Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Marina Koland.
Several acute and chronic inflammatory diseases of the joints affect individuals. Intra-articular (IA) drug delivery is often preferred when the joint disease is severe and painful since it places high concentrations of the drug directly at the desired site. Researchers have reported different strategies for increasing the local retention of the intra-articularly administered medication.
- thermosensitive
- intra-articular
- gels
- poloxamer
- joint disease
1. Introduction
The most prevalent joint disorders are osteoarthritis, rheumatoid arthritis, and gouty arthritis [1]. Severe chronic diseases such as rheumatoid arthritis are debilitating and even destructive enough to the joints to require surgery for replacement [2,3][2][3]. The commonly used drugs for the treatment of joint disorders include nonsteroidal anti-inflammatory drugs (NSAIDs), COX-2 enzyme inhibitors, corticosteroids, disease-modifying osteoarthritis drugs (DMOADs), and disease-modifying anti-rheumatic drugs (DMARDs) such as methotrexate, which are systemically administered. Some of these drugs are also occasionally injected into the joints (intra-articular injection) to treat synovitis, inflammation, and pain.
Intra-articular (IA) drug delivery is often preferred when the joint disease is severe and painful since it places high concentrations of the drug directly at the desired site, with a faster onset of action and increasing its bioavailability while reducing side effects associated with systemic delivery [4]. For pain management and joint lubrication, the most common drugs administered by IA are corticosteroids and hyaluronic acid (HA) [5]. However, intra-articular drug delivery for treating joint diseases has always been challenging. The retention of the drugs at the site of injection is poor, and they are rapidly cleared from the site. Small drug molecules are removed from the joint tissue by rapid uptake into the synovial capillaries and lymphatics [6].
Researchers have reported different strategies for increasing the local retention of the intra-articularly administered medication. They have designed drug delivery systems with slow-release effects that prolong the duration of action or reduce clearance from the administration site. These formulations include microspheres [7], nanoparticles [8], liposomes [9], and gels [10].
The IA administration of drug-incorporated hydrogels has drawn much attention recently. Their viscosity allows retention of the drug at the joint site, enables sustained release of the drug into the surrounding tissues, and provides prolonged effects. Their higher water content has a lubricating and soothing effect on the inflamed tissues, mimicking the soft tissues within the synovium [11].
Of particular interest in IA delivery of gels are in situ forming hydrogels, which can be injected as solutions and suspensions but gel under physiological conditions such as a change in pH, temperature, or ionic composition (Figure 1). The viscoelastic properties of the system enable the measurement of precise doses before administration and retention in the joints after injection due to gelation [12]. The gelation at the injection site provides a depot for the slow and continuous release of the incorporated drug. Such formulations increase drug bioavailability and achieve high local drug concentrations while minimizing systemic toxicity by decreasing dosing frequency.
