Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Wan Kam Chiu.
Lymph node status is important in predicting the prognosis and guiding adjuvant treatment in endometrial cancer. However, previous studies showed that systematic lymphadenectomy conferred no therapeutic values in clinically early-stage endometrial cancer but might lead to substantial morbidity and impact on the quality of life of the patients. The sentinel lymph node is the first lymph node that tumor cells drain to, and sentinel lymph node biopsy has emerged as an acceptable alternative to full lymphadenectomy in both low-risk and high-risk endometrial cancer.
- endometrial cancer
- sentinel lymph node biopsy
- lymphadenectomy
- micro-metastasis
- isolated tumor cells
- ultra-staging
1. Introduction
Endometrial cancer (EC) is the most common gynaecological cancer in developed countries, and the incidence has been rising with aging and increased obesity of the population. Surgery is the mainstay treatment for EC. Standard surgery includes total hysterectomy and bilateral salpingo-oophorectomy, with or without pelvic/para-aortic lymphadenectomy (LND) [2,3][1][2]. Lymph node (LN) assessment is important because LN metastasis is one of the most important prognostic factors for EC [4,5][3][4]. The 5-year overall survival (OS) for pelvic LN metastasis and para-aortic LN metastasis was found to be 57% and 49%, respectively [6][5]. The knowledge of LN status can also facilitate the use of adjuvant chemotherapy and radiotherapy to reduce the risk of distant and local recurrence [7,8][6][7]. However, the therapeutic value of LND has not been established. Two large randomized controlled trials (RCTs) in 2008 and 2009 showed that pelvic LND offered no therapeutic benefits compared with no LND in clinically early-stage EC [9,10][8][9]. A more recent multicenter retrospective study also demonstrated that LND had no survival benefit in an intermediate-risk group [11][10]. The Endometrial Cancer Lymphadenectomy (ECLAT) Trial is evaluating the survival effects of comprehensive LND in the absence of bulky nodes in patients with EC stages IB to II (all histological subtypes) and stage IA endometrioid International Federation of Gynecology and Obstetrics (FIGO) grade 3, serous, clear cell, or carcinosarcomas, and the results are expected in 2023 [12][11].
Sentinel lymph node (SLN) is the first LN reached by the metastasizing cells from the primary tumor before draining to the distal LNs [13][12]. In theory, if the SLN is negative, the remaining LNs in that lymphatic chain should also be negative. SLN biopsy (SLNB) is a minimally invasive technique used to identify the SLN and occult LN metastases. Nowadays, it replaces systematic LND in selected EC patients [2,3][1][2]. However, there are still myths about its usefulness in low-risk patients, and its safety in high-risk patients.
2. Lymphatic Drainage of Endometrial Cancer
