Colorectal cancer (CRC) is a malignant disease with an incidence of over 1.8 million new cases per year worldwide. CRC outcome is closely related to the respective stage of CRC and is more favorable at less advanced stages. Detection of early colorectal adenomas is the key to survival. In spite of implemented screening programs showing efficiency in the detection of early precancerous lesions and CRC in asymptomatic patients, a significant number of patients are still diagnosed in advanced stages. Research on CRC accomplished during the last decade has improved our understanding of the etiology and development of colorectal adenomas and revealed weaknesses in the general approach to their detection and elimination. Recent studies seek to find a reliable non-invasive biomarker detectable even in the blood.
- colorectal adenoma
- colorectal cancer
- biomarkers
- early detection
1. Colorectal Cancer (CRC)
Colorectal cancer (CRC) is a serious heterogeneous disease that stands in third place in cancer incidence and represents the second cause of death in the world (nearly1.8 million patients newly diagnosed and 1 million patients who die every year) [1]. CRC has become predominant cancer in Western countries, which could be partially explained by the aged population and adverse lifestyle habits such as smoking, increased consumption of red meat and alcohol, lack of physical activity related to obesity, and diabetes, connected usually with low diversity of intestine microflora. Risk factors also include positive family history reflecting individual genetic equipment [2]. The screening programs aim to identify patients with precancerous lesions or those with resectable CRC stages 0, I, and II, who have a generally better prognosis than symptomatic patients with the pre-existing disease [3]. Despite screening programs, many patients are diagnosed in the stages III and IV of CRC that lead to a worse overall prognosis [4]. According to data from National Cancer Institute from the United States of America (USA) [5], five years survival rates for stage IV account only for 12% at colon cancer (CC) and 13% at rectal cancer (RC), while detection at an early stage I can increase the chance to survive up to 92% at CC and 88% at RC [6].
Several screening methods such as stool testing, blood testing, and endoscopic and radiological examination are currently available [7][8]. Polyps or tumors can manifest by microscopic bleeding (so-called occult bleeding). First-line test detecting occult bleeding is a fecal occult blood test (FOBT). Guaiac fecal occult blood test (gFOBT) detects hemoglobin (Hb) by peroxidase activity. Nevertheless, gFOBT is insufficiently specific to human hemoglobin and connected with a risk of false-positive results and omission of small polyps or non-bleeding polyps [7]. Despite its disadvantages, it has been able to contribute to a 33% reduction in CRC mortality [9]. Nowadays, gFOBT has predominantly been replaced by a fecal immunochemical test (FIT) based on antibody assay, which provides qualitative and quantitate results on Hb concentration per gram feces. Positive results of gFOBT or FIT are followed by endoscopic examination [8].
Endoscopic methods include colonoscopy examination, sigmoidoscopy, or capsule endoscopy. Colonoscopy is the main investigative method of the large bowel, this technique provides visualization of the entire large intestine, precise localization, biopsy, or complete removal of a potential precancerous lesion in a single session [10]. Early polypectomy leads to a 76–90% reduction in CRC incidence [11]. The weakness of this method is its invasiveness, it is an unpleasant procedure requiring several days of diet restriction and bowel preparation. These could pose an obstacle for many people, and among other things, it is expensive with the necessary presence of a very well-trained examiner [12].
Sigmoidoscopy compared to colonoscopy reduces time-consuming examination and patient discomfort and provides a lower risk of complications without the need for sedation, but allows investigation of only the rectum and the sigmoid. The study of sigmoidoscopy screening of individuals between 55 and 64 years in the United Kingdom (UK), indicated subsequent CRC incidence reduction by 33% and mortality by 43% [13].
Colon capsule endoscopy (CCE) is a non-invasive method suitable for individuals who are unwilling to undergo colonoscopy because of discomfort or any other obstacles. Meta-analysis showed that CCE for any polyp has a specificity of 89% and sensitivity of 73%. Though CCE is not as accurate as colonoscopy, it could decrease the need for its application [14].
CRC screening by radiology using computed tomographic (CT) colonography is able to visualize the entire colorectum and with no need for sedation. Even though it still requires bowel preparation, it is a relatively non-invasive method. This technique can detect only large adenomas and tumors with size ≥10 mm, nevertheless with sensitivity of 90% [15].
Screening methods based on blood testing were enriched by a highly promising biomarker, methylated gene
(
SEPT9) in the last few years.
