days AEA: 3.5 mm2 CD: 5.7 mA/cm2 |
DA, TH, SOD and GSH-PX activities, nonenzymatic MDA activity | tDCS increased DA, SOD and GSH-Px; after MPTP induction, anodal tDCS increased TH and reduced MDA |
| Lee et al., 2018 [45] | 60 Male C57BL/6 mice (n = 15 in control group; n = 15 in anodal tDCS group; n = 15 in MPTP group; n = 15 in MPTP + tDCS group) | Anodal/Sham | AE: left motor cortex; R: between the shoulders |
0.1 mA, 30 min/day, 5 consecutive days AEA: 3.1 mm2 CD: 3.2 mA/cm2 |
TH-positive cells; TH; α-synuclein protein; loss of dopaminergic neuron cells; ratio of LC3-II/LC3-I; p62; PI3K; mTOR; AMPK; ULK | tDCS attenuated decrease of TH, p62, mTOR, PI3K, BDNF; attenuated increase of α-synuclein, LC3-II/LC3-I, AMPK and ULK |
| Lee et al., 2019 [17] | Male C57BL/6 mice (number n.r.) | Anodal/Sham | AE: on motor cortex; R: between the shoulders |
0.1 mA, 30 min/day, 5 days/week, 1 week; AEA: 3.1 mm2 CD: 3.2 mA/cm2 |
Expression of: TH, mitophagy-related proteins; marker of degradation phase of autophagy; mitochondrial biogenesis-related proteins; mitochondrial fission and fusion -related proteins; ATP concentration. Mitochondrial GDH activity |
tDCS preserved neurons and fibers in substantia nigra and striatum; attenuated mitochondrial GDH activity, ATP concentration; increased mitophagy-related and mitochondrial biogenesis proteins |
| Feng et al., 2020 [44] | 16 male Wistar (n = 8 in anodal group; n = 8 in sham group) | Anodal/Sham | AE: skull bregma; R: anterior chest |
300 μA, 20 min/day, 5 days/week, 4 weeks; AEA: 37.9 mm2; CD: 0.16 mA/cm2 |
Loss of dopaminergic nigrostriatal neurons and fibers | tDCS preserved neurons in the substantia nigra, but not fibers in the striatum |

2.2. rTMS
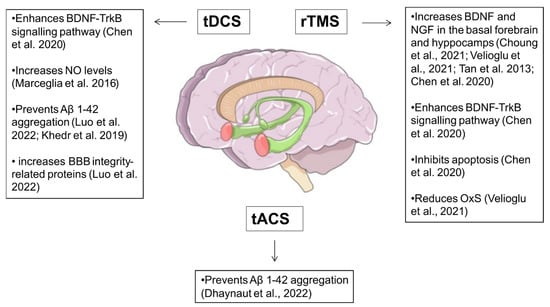
3. NIBS and Neuroprotection in Alzheimer’s Disease

| Study | NIBS Method |
Sample/Animals | Configuration | Parameters | Biological Outcomes | Biological Results |
|---|---|---|---|---|---|---|
| Tan et al., 2013 [73] | rTMS (LF) | 84 mice (n = 21 in control group; n = 21 rTMS group; n = 21 in Aβ injection; n = 21 Aβ injection + rTMS) | Whole brain stimulation | 400 pulses per session, 7 days/week, 2 weeks LF-rTMS: 20 trains (20 pulses at 1 Hz, 10 s inter-interval) |
Neuroplasticity-related proteins (BDNF, NGF and NMDA receptor) levels | LF-rTMS reversed NMDA receptor suppression, enhanced, BDNF and NGF levels |
| Marceglia et al., 2016 [83] | tDCS (anodal/sham) | 7 AD patients (n = 7 tDCS; n = 7 sham) | AE: bilateral temporo-parietal area; R: right arm |
1.5 mA, 15 min/day, 1 day AEA: 25 cm2 CD: 0.06 mA/cm2 |
total NO levels | tDCS increased NO levels |
| Khedr et al., 2019 [90] | tDCS (anodal) | 46 AD patients (n = 23 tDCS; n = 23 sham) |
AE: bilateral temporo-parietal area; R: left arm |
2 mA, 20 min each side (5 min in between), 5 days/week, 2 weeks AEA: 35 cm2 CD = 0.057 mA/cm2 |
AD brain damage biomarkers levels (TAU and Aβ 1-42) | tDCS increased Aβ 1-42 |