The lymphatic drainage of the endometrium is quite complex. The lymphatic drainage of the lower uterine segment is the same as that of the cervix, which drains through the parametria to the iliac and obturator nodes at the pelvic sidewall, common iliac LNs, para-aortic LNs and beyond (Figure 1). Alternative drainage near the uterine fundus develops along the gonadal vessels directly to the para-aortic nodes [14,15][13][14]. This implies that if the SLN is in the para-aortic region, it might be missed by the usual SLNB techniques that target the pelvic nodes (see Section 3). However, a prospective study of 742 patients reported that only 3% patients had isolated positive para-aortic nodes when pelvic nodes were negative [16][15].
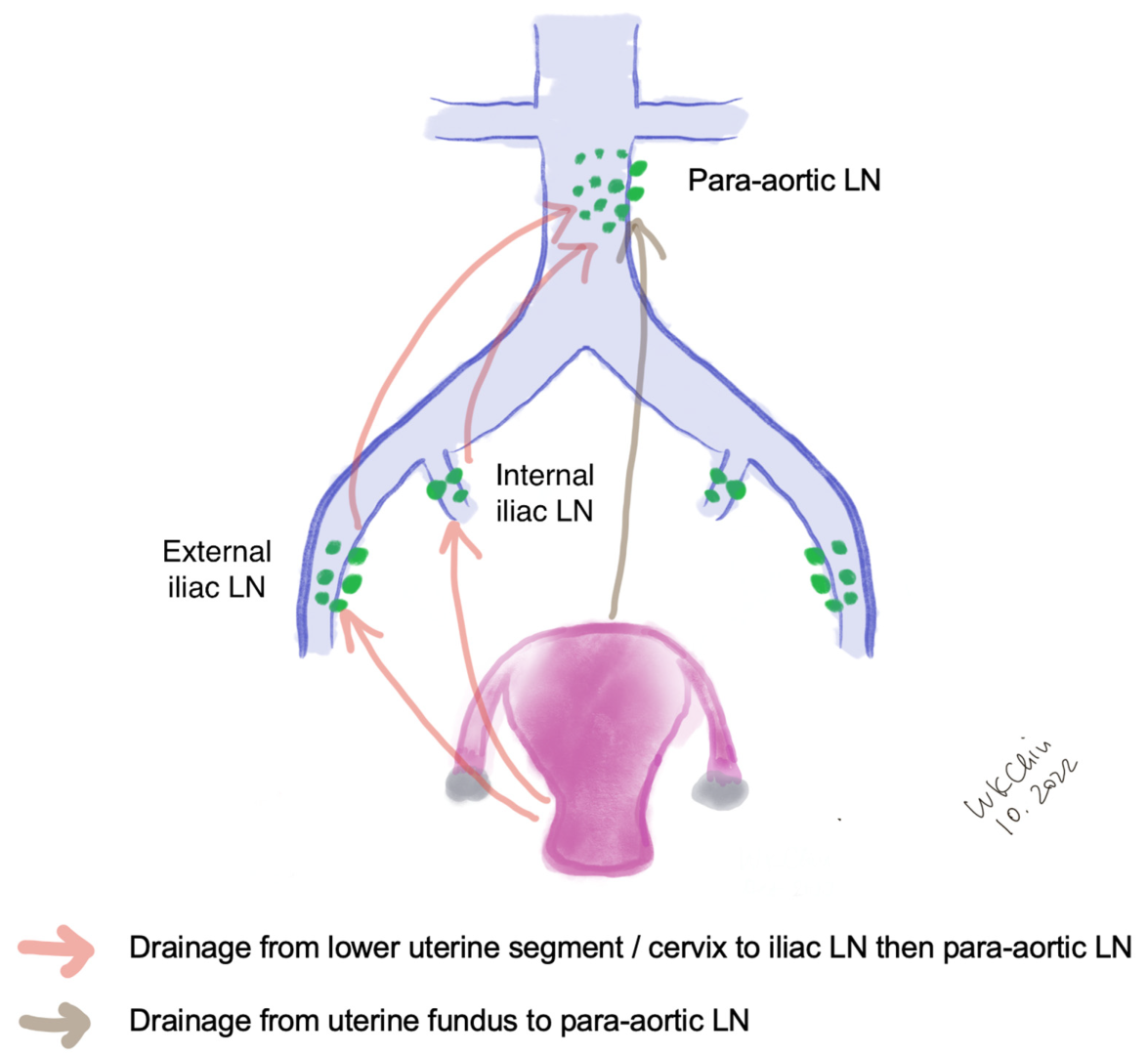
Figure 1.
Lymphatic drainage of endometrial cancer. (LN, lymph nodes).
3. Techniques of Sentinel Lymph Node Biopsy in Endometrial Cancer
Many factors such as age, depth of myometrial invasion and risk of lymphatic infiltration have been attributed to the successful rate of SLN mapping [17][16]. The performance of SLN biopsy is also affected by surgical expertise and the algorithm in SLN mapping failure [18][17].
3.1. Injection Sites
Different injection methods have been reported in the literature. Cervical injection is the most common approach. It had been shown that the cervical injection had a higher detection rate of pelvic SLN compared with the hysteroscopic injection at the endometrial tumor [19[18][19],20], while hysteroscopic injection had a slightly higher detection rate of para-aortic SLN especially isolated para-aortic LN compared to cervical injection [21][20]. This method is expensive because of the need for specialized equipment. Some reported that injection at dual sites (cervix and uterine fundal injection) increased the detection of SLN [22][21], and hysteroscopic injection and laparoscopic uterine fundal serosa injection also improved the para-aortic SLN detection [21,22][20][21]. Maramai et al. reported that in the case of failed bilateral mapping of SLN, cervical re-injection of ICG could significantly improve SLN detection rates from 73.3% to 94.5%, thus reducing the number of side-specific required lymphadenectomies [23][22].
To further improve SLN detection rate, some authors have combined preoperative lymphoscintigraphy with the injection of tracer. For example, Elisei et al. performed single-photon emission computerized tomography (SPECT) about three hours after Technetium (Tc)-99m-albumin cervical injection, and found that SPETCT could provide important anatomic information and enhance the intraoperative detection rate of SLNs [24][23]. At the same time, re-injection is another strategy that might improve the mapping rate of SLN in EC [23,25][22][24]. Cervical or hysteroscopic injections requires a long learning curve, and some studies suggested that an experienced physician was an important factor in improving the detection rate of SLN [25,26][24][25].
3.2. Injection Techniques and Tracers
Following general anesthesia, the tracer is injected into the submucosa (approximately 1–2 mm depth) and stroma (approximately 1–2 cm depth) of the cervix at 3 and 9 o’clock [21,23,24,25][20][22][23][24] (Figure 2a). According to the National Comprehensive Cancer Network (NCCN) guideline, injection at 3, 6, 9 and 12 o’clock of the cervix is another approach [3][2] (Figure 2b). The dye should be injected slowly at 5–10 sec per quadrant [27][26]. Retroperitoneal space needs to be opened. Retroperitoneal SLNs are then identified either by laparoscopic or open evaluation.
