Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Xabier Agirre.
Multiple Myeloma (MM) is a clonal B-cell neoplasm characterized by the uncontrolled proliferation and accumulation of malignant plasma cells (PCs) in the bone marrow. Aptamers are short oligonucleotide ligands that bind their targets with great affinity and specificity, and can be easily conjugated to different cargoes for their cell-specific delivery.
- aptamers
- multiple myeloma
- targeted therapies
1. Introduction
As for antibodies, an ideal target for MM-specific aptamer development would be one with a high and uniform expression in MM cells to have sufficient efficacy, while having a negligible to low expression on other normal cells to avoid on-target off-tumor effects. Hence, most aptamers being tested for MM are thus aimed at the same well-known molecules for which antibodies have been developed, showing some promising preclinical results for several of these aptamers (Figure 21; Table 21):
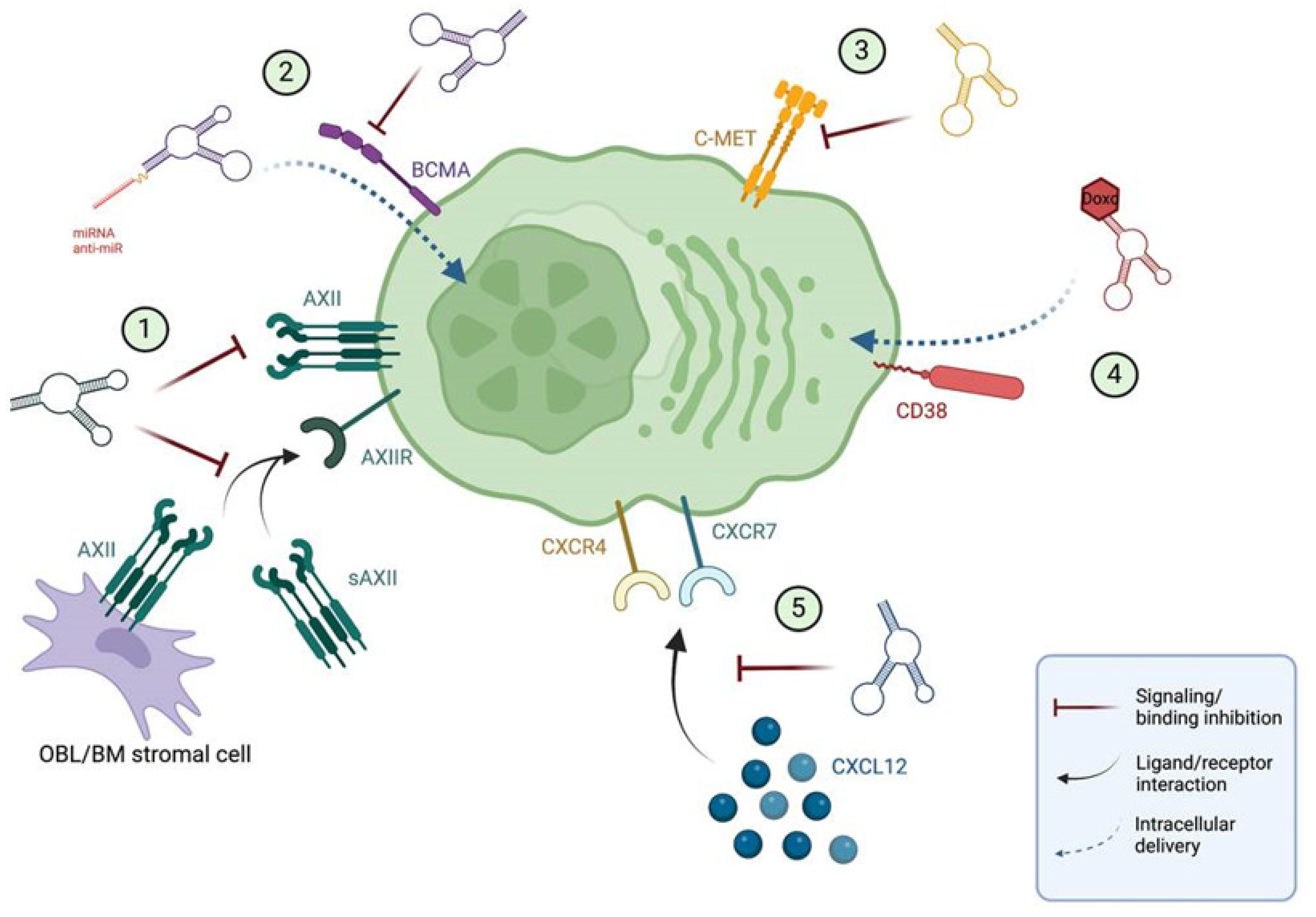
Figure 21. Current aptamers for precision medicine in MM. (1) Aptamer against AXII, (2) BCMA targeted aptamer, (3) aptamer against C-MET, (4) conjugated CD38-doxorubicin aptamer and (5) NOX-A12 RNA aptamer for CXCL12. All the aptamers except the NOX-A12 spiegelmer are directed towards receptors expressed in the MM plasma cell membrane. Receptor–ligand interactions are depicted with black arrows, while the mechanism of action of aptamers is depicted with red arrows for antagonist aptamers, and blue arrows for cargo delivery aptamers. BM: bone marrow; Doxo: doxorubicin; OBL: osteoblast; sAXII: soluble annexin A2. Image made in ©BioRender.
2. Aptamer against AXII
Annexin A2 (AXII) is a calcium-dependent, phospholipid binding member of the annexin family. AXII is overexpressed in MM plasma cell membranes, with its expression being negatively correlated with patient survival [28][1]. The interaction of AXII with its receptor AXIIR enhances MM cell adhesion and growth in the BM microenvironment, potentially supporting the homing and growth of MM cells in the BM. AXII can be secreted by various cell types in the BM, promoting