| Chen et al., 2020 [74] | rTMS (HF) | 30 mice (n = 15 rTMS; n = 15 sham) | Whole brain stimulation, | 600 pulses per session, 7 days/week, 2 weeks HF-rTMS 20 trains (30 pulses at 5 Hz, 2 s inter-interval) |
Synaptic plasticity-related proteins (PSD95), neurotrophic factors (BDNF, TrkB and AKT), autophagy marker proteins (p62 and LC3-II/LC3-I) | HF-rTMS increased BDNF and TrkB levels, and enhanced hippocampal cellular autophagy |
| Choung et al., 2021 [70] | rTMS (HF/LF/sham) |
24 mice (n = 8 HF-rTMS; n = 8 LF-rTMS; n = 8 sham) | Whole brain stimulation | 1600 pulses per session, 5 days/week, 2 weeks HF-rTMS: 40 trains (2 s duration at 20 Hz, 28 s inter-interval) LF-rTMS: continuous stimulation (1 Hz). |
BDNF, nestin and neuron protein levels | HF-rTMS increased BDNF, nestin and neuron expression levels in hippocampus and cortex, compared to sham |
| Velioglu et al., 2021 [71] | rTMS (HF) | 15 subjects | Left parietal cortex stimulation | 1640 pulses per session, 5 days/week, 2 weeks HF-rTMS: 42 trains (2 s duration at 20 Hz, 28 s inter-interval) |
BDNF and anti-oxidative stress proteins levels | HF-rTMS increased BDNF and anti-oxidative stress proteins levels |
| Luo et al., 2022 [88] | tDCS (anodal/sham) | 33 AD model mice (n = 11 tDCS; n = 11 not treated; n = 11 sham) | AE: frontal cortex; R: thorax |
150 µA, 30 min/day, 5 days/week, 2 weeks AEA:nr CD: nr |
Aβ plaques density in the hippocampus and frontal cortex, NVU integrity |
tDCS reduced Aβ plaques density and increased BBB integrity-related proteins |
References
- Ferrucci, R.; Mameli, F.; Guidi, I.; Mrakic-Sposta, S.; Vergari, M.; Marceglia, S.; Cogiamanian, F.; Barbieri, S.; Scarpini, E.; Priori, A. Transcranial Direct Current Stimulation Improves Recognition Memory in Alzheimer Disease. Neurology 2008, 71, 493–498.
- Lefaucheur, J.P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-Based Guidelines on the Therapeutic Use of Transcranial Direct Current Stimulation (TDCS); Elsevier Ireland Ltd.: Amsterdam, The Netherlands, 2017; Volume 128, pp. 56–92.
- Cespón, J.; Rodella, C.; Miniussi, C.; Pellicciari, M.C. Behavioural and Electrophysiological Modulations Induced by Transcranial Direct Current Stimulation in Healthy Elderly and Alzheimer’s Disease Patients: A Pilot Study. Clin. Neurophysiol. 2019, 130, 2038–2052.
- Chen, K.H.S.; Chen, R. Invasive and Noninvasive Brain Stimulation in Parkinson’s Disease: Clinical Effects and Future Perspectives. Clin. Pharmacol. Ther. 2019, 106, 763–775.
- Ferrucci, R.; Bocci, T.; Cortese, F.; Ruggiero, F.; Priori, A. Cerebellar Transcranial Direct Current Stimulation in Neurological Disease; BioMed Central Ltd.: London, UK, 2016; Volume 3.
- Guidetti, M.; Arlotti, M.; Bocci, T.; Bianchi, A.M.; Parazzini, M.; Ferrucci, R.; Priori, A. Electric Fields Induced in the Brain by Transcranial Electric Stimulation: A Review of In Vivo Recordings. Biomedicines 2022, 10, 2333.
- Liu, X.; Liu, H.; Liu, Z.; Rao, J.; Wang, J.; Wang, P.; Gong, X.; Wen, Y. Transcranial Direct Current Stimulation for Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2021, 13, 691.