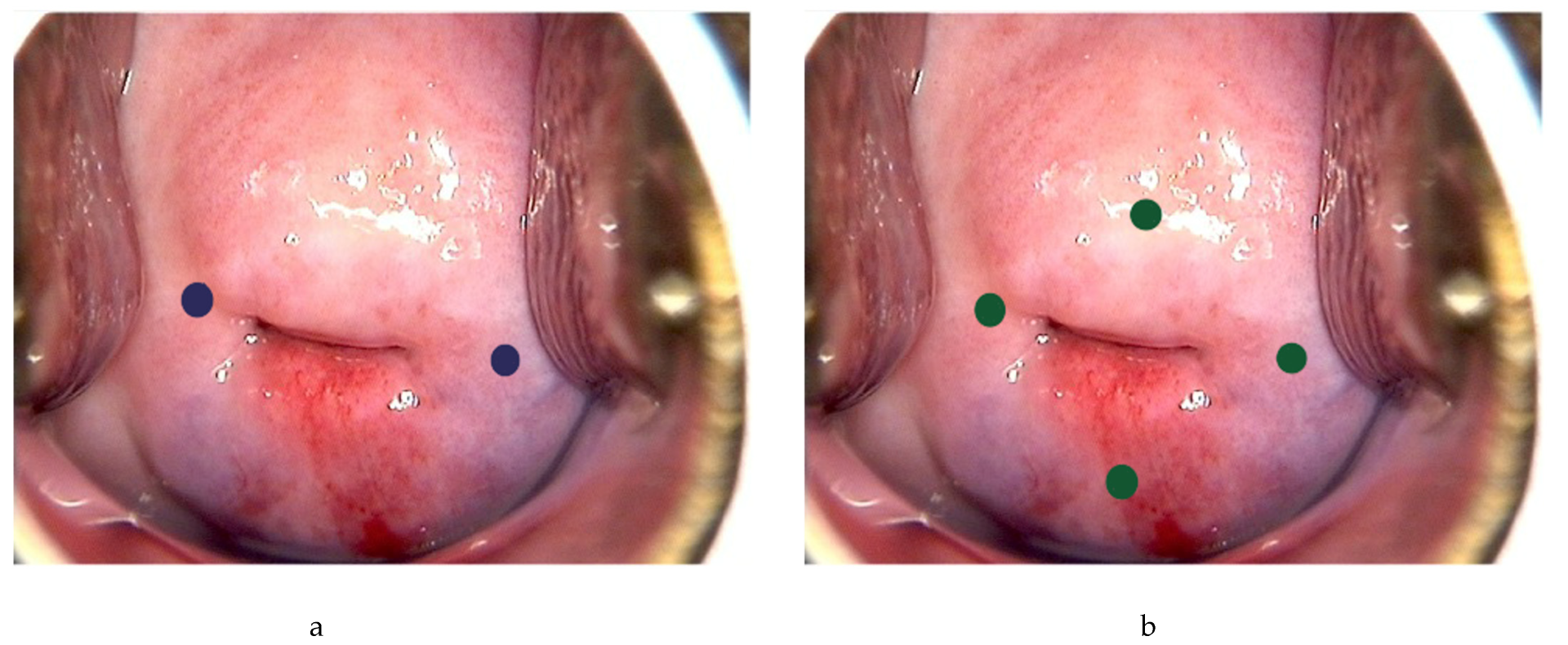
Figure 2. Injection of tracer at the cervix. Using ICG as example, 0.5–1 mL can be injected each superficially (1–3 mm at submucosa) and deeply (1–2 cm at stroma) at 3 and 9 o’ clock (blue dots) (a), or 0.5 mL each superficially and deeply at 3, 6, 9 and 12 o’clock of the cervix (green dots) (b).
Colored dyes, including isosulfan blue and methylene blue, are affordable and they do not require special equipment. However, they have a low detection rate of SLN compared to other methods [28][27]. There is also a degree of subjectivity with the visual assessment [29][28]. Isosulfan blue was also associated a 1% risk of allergic reactions including anaphylaxis [26[25][26],27], and methylene had also been found to carry a small risk of paradoxical methemoglobinemia and serotonin syndrome [30][29], making them less favored nowadays.
Tc-99m is a metastable isomer that has become one of the most commonly used medical radioisotopes in diagnostic procedures since its introduction in the 1960s [31][30]. It has a half-life of about six hours, which can avoid excessive radio-exposure to the patients and the doctors. Tc-99m is injected at 3 o’clock and 9 o’clock of the cervix, and the signal is then identified intraoperatively by a gamma probe, with or without the SPECT–CT scan. Tc-99m has a better identification rate and sensitivity than methylene blue in many malignant tumors [32][31]. It can allow preoperative detection of SLN, and facilitate the detection of uncommon sites of nodal metastasis. The risk of allergic reactions is very rare with an estimated rate of 1-10/100,000 [33][32]. However, it requires special equipment and support from radiology departments. Besides, there is a small risk of radiation exposure to the medical personnel and the patients, thus limiting its widespread use in clinical practice.