MM cell growth by creating a pro-tumorigenic niche, thus, targeting the AXII/AXIIR axis represents an attractive approach for the development of therapies targeted at the MM niche [29][2]. Zhou et al. [30][3] identified a ssDNA aptamer (wh6) that was able to bind AXII in the low nanomolar range through nine rounds of protein-based SELEX. They showed that the aptamer was able to specifically bind MM cells expressing AXII both in vitro and in vivo, and it could inhibit the AXII induced adhesion and progression in MM cell lines, indicating the suitability of the wh6 aptamer for targeted MM treatment.
3. BCMA targeted aptamer
B-cell maturation antigen (BCMA) is a member of the tumor necrosis factor (TNF) receptor superfamily, which is preferentially expressed by late-stage B lymphocytes while showing minimal expression in hematopoietic stem cells [31][4]. Under physiological conditions, the binding of its specific ligands BAFF and APRIL induces the activation of both canonical and non-canonical NF-κB pathways, promoting long-lived plasma cells survival. However, BCMA overexpression and increased activation are associated with MM progression in terms of the upregulation of NF-κB pathways and subsequent overexpression of critical genes for MM growth and survival [31,32][4][5]. In this direction, Catuogno et al. [32][5] selected a BCMA-targeted internalizing RNA aptamer (apt69.T) through a variation of the cell-SELEX approach. In vitro approaches using MM cell lines showed that the selected apt69.T aptamer was able to readily bind BCMA and inhibit the APRIL dependent downstream signaling pathway. Furthermore, it was able to internalize rapidly and successfully deliver therapeutic oligonucleotides to MM cells. For that, the BCMA aptamer was conjugated to miRNA and miRNA antagonists using a sticky-end based approach, leading to consequent upregulation of miR-137 and downregulation of miR-222 in MM cell lines. The upregulation of the tumor suppressor miR-137 was able to reduce MM cell viability, highlighting the feasibility of using MM-specific aptamers for the effective delivery of therapeutic oligonucleotides to MM cells.