- Dagan, M.; Herman, T.; Harrison, R.; Zhou, J.; Giladi, N.; Ruffini, G.; Manor, B.; Hausdorff, J.M. Multitarget Transcranial Direct Current Stimulation for Freezing of Gait in Parkinson’s Disease. Mov. Disord. 2018, 33, 642–646.
- Manor, B.; Dagan, M.; Herman, T.; Gouskova, N.A.; Vanderhorst, V.G.; Giladi, N.; Travison, T.G.; Pascual-Leone, A.; Lipsitz, L.A.; Hausdorff, J.M. Multitarget Transcranial Electrical Stimulation for Freezing of Gait: A Randomized Controlled Trial. Mov. Disord. 2021, 36, 2693–2698.
- Putzolu, M.; Ogliastro, C.; Lagravinese, G.; Bonassi, G.; Trompetto, C.; Marchese, R.; Avanzino, L.; Pelosin, E. Investigating the Effects of Transcranial Direct Current Stimulation on Obstacle Negotiation Performance in Parkinson Disease with Freezing of Gait: A Pilot Study. Brain Stimul. 2019, 12, 1583–1585.
- Valentino, F.; Cosentino, G.; Brighina, F.; Pozzi, N.G.; Sandrini, G.; Fierro, B.; Savettieri, G.; D’Amelio, M.; Pacchetti, C. Transcranial Direct Current Stimulation for Treatment of Freezing of Gait: A Cross-over Study. Mov. Disord. 2014, 29, 1064–1069.
- Rascol, O.; Payoux, P.; Ory, F.; Ferreira, J.J.; Brefel-Courbon, C.; Montastruc, J.-L. Limitations of Current Parkinson’s Disease Therapy. Ann. Neurol. 2003, 53 (Suppl. S3), S3–S15.
- Hadar, R.; Winter, R.; Edemann-Callesen, H.; Wieske, F.; Habelt, B.; Khadka, N.; Felgel-Farnholz, V.; Barroeta-Hlusicka, E.; Reis, J.; Tatarau, C.A.; et al. Prevention of Schizophrenia Deficits via Non-Invasive Adolescent Frontal Cortex Stimulation in Rats. Mol. Psychiatry 2019, 25, 896–905.
- McKinnon, C.; Gros, P.; Lee, D.J.; Hamani, C.; Lozano, A.M.; Kalia, L.V.; Kalia, S.K. Deep Brain Stimulation: Potential for Neuroprotection. Ann. Clin. Transl. Neurol. 2018, 6, 174–185.
- de Lau, L.M.; Breteler, M.M. Epidemiology of Parkinson’s Disease. Lancet Neurol. 2006, 5, 525–535.
- Balestrino, R.; Schapira, A.H.V. Parkinson Disease. Eur. J. Neurol. 2020, 27, 27–42.
- Lee, S.B.; Youn, J.; Jang, W.; Yang, H.O. Neuroprotective Effect of Anodal Transcranial Direct Current Stimulation on 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP)-Induced Neurotoxicity in Mice through Modulating Mitochondrial Dynamics. Neurochem. Int. 2019, 129, 104491.
- Hattori, N.; Mizuno, Y. Mitochondrial Dysfunction in Parkinson’s Disease. Exp. Neurobiol. 2015, 24, 103.
- Li, X.; Lu, C.; Wei, Y.; Hu, R.; Wang, Y.; Li, K. Transcranial Direct Current Stimulation Ameliorates Behavioral Deficits and Reduces Oxidative Stress in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Mouse Model of Parkinson’s Disease. Neuromodul. Technol. Neural Interface 2015, 18, 442–447.
- Laste, G.; Caumo, W.; Adachi, L.N.S.; Rozisky, J.R.; De MacEdo, I.C.; Filho, P.R.M.; Partata, W.A.; Fregni, F.; Torres, I.L.S. After-Effects of Consecutive Sessions of Transcranial Direct Current Stimulation (TDCS) in a Rat Model of Chronic Inflammation. Exp. Brain Res. 2012, 221, 75–83.