Indocyanine green (ICG) is a water-soluble tricarbocyanine dye that penetrates tissues for up to 15 mm. It absorbs light at about 800 nm and emits light at about 830 nm [34][33]. The usual recommendation is to dilute the ICG to 0.5 mg/mL to 1.25 mg/mL using sterile water; 2–4 mL are to be injected [35][34]. The light emitted by the ICG is then visualized using a near-infrared fluorescent imaging system. It has good visibility and allows penetration of signal through intact tissue. Because of its safety and effectiveness, it has been widely used in hepatobiliary surgery, cardiac surgery, urology and other fields [36][35]. A meta-analysis showed that the detection rate of SLN was 77.8% with blue dye, 80.9% with Tc-99m, 86.3% with blue dye and Tc-99m, 92.4% with ICG alone, respectively, and up to 96.7% using ICG and blue dye based on two studies and 100% using ICG and Tc-99m based on one study [37][36]. The major disadvantage of ICG is the cost due to the requirement of the near-infrared fluorescent imaging system. In addition, ICG contains sodium iodine; there is an estimated 1/42,000 risk of anaphylactic reaction and iodine allergy is a contraindication to ICG [38][37]. The pooled sensitivity was above 90% regardless the choice of tracers. There was no significant difference in the detection rate between different surgical approaches [39][38]. As ICG has limited toxicity, higher bilateral detection rate and higher detection rate in obese patients especially with BMI >30, it has become more popular compared to other tracers [28,40,41][27][39][40].
The advantages and disadvantages of different methods are summarized in Table 1.
Table 1. Summary of the SLN detection rate with different injection sites and tracers. (ICG, indocyanine green; NA, not available; SLN, sentinel lymph node; SPECT, single-photon emission computerized tomography).
| SLN Detection Rate | Other Advantages/Disadvantages | |||||
|---|---|---|---|---|---|---|
| Overall SLN | Pelvic SLN | Para-Aortic SLN | Isolated Para-Aortic SLN | Advantages | Disadvantages | |
| Cervical (dye) [20][19] | 89% | NA | NA | NA |
|
|
| Cervical (radioisotope) [20][19] | 96% | NA | NA | NA |
|
|
| Cervical (ICG) [19,21,42][18][20][41] | 82–95.1% | 77–78% | 19.5–59% | 0–6% |
|
|
| Hysteroscopic (radioisotope) [20][19] | 78% | NA | NA | NA |