4. Aptamer against C-MET
C-MET is a transmembrane tyrosine kinase known to be the receptor of the hepatocyte growth factor (HGF) cytokine. In MM, C-MET expression gradually increases during disease development, its high expression being correlated with poor MM patient outcomes [33][6]. Upon HGF binding, C-MET dimerizes, resulting in kinase auto-phosphorylation and the creation of a multi-substrate docking site necessary for the induction of downstream signaling cascades which ultimately contribute to MM development by promoting cell growth, migration and angiogenesis while inhibiting apoptosis [33][6]. SL1 [34][7] is the truncated version of the original CLN0003 ssDNA aptamer, which was selected against purified C-MET through a filter SELEX approach for the recognition of C-MET overexpressing tumors [35][8]. Accordingly, Zhang et al. [33][6] demonstrated that targeting C-MET via the SL1 aptamer [34,35][7][8] could be a potential therapeutic approach in MM, showing that SL1 was able to inhibit HGF-dependent C-MET signaling and suppress MM cell growth in vitro. Furthermore, SL1 showed synergism with bortezomib, highlighting the potential for novel combination therapies in MM.
5. Conjugated CD38-doxorubicin aptamer
CD38 is a cell surface glycoprotein which is highly and homogeneously expressed in MM cells, with minimal expression on normal myeloid and lymphoid cells. This highly versatile molecule contributes to MM development by acting as a receptor for proliferative signaling, as an adhesion molecule or as an ectoenzyme in the catabolism of NAD+ and NADP [36,37][9][10]; thus, in recent years, it has become one of the main targets for anti-MM targeted therapy development. Wen et al. [38][11] were able to identify a CD38 specific ssDNA aptamer via a hybrid protein- and cell-based SELEX approach. This aptamer was subsequently non-covalently conjugated to doxorubicin for the generation of CD38-specific aptamer–drug conjugates (ApDC). The ApDCs were readily internalized by MM cells, and after a pH-dependent release of the cargo in lysosomes, doxorubicin was able to exert its specific antitumor activity by inhibiting tumor growth without toxicity in both MM in vitro and in vivo models.
6. RNA aptamer for CXCL12
CXCL12, also known as stromal cell-derived factor-1 (SDF-1), is a chemoattractant chemokine that, upon binding to its receptors CXCR4 and CXCR7, induces the adhesion and homing of MM cells to the protective BM niche [39[12][13],40], and therefore, it is considered one of the major players in cell adhesion-mediated drug resistance (CAM-DR) [40][13]. MM cells present high levels of CXCL12, CXCR4 and CXCR7; therefore, CXCL12 neutralization represents an attractive option to modulate the BM niche for MM therapy and overcome CAM-DR [39,40][12][13].
NOX-A12 (olaptesed pegol) is the RNA spiegelmer aptamer that binds and antagonizes CXCL12, which was identified by protein SELEX against the D enantiomer of the natural L-CXCL12 protein [39][12]. Spiegelmers are synthetic RNA aptamers in which the natural D-configuration ribonucleotides have been replaced by their enantiomer (mirror-image) L-ribonucleotides. Like aptamers, they bind their targets with high affinity and specificity; however, due to the presence of unnatural ribonucleotides, they are not susceptible to nuclease degradation or hybridization to native oligonucleotides, and they do not exert immune responses [41,42][14][15]. In a study from 2014, Roccaro el al. [39][12] showed that in vivo CXCL12 neutralization by NOX-A12 could reduce MM cell homing and growth, thereby inhibiting disease progression by disrupting BM colonization by MM cells and inducing the release of MM plasma cells to circulation. Likewise, a phase IIa clinical trial from 2014 combining NOX-A12 with bortezomib and dexamethasone (NCT01521533) in MM refractory patients showed that NOX-A12 was able to mobilize plasma cells without toxic effects [43][16]. Nevertheless, the absence of more recent studies involving NOX-A12 in MM suggest that further research on this molecule in MM has been discontinued, despite the acceptable results obtained in the clinical trial. However, there are currently two open clinical trials with NOX-A12 in solid tumors: the GLORIA (NCT04121455) phase I/II trial in glioblastoma and the OPTIMUS (NCT04901741) phase II trial in metastatic pancreatic cancer, suggesting that NOX-A12 in combination with other therapies may still contribute to the improved management of human cancer patients.