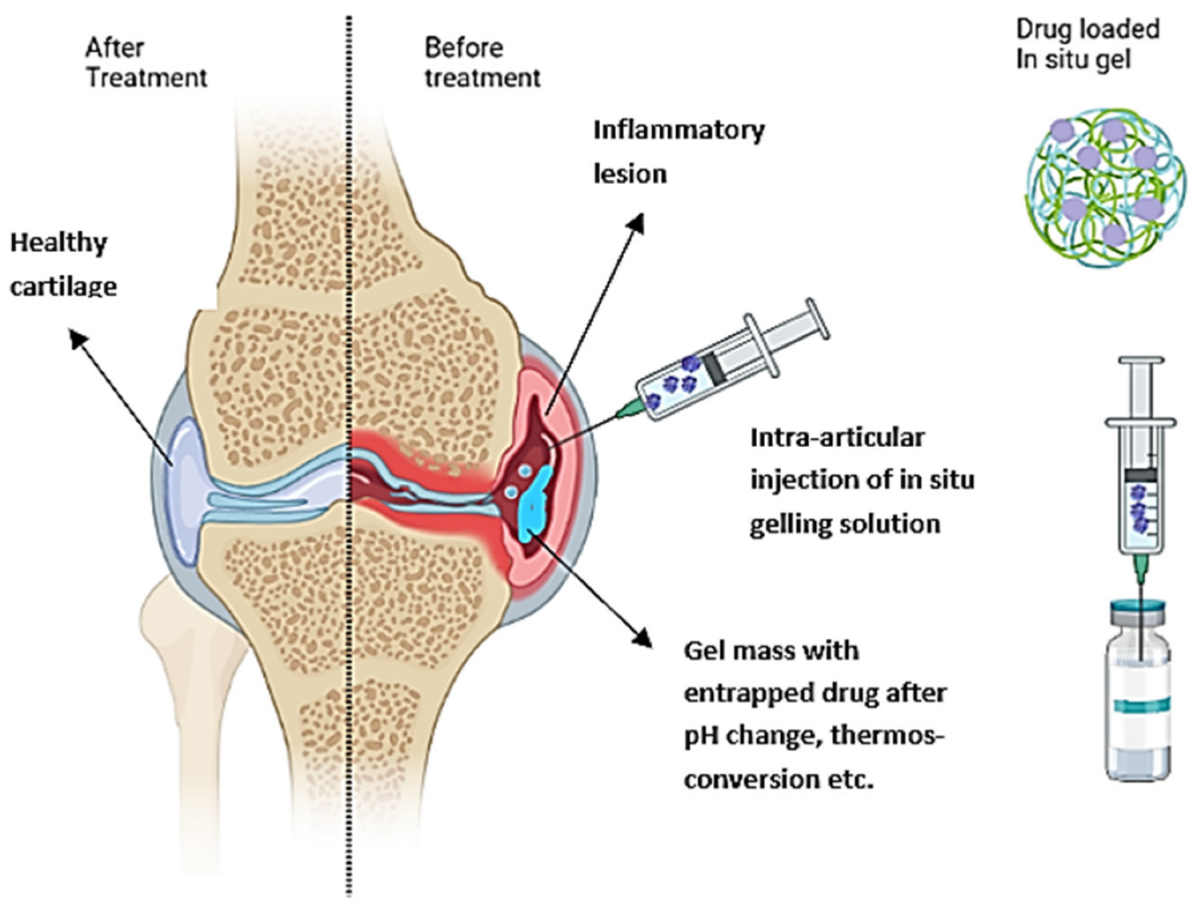
Figure 1. Schematic representation of the IA administration of an in situ gelling drug formulation into inflamed osteoarthritis (OA) joint and healthy tissue after treatment.
2. Intra-Articular Administration of Therapeutics
Intra-articular therapy includes long-term medications such as NSAIDs, corticosteroids, and other biologics [16][13]. In situ forming hydrogels are a simple and effective method to prolong the retention of drugs in the joints and their duration of action. Several drug delivery technologies are often combined with in situ gelling systems. Biodegradable nano-drug delivery systems are nontoxic, enhancing drug stability and delivering the drug in a controlled manner [17][14]. Utilizing micro- and nanoparticles increases the medication’s retention time at the joint, maintains the drug concentration at the desired level through a controlled release mechanism, and eliminates the potential side effects of the drug through low-dose administration. On the other hand, micro-sized particles are easily ingested by macrophages in synovial linings, but nano-sized particles readily escape from the joint cavity [18][15]. In situ forming hydrogels for intra-articular administration have only recently been launched. Many studies point to the possibility of hydrogels being used to treat osteoarthritis. After that, some treatment strategies and hydrogel formulation designs containing NSAIDs/bioactive molecules suitable for IA therapy are discussed.