|
|
| Hysteroscopic (ICG) [19,21,42][18][20][41] | 33–82.6% | 25–53% | 25–29% | 4–8% |
|
|
| Dual injection (cervical and fundal) with dual tracer (ICG and radioisotope) [22][21] | 100% | 98% | 66.7% | NA |
|
|
3.3. SLNB Algorithm
SLNB algorithm refers to (1) peritoneal and serosal inspection and peritoneal washings; (2) retroperitoneal evaluation localization of stained lymphatic channels from the parametria to the primary nodal basin, and removing all SLNs and any suspicious nodes, with frozen section if indicated (Figure 3a); (3) retroperitoneal dissection up to common iliac region, presacral region and/or para-aortic region to look for rare isolated para-aortic LN especially when no pelvic SLN is found (Figure 3b); (4) a side-specific systematic LND if SLN is not detected on the ipsilateral hemipelvis [18,27,43][17][26][42] (Figure 4). It has been shown that the rate of systematic LND was reduced from 65% to 23% since the introduction of such SLNB algorithm [27][26].
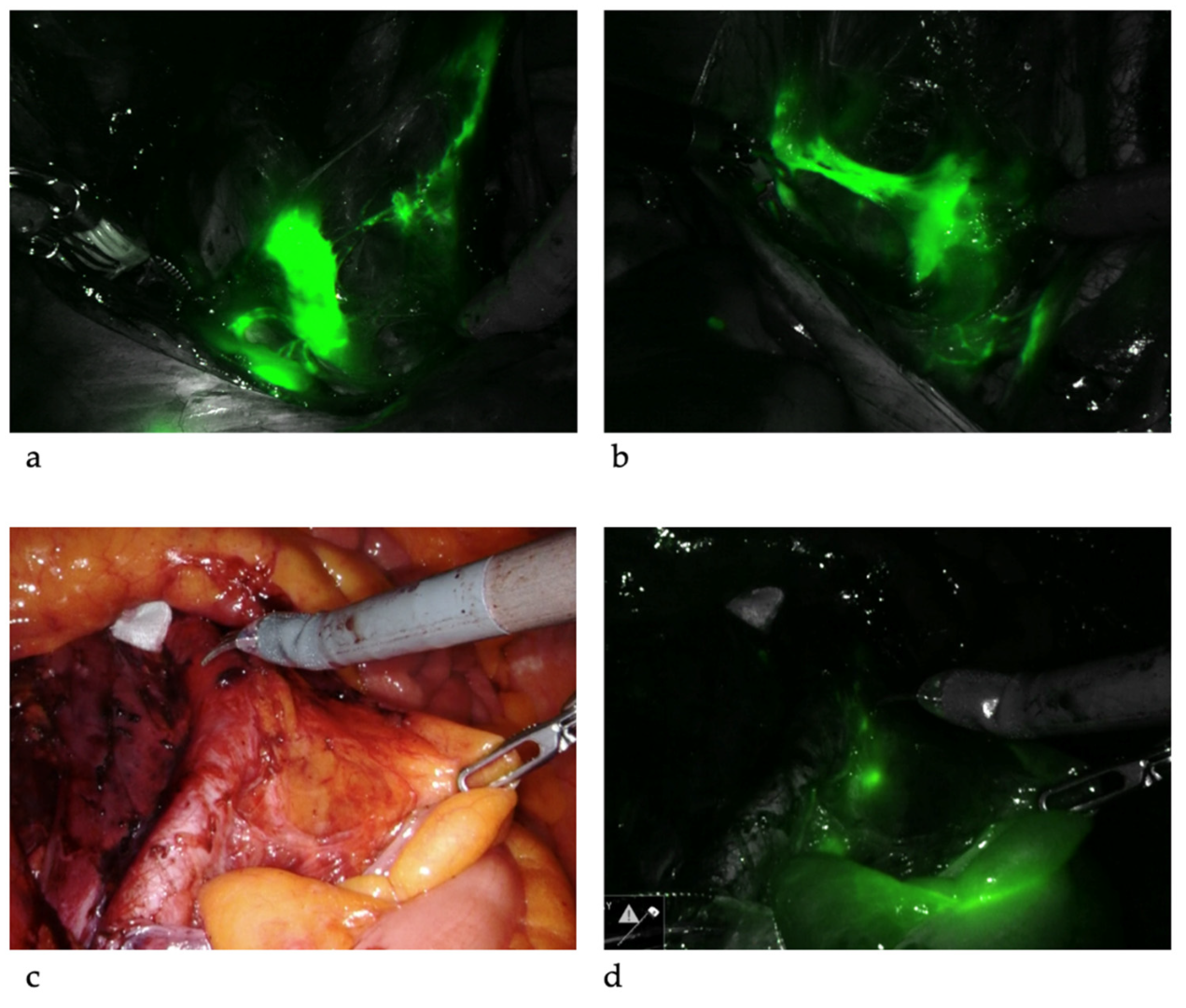
Figure 3. Sentinel lymph nodes. ICG was injected and SLNs were identified at left iliac (a) and right iliac (b) regions. When SLNs cannot be found in the pelvis, common iliac, presacral and para-aortic areas need to be explored. The picture illustrates a SLN at right common iliac near the presacral area (c,d).
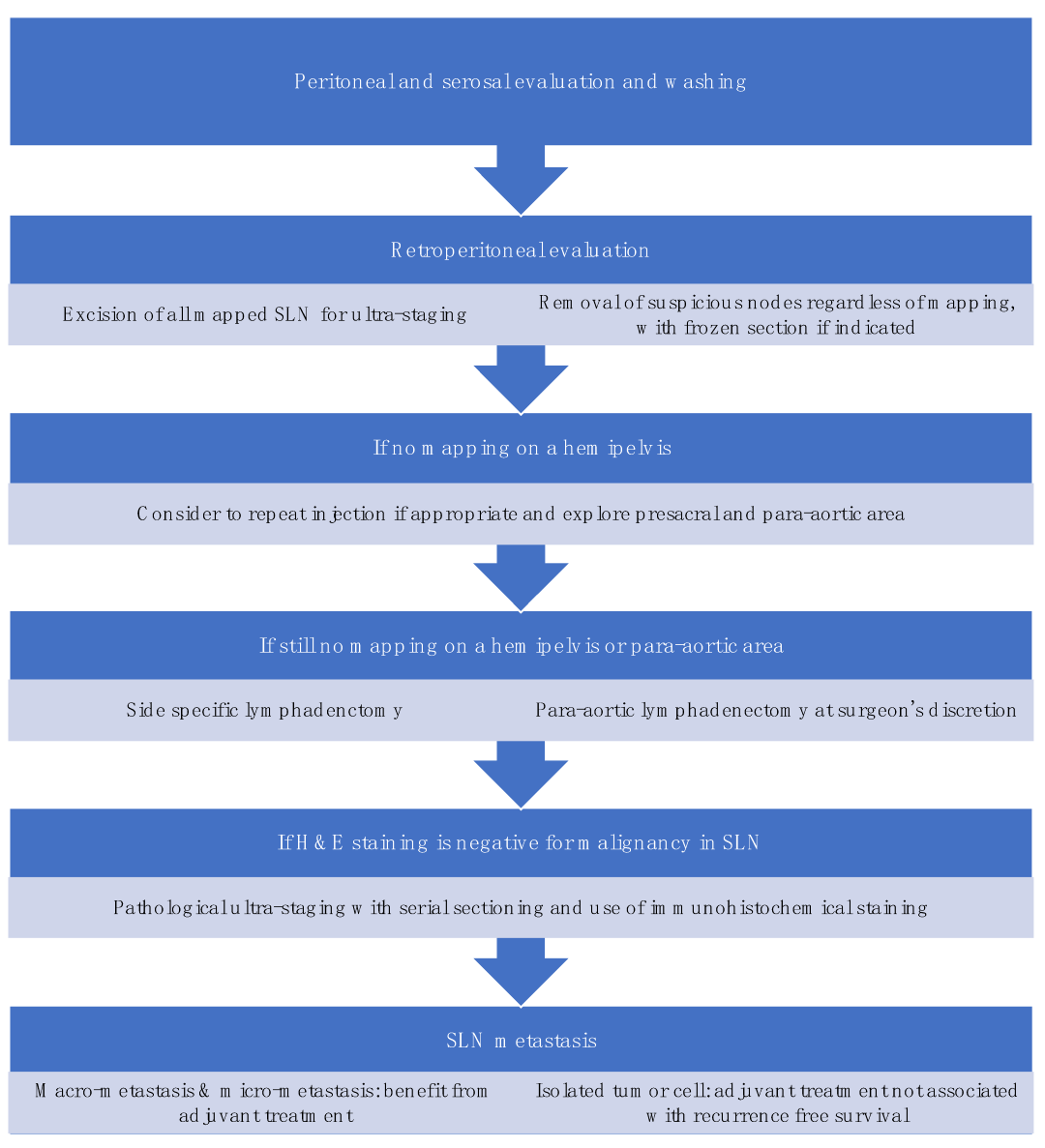
Standardization in techniques and algorithms for SLNB is important in the diagnostic accuracy of SLNB which ultimately affects the oncological outcomes of the patients. Moloney et al. developed a surgical competency tool for SLNB in minimally invasive surgery (MIS) for EC, and made a consensus recommendation on the use of tracer, injection sites and technique, the dissection for SLN, and importantly, the troubleshooting in SLN mapping failure [44][43].
References
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39.
- National Comprehensive Cancer Network. Uterine neoplasms2022; (Version 1.2022). Available online: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf (accessed on 10 September 2022).
- Randall, M.E.; Filiaci, V.; Muss, H.; Spirtos, N.M.; Mannel, R.S.; Fowler, J.; Thigpen, J.T.; Benda, J.A. Randomized Phase III Trial of Whole-Abdominal Irradiation Versus Doxorubicin and Cisplatin Chemotherapy in Advanced Endometrial Carcinoma: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2006, 24, 36–44.