- Lynch-Day, M.A.; Mao, K.; Wang, K.; Zhao, M.; Klionsky, D.J. The Role of Autophagy in Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009357.
- Guidetti, M.; Marceglia, S.; Loh, A.; Harmsen, I.E.; Meoni, S.; Foffani, G.; Lozano, A.M.; Moro, E.; Volkmann, J.; Priori, A. Clinical Perspectives of Adaptive Deep Brain Stimulation. Brain Stimul. 2021, 14, 1238–1247.
- Hariz, M.; Blomstedt, P. Deep Brain Stimulation for Parkinson’s Disease. J. Intern. Med. 2022, 292, 764–778.
- Barker, R.A.; Drouin-Ouellet, J.; Parmar, M. Cell-Based Therapies for Parkinson Disease—Past Insights and Future Potential. Nat. Rev. Neurol. 2015, 11, 492–503.
- Madrid, J.; Benninger, D.H. Non-Invasive Brain Stimulation for Parkinson’s Disease: Clinical Evidence, Latest Concepts and Future Goals: A Systematic Review. J. Neurosci. Methods 2021, 347, 108957.
- Ganguly, J.; Murgai, A.; Sharma, S.; Aur, D.; Jog, M. Non-Invasive Transcranial Electrical Stimulation in Movement Disorders. Front. Neurosci. 2020, 14, 522.
- Benninger, D.H.; Lomarev, M.; Lopez, G.; Wassermann, E.M.; Li, X.; Considine, E.; Hallett, M. Transcranial Direct Current Stimulation for the Treatment of Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2010, 81, 1105–1111.
- Simpson, M.W.; Mak, M. The Effect of Transcranial Direct Current Stimulation on Upper Limb Motor Performance in Parkinson’s Disease: A Systematic Review. J. Neurol. 2020, 267, 3479–3488.
- Broeder, S.; Nackaerts, E.; Heremans, E.; Vervoort, G.; Meesen, R.; Verheyden, G.; Nieuwboer, A. Transcranial Direct Current Stimulation in Parkinson’s Disease: Neurophysiological Mechanisms and Behavioral Effects. Neurosci. Biobehav. Rev. 2015, 57, 105–117.
- Fregni, F.; Boggio, P.S.; Santos, M.C.; Lima, M.; Vieira, A.L.; Rigonatti, S.P.; Silva, M.T.A.; Barbosa, E.R.; Nitsche, M.A.; Pascual-Leone, A. Noninvasive Cortical Stimulation with Transcranial Direct Current Stimulation in Parkinson’s Disease. Mov. Disord. 2006, 21, 1693–1702.
- Elsner, B.; Kugler, J.; Pohl, M.; Mehrholz, J. Transcranial Direct Current Stimulation (TDCS) for Idiopathic Parkinson’s Disease. Cochrane Database Syst. Rev. 2016, 2016, CD010916.
- Doruk, D.; Gray, Z.; Bravo, G.L.; Pascual-Leone, A.; Fregni, F. Effects of TDCS on Executive Function in Parkinson’s Disease. Neurosci. Lett. 2014, 582, 27–31.
- Manto, M.; Argyropoulos, G.P.D.; Bocci, T.; Celnik, P.A.; Corben, L.A.; Guidetti, M.; Koch, G.; Priori, A.; Rothwell, J.C.; Sadnicka, A.; et al. Consensus Paper: Novel Directions and Next Steps of Non-Invasive Brain Stimulation of the Cerebellum in Health and Disease. Cerebellum 2021, 21, 1092–1122.
- Sala, G.; Bocci, T.; Borzì, V.; Parazzini, M.; Priori, A.; Ferrarese, C. Direct Current Stimulation Enhances Neuronal Alpha-Synuclein Degradation in Vitro. Sci. Rep. 2021, 11, 2197.
- Fukai, M.; Bunai, T.; Hirosawa, T.; Kikuchi, M.; Ito, S.; Minabe, Y.; Ouchi, Y. Endogenous Dopamine Release under Transcranial Direct-Current Stimulation Governs Enhanced Attention: A Study with Positron Emission Tomography. Transl. Psychiatry 2019, 9, 115.