2.1. Types of Intra-Articular Treatments in Joint Diseases such as Osteoarthritis
Intra-articular therapy (IAT) is a restorative procedure that healthcare experts widely utilize. IAT helps individuals with joint synovitis, effusion, and discomfort from inflammatory arthritis and osteoarthritis [19][16]. Treatments for osteoarthritis (OA) can be divided into surgical, pharmacological, and nonpharmacological [20][17]. Patient education and self-management are two nonpharmacological modalities recommended by the European League Against Rheumatism (EULAR). Other nonpharmacological modalities include naturopathy, acupressure, walking aids, shoe and insole modification, and electromagnetic therapy [21][18]. These are beneficial; nevertheless, many patients fail to complete the treatment over time. Marrow stimulation, arthroscopic debridement, and articular cartilage replacement are utilized to heal cartilage defects and degeneration. These procedures are very sophisticated and depend on healthy cartilage [22][19]. Pharmacological therapies include symptomatic slow-acting drugs (glucosamine and chondroitin sulfate), opioid analgesics, nonsteroidal anti-inflammatory drugs (NSAIDs), and IA injections of substances such as corticosteroids, blood-derived products, and HA [23][20]. Osteoarthritis Research Society International (OARSI) recommends considering side effects while choosing medications for osteoarthritis patients. New treatments, such as nerve growth factor (NGF) antibodies, have been evaluated and have shown positive results in reducing pain in patients with hip and knee OA [24][21]. Patients in the late stages of OA, especially young, active patients with intermediate radiographic severity in OA, may benefit from surgical treatments, including joint replacement surgery, knee osteotomy, and knee-joint separation [25][22]. To give this, clinicians must have complete knowledge of all appropriate treatment options, including their risks, availability, and cost effectiveness [26][23].
2.1.1. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
NSAIDs are the pharmacological treatment of choice for osteoarthritis (OA). Numerous placebo-controlled trials have demonstrated that NSAIDs provide higher pain relief than placebo, with standardized mean differences in pain and function ratings of 0.33 standard deviations, indicating a moderate benefit [27][24]. Topical NSAIDs, for example, were strongly suggested for people with knee OA (Level 1A: 75% in favor and >50% strong recommendation). COX-2 inhibitors were indicated at Level 1B for people with gastrointestinal comorbidities, and NSAIDs were recommended at the second level; oral NSAIDs were not advised for those with cardiovascular comorbidities or infirmity. The use of corticosteroids has the drawback of inducing cartilage degeneration over time; as a result, NSAIDs could be an alternative to administering corticosteroids [28][25]. Berrin et al. developed an in situ gelling hydrogel containing nanoparticles for the prolonged local delivery of diclofenac sodium via emulsion–solvent evaporation. Compared to the conventional delivery system, the system described above released the drug at a controlled rate over thirty days. This slow release was accomplished by combining the formulations of polymeric nanoparticles and poloxomer 407-chitosan in situ hydrogels [29][26].
2.1.2. Hyaluronic Acid
Nonsulfated hyaluronic acid (HA) is a naturally occurring glycosaminoglycan with distinct physicochemical properties; it is made up of D-glucuronic acid and N-acetylglucosamine units that are repeated in an alternating fashion. HA is found in rooster combs, shark skin, bovine eyeballs, bovine nasal cartilage, rabbit brain, rabbit heart, etc. [30][27]. Viscous supplementation using intra-articular (IA) injections of HA has been widely and successfully utilized from 1987 to 1988 in Japan and Italy [31][28]. The intra-articular HA treatment typically entails a series of injections, administered by a specialized physician, and spaced apart by one week. Since 2004, a single-injection IA HA therapy (3 mL, 60 mg) has been suggested as an alternative to the multi-injection regimen (currently 3 × 2 mL, 20 mg per injection), dispensing the same quantity of HA. A single injection would reduce the number of medical visits, intrusive procedures, and other associated risks [32][29]. HA increases the viscosity and elasticity of synovial fluid. Synovial fluid with an average HA concentration works as a viscous lubricant and an elastic shock absorber during slow joint movements. Studies showed that treatment of HA produces chondroprotection, which is caused by HA binding to CD44, which inhibits interleukin (IL)-1β expression, leading to a decline in matrix metalloproteinase (MMP) −1, 2, 3, 9, and 13 production [33,34][30][31]. Liling et al. recently developed in situ gelling nanosystems for combined therapeutic efficacy. By co-loading hyaluronic acid (HA) and celecoxib, formulated as the HLC precursor, this study created a lyotropic liquid crystal (LLC) precursor. Medication retention in the articular cavity is effectively prolonged, leading to an anti-inflammatory effect that lasts longer. According to rheological tests, HLC gel with a cubic lattice structure has a spring-like effect to cushion joint shock. It is promising to protect cartilage by preventing mechanical wear and tear, lubricating joints, and reducing intense stress (about 50 percent). In vivo degradation studies proved that the system was biocompatible and biodegradable [35][32]. To summarize, intra-articular HA injections are acceptable, may be effective, and may alleviate pain in mild OA of the knee for up to 170 days.