- de Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; Colombo, A.; et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): Final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 295–309.
- Lewin, S.N.; Herzog, T.J.; Medel, N.I.B.; Deutsch, I.; Burke, W.M.; Sun, X.; Wright, J.D. Comparative performance of the 2009 international Federation of gynecology and obstetrics’ staging system for uterine corpus cancer. Obstet. Gynecol. 2010, 116, 1141–1149.
- Burke, W.M.; Orr, J.; Leitao, M.; Salom, E.; Gehrig, P.; Olawaiye, A.B.; Brewer, M.; Boruta, D.; Herzog, T.J.; Shahin, F.A.; et al. Endometrial cancer: A review and current management strategies: Part I. Gynecol. Oncol. 2014, 134, 385–392.
- Burke, W.M.; Orr, J.; Leitao, M.; Salom, E.; Gehrig, P.; Olawaiye, A.B.; Brewer, M.; Boruta, D.; Herzog, T.J.; Shahin, F.A.; et al. Endometrial cancer: A review and current management strategies: Part II. Gynecol. Oncol. 2014, 134, 393–402.
- Kitchener, H.; Swart, A.M.C.; Qian, Q.; Amos, C.; Parmar, M.K.B.; ASTEC Study Group. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): A randomised study. Lancet 2009, 373, 125–136.
- Panici, P.B.; Basile, S.; Maneschi, F.; Lissoni, A.A.; Signorelli, M.; Scambia, G.; Angioli, R.; Tateo, S.; Mangili, G.; Katsaros, D.; et al. Systematic Pelvic Lymphadenectomy vs No Lymphadenectomy in Early-Stage Endometrial Carcinoma: Randomized Clinical Trial. JNCI: J. Natl. Cancer Inst. 2008, 100, 1707–1716.
- Bougherara, L.; Azaïs, H.; Béhal, H.; Canlorbe, G.; Ballester, M.; Bendifallah, S.; Coutant, C.; Lavoue, V.; Ouldamer, L.; Graesslin, O.; et al. Does lymphadenectomy improve survival in patients with intermediate risk endometrial cancer? A multicentric study from the FRANCOGYN Research Group. Int. J. Gynecol. Cancer 2018, 29, 282–289.
- Emons, G.; Kim, J.-W.; Weide, K.; de Gregorio, N.; Wimberger, P.; Trillsch, F.; Gabriel, B.; Denschlag, D.; Kommoss, S.; Aydogdu, M.; et al. Endometrial Cancer Lymphadenectomy Trial (ECLAT) (pelvic and para-aortic lymphadenectomy in patients with stage I or II endometrial cancer with high risk of recurrence; AGO-OP.6). Int. J. Gynecol. Cancer 2021, 31, 1075–1079.