- Fonteneau, C.; Redoute, J.; Haesebaert, F.; Le Bars, D.; Costes, N.; Suaud-Chagny, M.F.; Brunelin, J. Frontal Transcranial Direct Current Stimulation Induces Dopamine Release in the Ventral Striatum in Human. Cereb. Cortex 2018, 28, 2636–2646.
- Tanaka, T.; Takano, Y.; Tanaka, S.; Hironaka, N.; Kobayashi, K.; Hanakawa, T.; Watanabe, K.; Honda, M. Transcranial Direct-Current Stimulation Increases Extracellular Dopamine Levels in the Rat Striatum. Front. Syst. Neurosci. 2013, 7, 6.
- Fritsch, B.; Reis, J.; Martinowich, K.; Schambra, H.M.; Ji, Y.; Cohen, L.G.; Lu, B. Direct Current Stimulation Promotes BDNF-Dependent Synaptic Plasticity: Potential Implications for Motor Learning. Neuron 2010, 66, 198–204.
- Spezia Adachi, L.N.; Caumo, W.; Laste, G.; Fernandes Medeiros, L.; Ripoll Rozisky, J.; De Souza, A.; Fregni, F.; Torres, I.L.S. Reversal of Chronic Stress-Induced Pain by Transcranial Direct Current Stimulation (TDCS) in an Animal Model. Brain Res. 2012, 1489, 17–26.
- Ranieri, F.; Podda, M.V.; Riccardi, E.; Frisullo, G.; Dileone, M.; Profice, P.; Pilato, F.; di Lazzaro, V.; Grassi, C. Modulation of LTP at Rat Hippocampal CA3-CA1 Synapses by Direct Current Stimulation. J. Neurophysiol. 2012, 107, 1868–1880.
- Zigova, T.; Pencea, V.; Wiegand, S.J.; Luskin, M.B. Intraventricular Administration of BDNF Increases the Number of Newly Generated Neurons in the Adult Olfactory Bulb. Mol. Cell. Neurosci. 1998, 11, 234–245.
- Benraiss, A.; Chmielnicki, E.; Lerner, K.; Roh, D.; Goldman, S.A. Adenoviral Brain-Derived Neurotrophic Factor Induces Both Neostriatal and Olfactory Neuronal Recruitment from Endogenous Progenitor Cells in the Adult Forebrain. J. Neurosci. 2001, 21, 6718–6731.
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 2001, 24, 677.
- Feng, X.J.; Huang, Y.T.; Huang, Y.Z.; Kuo, C.W.; Peng, C.W.; Rotenberg, A.; Juan, C.H.; Pei, Y.C.; Chen, Y.H.; Chen, K.Y.; et al. Early Transcranial Direct Current Stimulation Treatment Exerts Neuroprotective Effects on 6-OHDA-Induced Parkinsonism in Rats. Brain Stimul. 2020, 13, 655–663.
- Lee, S.B.; Kim, H.T.; Yang, H.O.; Jang, W. Anodal Transcranial Direct Current Stimulation Prevents Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP)-Induced Neurotoxicity by Modulating Autophagy in an in Vivo Mouse Model of Parkinson’s Disease. Sci. Rep. 2018, 8, 15165.
- Alam, G.; Richardson, J.R. Regulation of Tyrosine Hydroxylase: Relevance to Parkinson’s Disease. Genet. Neurol. Behav. Diet Park. Dis. 2020, 2, 51–66.
- Ischiropoulos, H.; Beckman, J.S. Oxidative Stress and Nitration in Neurodegeneration: Cause, Effect, or Association? J. Clin. Investig. 2003, 111, 163.
- Deas, E.; Wood, N.W.; Plun-Favreau, H. Mitophagy and Parkinson’s Disease: The PINK1–Parkin Link. Biochim. Biophys. Acta-Mol. Cell Res. 2011, 1813, 623–633.
- Rae, C.D.; Lee, V.H.C.; Ordidge, R.J.; Alonzo, A.; Loo, C. Anodal Transcranial Direct Current Stimulation Increases Brain Intracellular PH and Modulates Bioenergetics. Int. J. Neuropsychopharmacol. 2013, 16, 1695–1706.