2.1.3. Corticosteroid Injections
White and Norton launched the first clinical testing of IA corticosteroid injection in 1958, which the American College of Rheumatology currently supports [36][33]. Corticosteroids possess immunosuppressive and anti-inflammatory properties, but their pharmacological action is complicated. The Food and Drug Administration (FDA) has approved five injectable corticosteroids for IA injections. Methylprednisolone acetate, triamcinolone acetate, triamcinolone hexacetonide, betamethasone acetate, betamethasone sodium phosphate, and dexamethasone are among them. Through their direct action on nuclear steroid receptors, corticosteroids suppress the inflammatory and immunological response at several points [37][34]. Multiple studies have found that intra-articular corticosteroid injections have a duration of effect that can last anywhere from 1 week to 24 weeks. There is unanimity that steroid injections provide around one week of relief to patients. Corticosteroid injections might have harmful side effects. Joint infection after using corticosteroids is a very uncommon occurrence. Skin atrophy, pain, tendinopathy, and systemic hyperglycemia are just some of the additional issues that may arise and the mortality rate is estimated to be around 11% [38][35].
2.1.4. Platelet-Rich Plasma (PRP)
Since the 1990s and more recently, platelet-rich plasma (PRP), a naturally occurring mixture of highly concentrated platelets, associated growth factors, and other bioactive components produced by centrifugal separation of whole blood, has been used in maxillofacial and plastic surgery to treat bone, tendon, and ligament injuries [39][36]. The various solutions commonly contain PDGF, IGF-1, and TGF-B as their growth factors. Other growth factors may also be present. Studies using PRP have shown some degree of anabolic effects on chondrocytes, such as the deposition of type II collagen. IGF-1 promotes the synthesis of type II collagen, proteoglycans, and other extracellular matrix components in the joint, contributing to its anabolic effects. These components can enhance adhesion between chondrocytes and prevent proteolysis in the milieu of the extracellular matrix. IGF-1 is responsible for stimulating the production of type II collagen, proteoglycans, and other extracellular matrix components in the joint. On chondrocytes, PDGF and TGF-beta both have anabolic effects. However, the rise in chondrocyte production is brought on by PDGF. Pro-inflammatory cytokines such as NF-kB and IL-1 have dropped in PRP. TGF-beta also promotes the differentiation of stromal cells and mesenchymal stem cells into chondrocytes [40][37]. The most prevalent adverse effects were pain at the injection site, joint stiffness, vertigo, headache, nausea, dyspepsia, perspiration, and tachycardia [41][38]. With PRP, pain relief seems to start about two months after injection and could last for up to a year. Compared to hyaluronic acid, PRP was found to be beneficial based on ratings from the international functional knee documentation committee (IKDC) and VAS pain scores; however, publication bias may exist. Additionally, these advantages might be enhanced in younger patients and those who have diseases that specifically affect cartilage tissue [37][34].