SEPT9 is released from CRC cells into circulation and is detectable in peripheral blood. A recent study showed that
SEPT9 assay, approved by Food and Drug Administration (FDA) in the USA, has a higher specificity (94.5%) than FOBT at advanced stages of CRC, but not at asymptomatic patients with early neoplasia [16][17].
Several types of a lesion can be histologically described from the colonoscopy biopsy. A colon polyp is a small clump of cells that forms on the lining of the colon epithelium. There are two main classes of polyps, non-neoplastic and neoplastic (
) [18]. In general, the larger the neoplastic adenoma the greater the risk of cancer.
shows the recommended follow up after patient polypectomy [19][20][21]. Although the recommended surveillance guideline has been widely accepted, clinicians still detect the incidence of CRC (<10%) developed during the initial colonoscopy and the subsequent follow-up examination. This subgroup of CRCs is referred to as interval CRC (I-CRC) and represents one of the problems that screening programs face [22].
Classification of non-neoplastic and neoplastic polyps and polyposis [18].
| Non-Neoplastic | Neoplastic | |||
|---|---|---|---|---|
| Sporadic | Hereditary | Sporadic | Hereditary | |
| Hyperplastic polyps | Hyperplastic polyposis | Benigns adenomas: | Tubular | Familial adenomatous polyposis (FAP) |
| Villous | ||||
| Tubulovillous | ||||
| Inflammatory polyps | Juvenile polyposis | Serrated adenomas: | Sessile serrated | Hereditary non-polyposis colorectal cancer (HNPCC) |
| Juvenile polyps | Traditional serrated | |||
| Peutz-Jeghers syndrome | Malignant lesions: | Carcinoma in situ | ||
| MUTYH associated polyposis | ||||
| Lymphoid polyps | Intramucosal CRC | |||
|
Invasive CRC |
||||
Current surveillance recommendation [20][23].
| Neoplasia Found | Recommanded Interval for Colonoscopy Examination | Comment |
|---|---|---|
| Small rectal hyperplastic polyps | 10 years | Exception are patients with hyperplastic polyposis syndrome, who need more intensive follow up. |
| One or two small (<1 cm) tubular adenomas with only low-grade dysplasia | 5–10 years | The precise timing within this interval should be based on other clinical factors (such as prior colonoscopy findings, family history, and the preferences of the patient and judgment of the physician). |
| 3 to 10 adenomas, or any adenoma ≥ 1 cm, or any adenoma with villous features, or high-grade dysplasia | 3 years | Adenomas must have been completely removed. If the follow up colonoscopy is normal or shows only 1 or 2 small, tubular adenomas with low-grade dysplasia, then the interval for the subsequent examination should be 5 years. |
| More than 10 adenomas at one examination | < 3 years | The interval should be based on the clinician judgement and consider the possibility of an underlying familial syndrome. |
| Sessile adenomas that are removed piecemeal | 2 to 6 months | Once complete removal has been established, subsequent surveillance needs to be individualized based on the endoscopist’s judgment. Completeness of removal should be based on both endoscopic and pathologic assessments. |
Around 5–10% of CRC cases are related to heredity including most common syndromes such as hereditary non-polyposis colorectal cancer (HNPCC), familial adenomatous polyposis (FAP) and attenuated familial adenomatous polyposis (aFAP), MUTYH-associated polyposis (MAP), Juvenile polyposis syndrome (JPS), Peutz-Jeghers syndrome (PJS), Polymerase proofreading-associated polyposis (PPAP), PTEN hamartoma tumors syndrome (PHTS), Cowden syndrome, and Familial colorectal cancer type X, while more than 90% of CRC cases are of sporadic origin [6][7]. Syndromes are usually detected at an early age. However, sporadic CRC correlates with increasing age due to the accumulation of mutations in intestine cells [24][25].
In the study by Brenner et al. [25], 10 years of cumulative risk of CRC among both sex with advanced adenomas increases from 25.4–25.2% at age 55 years to 42.9–39.7% at age 80 years. The development of carcinoma from adenoma tissue can last 5 to 20 years, and it is not influenced purely by one pathway [26,27]. This transition is a complex, multifactorial process that has been characterized by chromosomal instability (CIN), microsatellite instability (MSI), and DNA methylation in CpG islands areas (CIMP). All these pathways may overlap with each other and are responsible for genetic instability in adenoma that could undergo malignant transformation [28] (In the study by Brenner et al. [26], 10 years of cumulative risk of CRC among both sex with advanced adenomas increases from 25.4–25.2% at age 55 years to 42.9–39.7% at age 80 years. The development of carcinoma from adenoma tissue can last 5 to 20 years, and it is not influenced purely by one pathway [27][28]. This transition is a complex, multifactorial process that has been characterized by chromosomal instability (CIN), microsatellite instability (MSI), and DNA methylation in CpG islands areas (CIMP). All these pathways may overlap with each other and are responsible for genetic instability in adenoma that could undergo malignant transformation [29] (
Figure 1). The events contributing to these processes are constantly subject to intensive investigations [27].). The events contributing to these processes are constantly subject to intensive investigations [28].