- Filichia, E.; Hoffer, B.; Qi, X.; Luo, Y. Inhibition of Drp1 Mitochondrial Translocation Provides Neural Protection in Dopaminergic System in a Parkinson’s Disease Model Induced by MPTP. Sci. Rep. 2016, 6, 32656.
- Majeski, A.E.; Fred Dice, J. Mechanisms of Chaperone-Mediated Autophagy. Int. J. Biochem. Cell Biol. 2004, 36, 2435–2444.
- Cuervo, A.M.; Stafanis, L.; Fredenburg, R.; Lansbury, P.T.; Sulzer, D. Impaired Degradation of Mutant Alpha-Synuclein by Chaperone-Mediated Autophagy. Science 2004, 305, 1292–1295.
- Lu, J.; Wu, M.; Yue, Z. Autophagy and Parkinson’s Disease. Adv. Exp. Med. Biol. 2020, 1207, 21–51.
- Vekrellis, K.; Stefanis, L. Targeting Intracellular and Extracellular Alpha-Synuclein as a Therapeutic Strategy in Parkinson’s Disease and Other Synucleinopathies. Expert Opin. Ther. Targets 2012, 16, 421–432.
- Chen, A.; Xiong, L.J.; Tong, Y.; Mao, M. Neuroprotective Effect of Brain-Derived Neurotrophic Factor Mediated by Autophagy through the PI3K/Akt/MTOR Pathway. Mol. Med. Rep. 2013, 8, 1011–1016.
- Hsieh, T.H.; He, X.K.; Liu, H.H.; Chen, J.J.J.; Peng, C.W.; Liu, H.L.; Rotenberg, A.; Chen, K.T.; Chang, M.Y.; Chiang, Y.H.; et al. Early Repetitive Transcranial Magnetic Stimulation Exerts Neuroprotective Effects and Improves Motor Functions in Hemiparkinsonian Rats. Neural Plast. 2021, 2021, 1763533.
- Ba, M.; Ma, G.; Ren, C.; Sun, X.; Kong, M. Repetitive Transcranial Magnetic Stimulation for Treatment of Lactacystin-Induced Parkinsonian Rat Model. Oncotarget 2017, 8, 50921–50929.
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer Disease. Nat. Rev. Dis. Prim. 2021, 7, 33.
- Chou, Y.H.; Ton That, V.; Sundman, M. A Systematic Review and Meta-Analysis of RTMS Effects on Cognitive Enhancement in Mild Cognitive Impairment and Alzheimer’s Disease. Neurobiol. Aging 2020, 86, 1–10.
- Cammisuli, D.M.; Cignoni, F.; Ceravolo, R.; Bonuccelli, U.; Castelnuovo, G. Transcranial Direct Current Stimulation (TDCS) as a Useful Rehabilitation Strategy to Improve Cognition in Patients With Alzheimer’s Disease and Parkinson’s Disease: An Updated Systematic Review of Randomized Controlled Trials. Front. Neurol. 2022, 12, 2648.
- Rajji, T.K. Transcranial Magnetic and Electrical Stimulation in Alzheimer’s Disease and Mild Cognitive Impairment: A Review of Randomized Controlled Trials. Clin. Pharmacol. Ther. 2019, 106, 776–780.
- Teselink, J.; Bawa, K.K.; Koo, G.K.; Sankhe, K.; Liu, C.S.; Rapoport, M.; Oh, P.; Marzolini, S.; Gallagher, D.; Swardfager, W.; et al. Efficacy of Non-Invasive Brain Stimulation on Global Cognition and Neuropsychiatric Symptoms in Alzheimer’s Disease and Mild Cognitive Impairment: A Meta-Analysis and Systematic Review. Ageing Res. Rev. 2021, 72, 101499.
- Lefaucheur, J.P. A Comprehensive Database of Published TDCS Clinical Trials (2005–2016). Neurophysiol. Clin. 2016, 46, 319–398.
- Xiao, N.; Le, Q.T. Neurotrophic Factors and Their Potential Applications in Tissue Regeneration. Arch. Immunol. Ther. Exp. (Warsz) 2016, 64, 89–99.
- Mitra, S.; Behbahani, H.; Eriksdotter, M. Innovative Therapy for Alzheimer’s Disease-With Focus on Biodelivery of NGF. Front. Neurosci. 2019, 13, 38.