2.1.5. Stem Cell Therapy
Mesenchymal stem cells (MSCs) are a possible source for the therapy of OA due to their ability to differentiate into chondrocytes and regulate the immune system [42][39]. MSCs were first isolated from bone marrow and later from other tissues, such as the placenta, umbilical cord, cord blood, amniotic fluid, dental pulp, and adipose tissue [43][40]. There are three essential elements for cartilage tissue engineering: cells, scaffold, and environment. Adult stem cells, specifically multipotent mesenchymal stem cells, are the cell type of choice for tissue engineering due to their ease of isolation, expansion, and multilineage differentiation potential. MSCs can be employed as progenitor cells to create cartilage implants that can heal chondral and osteochondral lesions and as trophic generators of bioactive molecules to stimulate endogenous regeneration processes in the OA joint [30][27]. MSCs have been successfully employed in preclinical models to resurface degenerated cartilage. In early phase clinical trials, intra-articular (IA) administration of MSCs leads to pain reduction and cartilage protection or healing [44][41]. Direct IA injection of MSCs would eliminate surgeries and adverse effects such as periosteal hypertrophy and ossification, immunological reactivity, and disease transmission from xenograft coverage. More relevantly, the injection is simple and easy to use, which may lead to better treatment options, especially for older people with multiple health problems. Despite this promise, no clinical studies have been carried out; only a few case reports have been published. In situ hydrogel-forming materials provide an extracellular matrix for administered stem cells. The matrix offers a favorable environment for the cells with mechanical support [45][42].
Various therapeutics administered by IA injection in the management of joint diseases are summarized in Table 1.
Table 1.
Types of intra-articular treatments used in the management of joint disorders.
| NSAIDs | Hyaluronic Acid | Corticosteroids | Platelet-Rich Plasma | Mesenchymal Stem Cells |
|
|---|---|---|---|---|---|
| Constituents | Aspirin, Ibuprofen, Naproxen, Celecoxib, Diclofenac, Ketoprofen | Hyaluronic acid | Betamethasone acetate, Dexamethasone acetate, Triamcinolone hexacetonide, Betamethasone sodium phosphate, Prednisone tebutate |
Cells and coagulation factors, anticoagulants, fibrinogen, activators, platelet-rich fibrin, leukocyte-rich plasma |
Suspension of mesenchymal stem cells |
| Advantages | Inexpensive Noninfectious Used as monotherapy |
Relatively safe Benefit up to 60 days |
Low dose is required: eg. triamcinolone acetonide (5 mg). Adverse effect is very low Provide pain relief and reduce joint effusions |
Simple Low cost Minimally invasive Reduce inflammation, pain relief, improved function, and possible cartilage regeneration |
Safe and encouraging results for articular cartilage repair and regeneration |
| Disadvantages | Dose-dependent toxicity. Using NSAIDs in the short term. Long-term usage may cause liver toxicity |
Risk of infection Local adverse events after injection due to injection technique, partially flexed knee, etc. Other side effects are acute synovitis, joint swelling for up to 3 weeks, haemarthrosis, pseudogout, muscle pain |
Short-lived beneficial effect: not more than one week. IA corticosteroids seem to produce time and dose-dependent deleterious effects on articular cartilage with erosion, decreased glycosaminoglycans content, and joint narrowing Local side effects include post-injection flare, infection, and skin hypopigmentation |
PRP formulations are complex Mechanisms of action in a joint with OA remain unanswered. More clinical evidence required Optimal therapeutic protocol not yet been established related to timing, dosage, volume, frequency, and composition |
Preparation is complex Exact mechanism of action of MSCs is debated Long-term clinical trial studies are required |
| References | [21,22,26,27][18][19][23][24] | [26,31,32,33][23][28][29][30] | [19,23,46,[20][4347][16]][44] | [26,2339,]41,47][[36][38][44] | [26,37,][40,23][34][37]42[39] |
References
- Burt, H.M.; Tsallas, A.; Gilchrist, S.; Liang, L.S. Intra-Articular Drug Delivery Systems: Overcoming the Shortcomings of Joint Disease Therapy. Expert Opin. Drug Deliv. 2009, 6, 17–26.