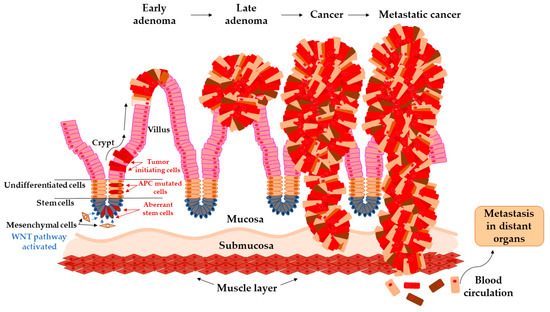
Arise of tumor-initiating cells from aberrant colon crypt and subsequent transition of early adenoma to metastatic cancer.
Considering the current knowledge about the CRC development and with an application of screening programs, we are still missing identification of patients with asymptomatic disease progression in early stages, where detection plays a key role in cancer survival. Recent studies seek to find new non-invasive biomarkers measurable even in early stages of CRC from an area of non-coding RNA, inflammatory biomarkers, or cell-free DNA [30].
Transition of Adenoma to Carcinoma in Colon
2. Transition of Adenoma to Carcinoma in Colon
The colon epithelium is constantly and rapidly renewing tissue. Old cells on the top of the villus are released into the lumen and replaced with new cells raised from colonic crypts. On the bottom of colonic crypts are stem cells that proliferate and differentiate into the cellular compartment of colon epithelium [31]. Vogelstein et al. [32] proposed the classical model of tumor evolution in the large bowel (
). Cells with high WNT signaling activity arise from aberrant crypts and evolve into a tubular or tubule-villous polyp. The subsequent proliferation of polyp may lead to the development of early adenoma with a low grade of dysplasia. Early adenoma expanses into advanced adenoma with a high grade of dysplasia and with increasing accumulation of mutations in daughter cells progressing ultimately further into carcinoma [2][32][33].
Each mutation that provides tumor cell-selective growth advantage is called driver mutation. This advantage slightly increases the growth rate of clonal expansion around 0.4% and is increasing with every new driving mutation [34]. Driver mutations enhance the accumulation of a large number of somatic mutations due to altering the cell condition and reduce the population fitness landscape. The predominant mutations, so-called passenger mutations, are mutations without selective growth advantage. With each clonal expansion of cancer cells, heterogeneous passenger mutations are generated that constitute the enormous variations of unique tumors [35].
Thanks to the next-generation sequencing (NGS) technique, thousands of mutations in the human genome were identified and some of them contribute to malignant evolution [36]. The driver mutations in the
gene, predominantly frameshift at codon 1,554 [37], provide cell-selective growth advantage [32], and cause loss of cell ability to control the concentration level of protein β-catenin in the cytoplasm. β-catenin implements in the WNT signaling pathway and its concentration imbalances lead to uncontrolled growth and cell division [38]. Following mutations in
or
genes induce transformation into a malignant tumor, which overgrows into basal tissue and has an ability to metastasize into lymph nodes and distant organs [28].
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis Primers 2015, 1, 15065.
- Pande, R.; Froggatt, P.; Baragwanath, P.; Harmston, C. Survival outcome of patients with screening versus symptomatically detected colorectal cancers. Colorectal Dis. 2013, 15, 74–79.
- Altobelli, E.; Rapacchietta, L.; Marziliano, C.; Campagna, G.; Profeta, V.F.; Fagnano, R. Differences in colorectal cancer surveillance epidemiology and screening in the WHO European Region. Oncol. Lett. 2019, 17, 2531–2542.
- National Cancer Institute, Surveillance Research Program, Surveillance Systems Branch, Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence – SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2015 Sub (1973–2013 varying) – Linked To County Attributes – Total U.S., 1969–2014 Counties. 2016. Available online: https://seer.cancer.gov/data-software/documentation/seerstat/nov2016/ (accessed on 4 May 2020).
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103.
- Binefa, G.; Rodriguez-Moranta, F.; Teule, A.; Medina-Hayas, M. Colorectal cancer: From prevention to personalized medicine. World J. Gastroenterol. 2014, 20, 6786–6808.