- Ng, T.K.S.; Ho, C.S.H.; Tam, W.W.S.; Kua, E.H.; Ho, R.C.M. Decreased Serum Brain-Derived Neurotrophic Factor (BDNF) Levels in Patients with Alzheimer’s Disease (AD): A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 257.
- Straten, G.; Eschweiler, G.W.; Maetzler, W.; Laske, C.; Leyhe, T. Glial Cell-Line Derived Neurotrophic Factor (GDNF) Concentrations in Cerebrospinal Fluid and Serum of Patients with Early Alzheimer’s Disease and Normal Controls. J. Alzheimer’s Dis. 2009, 18, 331–337.
- Rockenstein, E.; Ubhi, K.; Doppler, E.; Novak, P.; Moessler, H.; Li, B.; Blanchard, J.; Grundke-Iqbal, I.; Iqbal, K.; Mante, M.; et al. Regional Comparison of the Neurogenic Effects of CNTF-Derived Peptides and Cerebrolysin in AβPP Transgenic Mice. J. Alzheimer’s Dis. 2011, 27, 743–752.
- Knipper, M.; da Penha Berzaghi, M.; Blöchl, A.; Breer, H.; Thoenen, H.; Lindholm, D. Positive Feedback between Acetylcholine and the Neurotrophins Nerve Growth Factor and Brain-Derived Neurotrophic Factor in the Rat Hippocampus. Eur. J. Neurosci. 1994, 6, 668–671.
- Choung, J.S.; Kim, J.M.; Ko, M.H.; Cho, D.S.; Kim, M.Y. Therapeutic Efficacy of Repetitive Transcranial Magnetic Stimulation in an Animal Model of Alzheimer’s Disease. Sci. Rep. 2021, 11, 437.
- Velioglu, H.A.; Hanoglu, L.; Bayraktaroglu, Z.; Toprak, G.; Guler, E.M.; Bektay, M.Y.; Mutlu-Burnaz, O.; Yulug, B. Left Lateral Parietal RTMS Improves Cognition and Modulates Resting Brain Connectivity in Patients with Alzheimer’s Disease: Possible Role of BDNF and Oxidative Stress. Neurobiol. Learn. Mem. 2021, 180, 107410.
- Chen, X.; Chen, S.; Liang, W.; Ba, F. Administration of Repetitive Transcranial Magnetic Stimulation Attenuates A β 1-42-Induced Alzheimer’s Disease in Mice by Activating β-Catenin Signaling. Biomed Res. Int. 2019, 2019, 1431760.
- Tan, T.; Xie, J.; Liu, T.; Chen, X.; Zheng, X.; Tong, Z.; Tian, X. Low-Frequency (1Hz) Repetitive Transcranial Magnetic Stimulation (RTMS) Reverses Aβ1–42-Mediated Memory Deficits in Rats. Exp. Gerontol. 2013, 48, 786–794.
- Chen, X.; Dong, G.Y.; Wang, L.X. High-Frequency Transcranial Magnetic Stimulation Protects APP/PS1 Mice against Alzheimer’s Disease Progress by Reducing APOE and Enhancing Autophagy. Brain Behav. 2020, 10, e01740.
- Ohira, K.; Hayashi, M. A New Aspect of the TrkB Signaling Pathway in Neural Plasticity. Curr. Neuropharmacol. 2009, 7, 276.
- Marceglia, S.; Mrakic-Sposta, S.; Rosa, M.; Ferrucci, R.; Mameli, F.; Vergari, M.; Arlotti, M.; Ruggiero, F.; Scarpini, E.; Galimberti, D.; et al. Transcranial Direct Current Stimulation Modulates Cortical Neuronal Activity in Alzheimer’s Disease. Front. Neurosci. 2016, 10, 134.
- Luo, Y.; Yang, H.; Yan, X.; Wu, Y.; Wei, G.; Wu, X.; Tian, X.; Xiong, Y.; Wu, G.; Wen, H. Transcranial Direct Current Stimulation Alleviates Neurovascular Unit Dysfunction in Mice With Preclinical Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 857415.