- Wasserman, A.M. Diagnosis and Management of Rheumatoid Arthritis. Am. Fam. Physician 2011, 84, 1245–1252.
- Smolen, J.S.; Steiner, G. Therapeutic Strategies for Rheumatoid Arthritis. Nat. Rev. Drug Discov. 2003, 2, 473–488.
- Rai, M.F.; Pham, C.T. Intra-Articular Drug Delivery Systems for Joint Diseases. Curr. Opin. Pharmacol. 2018, 40, 67.
- Ma, L.; Zheng, X.; Lin, R.; Sun, A.R.J.; Song, J.; Ye, Z.; Liang, D.; Zhang, M.; Tian, J.; Zhou, X.; et al. Knee Osteoarthritis Therapy: Recent Advances in Intra-Articular Drug Delivery Systems. Drug Des. Devel. Ther. 2022, 16, 1311–1347.
- Evans, C.H.; Kraus, V.B.; Setton, L.A. Progress in Intra-Articular Therapy. Nat. Rev. Rheumatol. 2014, 10, 11–22.
- Zhang, X.; Shi, Y.; Zhang, Z.; Yang, Z.; Huang, G. Intra-Articular Delivery of Tetramethylpyrazine Microspheres with Enhanced Articular Cavity Retention for Treating Osteoarthritis. Asian J. Pharm. Sci. 2018, 13, 229–238.
- Agarwal, R.; Volkmer, T.M.; Wang, P.; Lee, L.A.; Wang, Q.; García, A.J. Synthesis of Self-Assembled IL-1Ra-Presenting Nanoparticles for the Treatment of Osteoarthritis. J. Biomed. Mater. Res. A 2016, 104, 595–599.
- Pawar, V.A.; Manjappa, A.S.; Murumkar, P.R.; Gajaria, T.K.; Devkar, R.V.; Mishra, A.K.; Yadav, M.R. Drug-Fortified Liposomes as Carriers for Sustained Release of NSAIDs: The Concept and Its Validation in the Animal Model for the Treatment of Arthritis. Eur. J. Pharm. Sci. 2018, 125, 11–22.
- Qi, X.; Qin, X.; Yang, R.; Qin, J.; Li, W.; Luan, K.; Wu, Z.; Song, L. Intra-Articular Administration of Chitosan Thermosensitive In Situ Hydrogels Combined With Diclofenac Sodium-Loaded Alginate Microspheres. J. Pharm. Sci. 2016, 105, 122–130.
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071.
- Abdelkader, H.; Mansour, H.F. Comparative Studies for Ciprofloxacin Hydrochloride Pre-Formed Gels and Thermally Triggered (in Situ) Gels: In Vitro and in Vivo Appraisal Using a Bacterial Keratitis Model in Rabbits. Pharm. Dev. Technol. 2015, 20, 410–416.
- Anita, C.; Munira, M.; Mural, Q.; Shaily, L. Topical Nanocarriers for Management of Rheumatoid Arthritis: A Review. Biomed. Pharmacother. 2021, 141, 111880.
- Dyondi, D.; Lakhawat, R.; Banerjee, R. Biodegradable Nanoparticles for Intra-Articular Therapy. Dyn. Biochem. Process Biotechnol. Mol. Biol. 2009, 3, 33–41.
- Oliveira, I.M.; Fernandes, D.C.; Cengiz, I.F.; Reis, R.L.; Oliveira, J.M. Hydrogels in the Treatment of Rheumatoid Arthritis: Drug Delivery Systems and Artificial Matrices for Dynamic in Vitro Models. J. Mater. Sci. Mater. Med. 2021, 32, 1–13.