- Schreuders, E.H.; Grobbee, E.J.; Spaander, M.C.W.; Kuipers, E.J. Advances in Fecal Tests for Colorectal Cancer Screening. Curr. Treat. Options. Gastroenterol. 2016, 14, 52–162.
- Mandel, J.S.; Bond, J.H.; Church, T.R.; Snover, D.C.; Bradley, G.M.; Schuman, L.M.; Ederer, F. Reducing Mortality from Colorectal Cancer by Screening for Fecal Occult Blood. N. Engl. J. Med. 1993, 328, 1365–1371.
- Ladabaum, U.; Dominitz, J.A.; Kahi, C.; Schoen, R.E. Strategies for Colorectal Cancer Screening. Gastroenterology 2020, 158, 418–432.
- Winawer, S.J.; Zauber, A.G.; Ho, M.N.; O’Brien, M.J.; Gottlieb, L.S.; Sternberg, S.S.; Waye, J.D.; Schapiro, M.; Bond, J.H.; Panish, J.F.; et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N. Engl. J. Med. 1993, 329, 1977–1981.
- Lhewa, D.Y.; Strate, L.L. Pros and cons of colonoscopy in management of acute lower gastrointestinal bleeding. World J. Gastroenterol. 2012, 18, 1185–1190.
- Atkin, W.S.; Edwards, R.; Kralj-Hans, I.; Wooldrage, K.; Hart, A.R.; Northover, J.M.; Parkin, D.M.; Wardle, J.; Duffy, S.W.; Cuzick, J. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: A multicentre randomised controlled trial. Lancet 2010, 375, 1624–1633.
- Rokkas, T.; Papaxoinis, K.; Triantafyllou, K.; Ladas, S.D. A meta-analysis evaluating the accuracy of colon capsule endoscopy in detecting colon polyps. Gastrointest. Endosc. 2010, 71, 792–798.
- Johnson, C.D.; Chen, M.-H.; Toledano, A.Y.; Heiken, J.P.; Dachman, A.; Kuo, M.D.; Menias, C.O.; Siewert, B.; Cheema, J.I.; Obregon, R.G.; et al. Accuracy of CT Colonography for Detection of Large Adenomas and Cancers. N. Engl. J. Med. 2008, 359, 1207–1217.
- Sun, J.; Fei, F.; Zhang, M.; Li, Y.; Zhang, X.; Zhu, S.; Zhang, S. The role of (m)SEPT9 in screening, diagnosis, and recurrence monitoring of colorectal cancer. BMC Cancer 2019, 19, 450.
- Song, L.; Jia, J.; Peng, X.; Xiao, W.; Li, Y. The performance of the SEPT9 gene methylation assay and a comparison with other CRC screening tests: A meta-analysis. Sci. Rep. 2017, 7, 3032.
- Rubio, C.A.; Jaramillo, E.; Lindblom, A.; Fogt, F. Classification of Colorectal Polyps: Guidelines for the Endoscopist. Endoscopy 2002, 34, 226–236.
- Hassan, C.; Quintero, E.; Dumonceau, J.M.; Regula, J.; Brandao, C.; Chaussade, S.; Dekker, E.; Dinis-Ribeiro, M.; Ferlitsch, M.; Gimeno-Garcia, A.; et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2013, 45, 842–851.
- Levin, B.; Lieberman, D.A.; McFarland, B.; Smith, R.A.; Brooks, D.; Andrews, K.S.; Dash, C.; Giardiello, F.M.; Glick, S.; Levin, T.R.; et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J. Clin. 2008, 58, 130–160.
- Shussman, N.; Wexner, S.D. Colorectal polyps and polyposis syndromes. Gastroenterol. Rep. (Oxf) 2014, 2, 1–15.
- Dong, S.-H.; Huang, J.-Q.; Chen, J.-S. Interval colorectal cancer: A challenging field in colorectal cancer. Future Oncol. 2018, 14, 1307–1316.
- Winawer, S.J.; Zauber, A.G.; Fletcher, R.H.; Stillman, J.S.; O’Brien, M.J.; Levin, B.; Smith, R.A.; Lieberman, D.A.; Burt, R.W.; Levin, T.R.; et al. Guidelines for colonoscopy surveillance after polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. CA Cancer J. Clin. 2006, 56, 143–159, quiz 184-5.