- Tanis, P.J.; E Nieweg, O.; Olmos, R.A.V.; Rutgers, E.J.T.; Kroon, B.B. History of sentinel node and validation of the technique. Breast Cancer Res. 2001, 3, 109–112.
- Levenback, C.F. Status of Sentinel Lymph Node Biopsy in Gynecological Cancers. Ann. Surg. Oncol. 2007, 15, 18–20.
- Gonçalves, E.; Figueiredo, O.; Costa, F. Sentinel lymph node in endometrial cancer: An overview. Gynecol. Surg. 2013, 10, 231–239.
- Kumar, S.; Podratz, K.C.; Bakkum-Gamez, J.N.; Dowdy, S.C.; Weaver, A.L.; McGree, M.E.; Cliby, W.A.; Keeney, G.L.; Thomas, G.; Mariani, A. Prospective assessment of the prevalence of pelvic, paraaortic and high paraaortic lymph node metastasis in endometrial cancer. Gynecol. Oncol. 2014, 132, 38–43.
- Bravo, W.R.M.; Vicente, A.M.G.; Álvarez, E.N.; García, B.G.; López-de la Manzanara, C.; García, J.M.C.; Castrejón, Á.S. Failure of scintigraphy lymphatic mapping in endometrial cancer. Causes and solutions. Rev. Española Med. Nucl. Imagen Mol. 2022, 41, 78–85.
- Koskas, M.; Amant, F.; Mirza, M.R.; Creutzberg, C.L. Cancer of the corpus uteri: 2021 update. Int. J. Gynecol. Obstet. 2021, 155, 45–60.
- Rossi, E.C.; Jackson, A.; Ivanova, A.; Boggess, J.F. Detection of Sentinel Nodes for Endometrial Cancer with Robotic Assisted Fluorescence Imaging: Cervical Versus Hysteroscopic Injection. Int. J. Gynecol. Cancer 2013, 23, 1704–1711.
- Niikura, H.; Kaiho-Sakuma, M.; Tokunaga, H.; Toyoshima, M.; Utsunomiya, H.; Nagase, S.; Takano, T.; Watanabe, M.; Ito, K.; Yaegashi, N. Tracer injection sites and combinations for sentinel lymph node detection in patients with endometrial cancer. Gynecol. Oncol. 2013, 131, 299–303.
- Ditto, A.; Casarin, J.; Pinelli, C.; Perrone, A.M.; Scollo, P.; Martinelli, F.; Bogani, G.; Maggiore, U.L.R.; Signorelli, M.; Chiappa, V.; et al. Hysteroscopic versus cervical injection for sentinel node detection in endometrial cancer: A multicenter prospective randomised controlled trial from the Multicenter Italian Trials in Ovarian cancer (MITO) study group. Eur. J. Cancer 2020, 140, 1–10.
- Torrent, A.; Amengual, J.; Sampol, C.M.; Ruiz, M.; Rioja, J.; Matheu, G.; Poca, P.; Cordoba, O. Sentinel Lymph Node Biopsy in Endometrial Cancer: Dual Injection, Dual Tracer—A Multidisciplinary Exhaustive Approach to Nodal Staging. Cancers 2022, 14, 929.
- Maramai, M.; Achilarre, M.; Aloisi, A.; Betella, I.; Bogliolo, S.; Garbi, A.; Maruccio, M.; Quatrale, C.; Aletti, G.; Mariani, A.; et al. Cervical re-injection of indocyanine green to improve sentinel lymph node detection in endometrial cancer. Gynecol. Oncol. 2021, 162, 38–42.
- Elisei, F.; Crivellaro, C.; Giuliani, D.; Dolci, C.; De Ponti, E.; Montanelli, L.; La Manna, M.; Guerra, L.; Arosio, M.; Landoni, C.; et al. Sentinel-node mapping in endometrial cancer patients: Comparing SPECT/CT, gamma-probe and dye. Ann. Nucl. Med. 2016, 31, 93–99.
- Capozzi, V.A.; Valentina, C.; Giulio, S.; Alessandra, C.; Giulia, G.; Giulia, A.; Vito, C.; Roberto, B. Sentinel node mapping in endometrial cancer: Tips and tricks to improve bilateral detection rate. The sentitricks study, a monocentric experience. Taiwan J. Obstet. Gynecol. 2021, 60, 31–35.
- Bogani, G.; Ditto, A.; Martinelli, F.; Signorelli, M.; Perotto, S.; Lorusso, D.; Raspagliesi, F. A critical assessment on the role of sentinel node mapping in endometrial cancer. J. Gynecol. Oncol. 2015, 26, 252–254.
- Abu-Rustum, N.R. Sentinel Lymph Node Mapping for Endometrial Cancer: A Modern Approach to Surgical Staging. J. Natl. Compr. Cancer Netw. 2014, 12, 288–297.
- Sinno, A.K.; Fader, A.N.; Roche, K.L.; Giuntoli, R.L., II; Tanner, E.J. A comparison of colorimetric versus fluorometric sentinel lymph node mapping during robotic surgery for endometrial cancer. Gynecol. Oncol. 2014, 134, 281–286.