- Uson, J.; Rodriguez-Garciá, S.C.; Castellanos-Moreira, R.; O’Neill, T.W.; Doherty, M.; Boesen, M.; Pandit, H.; Möller Parera, I.; Vardanyan, V.; Terslev, L.; et al. EULAR Recommendations for Intra-Articular Therapies. Ann. Rheum. Dis. 2021, 80, 1299–1305.
- Kou, L.; Xiao, S.; Sun, R.; Bao, S.; Yao, Q.; Chen, R. Biomaterial-Engineered Intra-Articular Drug Delivery Systems for Osteoarthritis Therapy. Drug Deliv. 2019, 26, 870–885.
- Rannou, F.; Poiraudeau, S. Non-Pharmacological Approaches for the Treatment of Osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2010, 24, 93–106.
- Simon, T.M.; Jackson, D.W. Articular Cartilage: Injury Pathways and Treatment Options. Sports Med. Arthrosc. Rev. 2018, 26, 146–154.
- Shi, J.; Fan, K.; Yan, L.; Fan, Z.; Li, F.; Wang, G.; Liu, H.; Liu, P.; Yu, H.; Li, J.J.; et al. Cost Effectiveness of Pharmacological Management for Osteoarthritis: A Systematic Review. Appl. Health Econ. Health Policy 2022, 20, 351.
- Chen, J.; Li, J.; Li, R.; Wang, H.; Yang, J.; Xu, J.; Zha, Z. Efficacy and Safety of Tanezumab on Osteoarthritis Knee and Hip Pains: A Meta-Analysis of Randomized Controlled Trials. Pain Med. 2017, 18, 374–385.
- van der Woude, J.A.D.; Wiegant, K.; van Heerwaarden, R.J.; Spruijt, S.; van Roermund, P.M.; Custers, R.J.H.; Mastbergen, S.C.; Lafeber, F.P.J.G. Knee Joint Distraction Compared with High Tibial Osteotomy: A Randomized Controlled Trial. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 876–886.
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI Guidelines for the Non-Surgical Management of Knee, Hip, and Polyarticular Osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589.
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578.
- Sayed Aly, M.N. Intra-Articular Drug Delivery: A Fast Growing Approach. Recent Patents Drug Deliv. Formul. 2008, 2, 231–237.
- Küçüktürkmen, B.; Öz, U.C.; Bozkir, A. In Situ Hydrogel Formulation for Intra-Articular Application of Diclofenac Sodium-Loaded Polymeric Nanoparticles. Turk. J. Pharm. Sci. 2017, 14, 56.
- Iannitti, T.; Lodi, D.; Palmieri, B. Intra-Articular Injections for the Treatment of Osteoarthritis: Focus on the Clinical Use of Hyaluronic Acid. Drugs R D 2011, 11, 13–27.
- Baron, D.; Flin, C.; Porterie, J.; Despaux, J.; Vincent, P. Hyaluronic Acid Single Intra-Articular Injection in Knee Osteoarthritis: A Multicenter Open Prospective Study (ART-ONE 75) with Placebo Post Hoc Comparison. Curr. Ther. Res. Clin. Exp. 2018, 88, 35.
- Suppan, V.K.L.; Tew, M.M.; Wong, B.C.; Chan, H.K.; Chew, Y.W.; Tan, C.S.; Nanta Kumar, V.K.; Shafie, A.A.; Sadashiva Rao, A. One-Year Follow-up of Efficacy and Cost of Repeated Doses versus Single Larger Dose of Intra-Articular Hyaluronic Acid for Knee Osteoarthritis. J. Orthop. Surg. 2020, 28, 2309499019895029.
- Altman, R.D.; Manjoo, A.; Fierlinger, A.; Niazi, F.; Nicholls, M. The Mechanism of Action for Hyaluronic Acid Treatment in the Osteoarthritic Knee: A Systematic Review. BMC Musculoskelet. Disord. 2015, 16, 321.