- Whiffin, N.; Hosking, F.J.; Farrington, S.M.; Palles, C.; Dobbins, S.E.; Zgaga, L.; Lloyd, A.; Kinnersley, B.; Gorman, M.; Tenesa, A.; et al. Identification of susceptibility loci for colorectal cancer in a genome-wide meta-analysis. Hum. Mol. Genet. 2014, 23, 4729–4737.
- Archambault, A.N.; Su, Y.R.; Jeon, J.; Thomas, M.; Lin, Y.; Conti, D.V.; Win, A.K.; Sakoda, L.C.; Lansdorp-Vogelaar, I.; Peterse, E.F.P.; et al. Cumulative Burden of Colorectal Cancer-Associated Genetic Variants Is More Strongly Associated With Early-Onset vs. Late-Onset Cancer. Gastroenterology 2020, 158, 1274–1286.e12.
- Brenner, H.; Hoffmeister, M.; Stegmaier, C.; Brenner, G.; Altenhofen, L.; Haug, U. Risk of progression of advanced adenomas to colorectal cancer by age and sex: Estimates based on 840,149 screening colonoscopies. Gut 2007, 56, 1585–1589.
- Loeve, F.; Boer, R.; Zauber, A.G.; Van Ballegooijen, M.; Van Oortmarssen, G.J.; Winawer, S.J.; Habbema, J.D. National Polyp Study data: Evidence for regression of adenomas. Int. J. Cancer 2004, 111, 633–639.
- Cross, W.; Kovac, M.; Mustonen, V.; Temko, D.; Davis, H.; Baker, A.M.; Biswas, S.; Arnold, R.; Chegwidden, L.; Gatenbee, C.; et al. The evolutionary landscape of colorectal tumorigenesis. Nat. Ecol. Evol. 2018, 2, 1661–1672.
- Muller, M.F.; Ibrahim, A.E.; Arends, M.J. Molecular pathological classification of colorectal cancer. Virchows Arch. 2016, 469, 125–134.
- Pellino, G.; Gallo, G.; Pallante, P.; Capasso, R.; De Stefano, A.; Maretto, I.; Malapelle, U.; Qiu, S.; Nikolaou, S.; Barina, A.; et al. Noninvasive Biomarkers of Colorectal Cancer: Role in Diagnosis and Personalised Treatment Perspectives. Gastroenterol. Res. Pract. 2018, 2018, 2397863.
- Cernat, L.; Blaj, C.; Jackstadt, R.; Brandl, L.; Engel, J.; Hermeking, H.; Jung, A.; Kirchner, T.; Horst, D. Colorectal Cancers Mimic Structural Organization of Normal Colonic Crypts. PLoS ONE 2014, 9, e104284.
- Kinzler, K.W.; Vogelstein, B. Cancer-susceptibility genes. Gatekeepers and caretakers. Nature 1997, 386, 761–763.
- Tomasetti, C.; Vogelstein, B.; Parmigiani, G. Half or more of the somatic mutations in cancers of self-renewing tissues originate prior to tumor initiation. Proc. Natl. Acad. Sci. USA 2013, 110, 1999–2004.
- Khurana, E.; Fu, Y.; Colonna, V.; Mu, X.J.; Kang, H.M.; Lappalainen, T.; Sboner, A.; Lochovsky, L.; Chen, J.; Harmanci, A.; et al. Integrative annotation of variants from 1092 humans: Application to cancer genomics. Science 2013, 342, 1235587.
- Bozic, I.; Antal, T.; Ohtsuki, H.; Carter, H.; Kim, D.; Chen, S.; Karchin, R.; Kinzler, K.W.; Vogelstein, B.; Nowak, M.A. Accumulation of driver and passenger mutations during tumor progression. Proc. Natl. Acad. Sci. USA 2010, 107, 18545–18550.
- Ciriello, G.; Miller, M.L.; Aksoy, B.A.; Senbabaoglu, Y.; Schultz, N.; Sander, C. Emerging landscape of oncogenic signatures across human cancers. Nat. Genet. 2013, 45, 1127–1133.
- Rowan, A.J.; Lamlum, H.; Ilyas, M.; Wheeler, J.; Straub, J.; Papadopoulou, A.; Bicknell, D.; Bodmer, W.F.; Tomlinson, I.P.M. APC mutations in sporadic colorectal tumors: A mutational “hotspot” and interdependence of the “two hits”. Proc. Natl. Acad. Sci. USA 2000, 97, 3352–3357.
- Kwong, L.N.; Dove, W.F. APC and its modifiers in colon cancer. Adv. Exp. Med. Biol. 2009, 656, 85–106.