- Ruscito, I.; Gasparri, M.L.; Braicu, E.I.; Bellati, F.; Raio, L.; Sehouli, J.; Mueller, M.D.; Panici, P.B.; Papadia, A. Sentinel Node Mapping in Cervical and Endometrial Cancer: Indocyanine Green Versus Other Conventional Dyes—A Meta-Analysis. Ann. Surg. Oncol. 2016, 23, 3749–3756.
- Holloway, R.W.; Abu-Rustum, N.R.; Backes, F.J.; Boggess, J.F.; Gotlieb, W.H.; Lowery, W.J.; Rossi, E.C.; Tanner, E.J.; Wolsky, R.J. Sentinel lymph node mapping and staging in endometrial cancer: A Society of Gynecologic Oncology literature review with consensus recommendations. Gynecol. Oncol. 2017, 146, 405–415.
- Richards, P.; Tucker, W.D.; Srivastava, S.C. Technetium-99m: An historical perspective. Int. J. Appl. Radiat. Isot. 1982, 33, 793–799.
- Tausch, C.; Baege, A.; Rageth, C. Mapping lymph nodes in cancer management—Role of 99mTc-tilmanocept injection. OncoTargets Ther. 2014, 7, 1151–1158.
- Salvatori, M.; Treglia, G.; Mores, N. Further considerations on adverse reactions to radiopharmaceuticals. Eur. J. Pediatr. 2012, 39, 1360–1362.
- Lin, J.; Lin, L.-S.; Chen, D.-R.; Lin, K.-J.; Wang, Y.-F.; Chang, Y.-J. Indocyanine green fluorescence method for sentinel lymph node biopsy in breast cancer. Asian J. Surg. 2020, 43, 1149–1153.
- Jewell, E.L.; Huang, J.J.; Abu-Rustum, N.R.; Gardner, G.J.; Brown, C.L.; Sonoda, Y.; Barakat, R.R.; Levine, D.A.; Leitao, M.M. Detection of sentinel lymph nodes in minimally invasive surgery using indocyanine green and near-infrared fluorescence imaging for uterine and cervical malignancies. Gynecol. Oncol. 2014, 133, 274–277.
- Lau, C.T.; Au, D.M.; Wong, K.K.Y. Application of indocyanine green in pediatric surgery. Pediatr. Surg. Int. 2019, 35, 1035–1041.
- Nagar, H.; Wietek, N.; Goodall, R.J.; Hughes, W.; Schmidt-Hansen, M.; Morrison, J. Sentinel node biopsy for diagnosis of lymph node involvement in endometrial cancer. Cochrane Database Syst. Rev. 2021, 6, CD013021.
- Holloway, R.W.; Bravo, R.A.M.; Rakowski, J.A.; James, J.A.; Jeppson, C.N.; Ingersoll, S.B.; Ahmad, S. Detection of sentinel lymph nodes in patients with endometrial cancer undergoing robotic-assisted staging: A comparison of colorimetric and fluorescence imaging. Gynecol. Oncol. 2012, 126, 25–29.
- Rozenholc, A.; Samouelian, V.; Warkus, T.; Gauthier, P.; Provencher, D.; Gauthier, F.; Drakopoulos, P.; Cormier, B. Green versus blue: Randomized controlled trial comparing indocyanine green with methylene blue for sentinel lymph node detection in endometrial cancer. Gynecol. Oncol. 2019, 153, 500–504.
- Papadia, A.; Gasparri, M.L.; Buda, A.; Mueller, M.D. Sentinel lymph node mapping in endometrial cancer: Comparison of fluorescence dye with traditional radiocolloid and blue. J. Cancer Res. Clin. Oncol. 2017, 143, 2039–2048.
- Tanner, E.J.; Sinno, A.; Stone, R.L.; Levinson, K.L.; Long, K.C.; Fader, A.N. Factors associated with successful bilateral sentinel lymph node mapping in endometrial cancer. Gynecol. Oncol. 2015, 138, 542–547.
- Rossi, E.C.; Kowalski, L.D.; Scalici, J.; Cantrell, L.; Schuler, K.; Hanna, R.K.; Method, M.; Ade, M.; Ivanova, A.; Boggess, J.F. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): A multicentre, prospective, cohort study. Lancet Oncol. 2017, 18, 384–392.
- Tanner, E.; Puechl, A.; Levinson, K.; Havrilesky, L.J.; Sinno, A.; Secord, A.A.; Fader, A.N.; Lee, P.S. Use of a novel sentinel lymph node mapping algorithm reduces the need for pelvic lymphadenectomy in low-grade endometrial cancer. Gynecol Oncol. 2017, 147, 535–540.
- Moloney, K.; Janda, M.; Frumovitz, M.; Leitao, M.; Abu-Rustum, N.R.; Rossi, E.; Nicklin, J.L.; Plante, M.; Lecuru, F.R.; Buda, A.; et al. Development of a surgical competency assessment tool for sentinel lymph node dissection by minimally invasive surgery for endometrial cancer. Int. J. Gynecol. Cancer 2021, 31, 647–655.
More