- Nawrat, P.; Surazyński, A.; Karna, E.; Pałka, J.A. The Effect of Hyaluronic Acid on Interleukin-1-Induced Deregulation of Collagen Metabolism in Cultured Human Skin Fibroblasts. Pharmacol. Res. 2005, 51, 473–477.
- Mei, L.; Wang, H.; Chen, J.; Zhang, Z.; Li, F.; Xie, Y.; Huang, Y.; Peng, T.; Cheng, G.; Pan, X.; et al. Self-Assembled Lyotropic Liquid Crystal Gel for Osteoarthritis Treatment via Anti-Inflammation and Cartilage Protection. Biomater. Sci. 2021, 9, 7205–7218.
- Uthman, I.; Raynauld, J.P.; Haraoui, B. Intra-Articular Therapy in Osteoarthritis. Postgrad. Med. J. 2003, 79, 449–453.
- Richards, M.M.; Maxwell, J.S.; Weng, L.; Angelos, M.G.; Golzarian, J. Intra-Articular Treatment of Knee Osteoarthritis: From Anti-Inflammatories to Products of Regenerative Medicine. Phys. Sportsmed. 2016, 44, 101.
- McGarry, J.G.; Daruwalla, Z.J. The Efficacy, Accuracy and Complications of Corticosteroid Injections of the Knee Joint. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 1649–1654.
- Hall, M.P.; Band, P.A.; Meislin, R.T.; Jazrawi, L.M.; Cardone, D.A. Platelet-Rich Plasma: Current Concepts and Application in Sports Medicine. J. Am. Acad. Orthop. Surg. 2009, 17, 602–608.
- Demange, M.K.; Sisto, M.; Rodeo, S. Future Trends for Unicompartmental Arthritis of the Knee: Injectables & Stem Cells. Clin. Sports Med. 2014, 33, 161–174.
- Khoshbin, A.; Leroux, T.; Wasserstein, D.; Marks, P.; Theodoropoulos, J.; Ogilvie-Harris, D.; Gandhi, R.; Takhar, K.; Lum, G.; Chahal, J. The Efficacy of Platelet-Rich Plasma in the Treatment of Symptomatic Knee Osteoarthritis: A Systematic Review with Quantitative Synthesis. Arthroscopy 2013, 29, 2037–2048.
- Zhu, C.; Wu, W.; Qu, X. Mesenchymal Stem Cells in Osteoarthritis Therapy: A Review. Am. J. Transl. Res. 2021, 13, 448.
- Chen, F.H.; Rousche, K.T.; Tuan, R.S. Technology Insight: Adult Stem Cells in Cartilage Regeneration and Tissue Engineering. Nat. Clin. Pract. Rheumatol. 2006, 2, 373–382.
- Mancuso, P.; Raman, S.; Glynn, A.; Barry, F.; Murphy, J.M. Mesenchymal Stem Cell Therapy for Osteoarthritis: The Critical Role of the Cell Secretome. Front. Bioeng. Biotechnol. 2019, 7, 9.
- Dashnyam, K.; Lee, J.H.; Mandakhbayar, N.; Jin, G.Z.; Lee, H.H.; Kim, H.W. Intra-Articular Biomaterials-Assisted Delivery to Treat Temporomandibular Joint Disorders. J. Tissue Eng. 2018, 9, 2041731418776514.
- Jones, I.A.; Togashi, R.; Wilson, M.L.; Heckmann, N.; Vangsness, C.T. Intra-Articular Treatment Options for Knee Osteoarthritis. Nat. Rev. Rheumatol. 2018, 15, 77–90.
- Ayhan, E.; Kesmezacar, H.; Akgun, I. Intraarticular Injections (Corticosteroid, Hyaluronic Acid, Platelet Rich Plasma) for the Knee Osteoarthritis. World J. Orthop. 2014, 5, 351.
More