- Khedr, E.M.; Salama, R.H.; Abdel Hameed, M.; Abo Elfetoh, N.; Seif, P. Therapeutic Role of Transcranial Direct Current Stimulation in Alzheimer Disease Patients: Double-Blind, Placebo-Controlled Clinical Trial. Neurorehabil. Neural Repair 2019, 33, 384–394.
- Obulesu, M.; Lakshmi, M.J. Apoptosis in Alzheimer’s Disease: An Understanding of the Physiology, Pathology and Therapeutic Avenues. Neurochem. Res. 2014, 39, 2301–2312.
- Paradis, E.; Douillard, H.; Koutroumanis, M.; Goodyer, C.; LeBlanc, A. Amyloid Beta Peptide of Alzheimer’s Disease Downregulates Bcl-2 and Upregulates Bax Expression in Human Neurons. J. Neurosci. 1996, 16, 7533–7539.
- Feng, Y.; Wang, X. Antioxidant Therapies for Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2012, 2012, 472932.
- Selley, M.L. Increased Concentrations of Homocysteine and Asymmetric Dimethylarginine and Decreased Concentrations of Nitric Oxide in the Plasma of Patients with Alzheimer’s Disease. Neurobiol. Aging 2003, 24, 903–907.
- Guix, F.X.; Uribesalgo, I.; Coma, M.; Muñoz, F.J. The Physiology and Pathophysiology of Nitric Oxide in the Brain. Prog. Neurobiol. 2005, 76, 126–152.
- Katusic, Z.S.; Austin, S.A. Endothelial Nitric Oxide: Protector of a Healthy Mind. Eur. Heart J. 2014, 35, 888–894.
- Trivedi, D.P.; Hallock, K.J.; Bergethon, P.R. Electric Fields Caused by Blood Flow Modulate Vascular Endothelial Electrophysiology and Nitric Oxide Production. Bioelectromagnetics 2013, 34, 22–30.
- Kirabali, T.; Rust, R.; Rigotti, S.; Siccoli, A.; Nitsch, R.M.; Kulic, L. Distinct Changes in All Major Components of the Neurovascular Unit across Different Neuropathological Stages of Alzheimer’s Disease. Brain Pathol. 2020, 30, 1056–1070.
- Bai, H.; Forrester, J.V.; Zhao, M. DC Electric Stimulation Upregulates Angiogenic Factors in Endothelial Cells through Activation of VEGF Receptors. Cytokine 2011, 55, 110–115.
- Rueger, M.A.; Keuters, M.H.; Walberer, M.; Braun, R.; Klein, R.; Sparing, R.; Fink, G.R.; Graf, R.; Schroeter, M. Multi-Session Transcranial Direct Current Stimulation (TDCS) Elicits Inflammatory and Regenerative Processes in the Rat Brain. PLoS ONE 2012, 7, e43776.
- Cancel, L.M.; Arias, K.; Bikson, M.; Tarbell, J.M. Direct Current Stimulation of Endothelial Monolayers Induces a Transient and Reversible Increase in Transport Due to the Electroosmotic Effect. Sci. Rep. 2018, 8, 9265.
- Teter, B. Rodent Aging. In Encyclopedia of Neuroscience; Academic Press: Oxford, UK, 2009; pp. 397–406.
- Dhaynaut, M.; Sprugnoli, G.; Cappon, D.; Macone, J.; Sanchez, J.S.; Normandin, M.D.; Guehl, N.J.; Koch, G.; Paciorek, R.; Connor, A.; et al. Impact of 40 Hz Transcranial Alternating Current Stimulation on Cerebral Tau Burden in Patients with Alzheimer’s Disease: A Case Series. J. Alzheimer’s Dis. 2022, 85, 1667–1676.
- Luo, Y.; Yang, W.; Li, N.; Yang, X.; Zhu, B.; Wang, C.; Hou, W.; Wang, X.; Wen, H.; Tian, X. Anodal Transcranial Direct Current Stimulation Can Improve Spatial Learning and Memory and Attenuate Aβ 42 Burden at the Early Stage of Alzheimer’s Disease in APP/PS1 Transgenic Mice. Front. Aging Neurosci. 2020, 12, 134.