Table 21.
Current aptamers for MM precision medicine.
| Target | Class | Identification | Internalization in mm Cells | Therapeutic Application | Effect | Stage | Reference |
|---|---|---|---|---|---|---|---|
| AXII | ssDNA | Recombinant protein SELEX | Not determined | Aptamer alone. | Inhibition of MM cell-line adhesion and proliferation | Preclinical | [40][13] |
| BCMA | RNA | Cell-SELEX | Yes | Aptamer alone. | Inhibition of BCMA pathway in vitro. | Preclinical | [42][15] |
| Aptamer miRNA (miR-137) chimera. | Upregulation of tumor suppressor miR-137 leading to reduced viability in vitro. | ||||||
| Aptamer anti-miRNA (anti-miR-222) chimera. | Inhibition of oncogenic miR-222 in vitro. | ||||||
| C-MET | ssDNA | Recombinant protein SELEX | Not determined | Aptamer alone. | Suppression of HGF-induced C-MET activation, inhibition of MM cell line proliferation and increased apoptosis, inhibition of cell migration and adhesion. | Preclinical | [20,43,44][16][17][18] |
| Combination therapy with bortezomib. | Synergy with bortezomib. | ||||||
| CD38 | ssDNA | Hybrid protein and cell SELEX | Yes | Aptamer-Doxorubicin conjugate. | Inhibition of MM cell-line proliferation in vitro, tumor inhibition in xenograft models | Preclinical | [45][19] |
| CXCL12 (NOX-A12) | spiegelmer | Protein SELEX against target enantiomer | No | Aptamer alone. | Inhibition of CXCR4 and CXCR7 activity in vitro, reduction of tumor growth in vivo, release of plasma cells into circulation in vivo. | Preclinical | [46,47,48][20][21][22] |
| Combination therapy with bortezomib. | Synergy with bortezomib in vivo. | Preclinical | |||||
| Combination therapy with dexamethasone and bortezomib. | Plasma cell mobilization. | Clinical Trial. Phase II completed (NTC01521533) |
References
- Seckinger, A.; Mei, T.; Ro, A.; Jauch, A.; Schnettler, R.; Ewerbeck, V.; Goldschmidt, H.; Klein, B.; Hose, D. Clinical and Prognostic Role of Annexin A2 in Multiple Myeloma. Blood 2012, 120, 1087–1095.
- D’Souza, S.; Kurihara, N.; Shiozawa, Y.; Joseph, J.; Taichman, R.; Galson, D.L.; Roodman, G.D. Annexin II Interactions with the Annexin II Receptor Enhance Multiple Myeloma Cell Adhesion and Growth in the Bone Marrow Microenvironment. Blood 2012, 119, 1888–1896.
- Zhou, W.; Zhang, Y.; Zeng, Y.; Peng, M.; Li, H.; Sun, S.; Ma, B.; Wang, Y.; Ye, M.; Liu, J. Screening and Characterization of an Annexin A2 Binding Aptamer That Inhibits the Proliferation of Myeloma Cells. Biochimie 2018, 151, 150–158.
- Shah, N.; Chari, A.; Scott, E.; Mezzi, K.; Usmani, S.Z. B-Cell Maturation Antigen (BCMA) in Multiple Myeloma: Rationale for Targeting and Current Therapeutic Approaches. Leukemia 2020, 34, 985–1005.
- Catuogno, S.; Di Martino, M.T.; Nuzzo, S.; Esposito, C.L.; Tassone, P.; de Franciscis, V. An Anti-BCMA RNA Aptamer for MiRNA Intracellular Delivery. Mol. Ther.-Nucleic Acids 2019, 18, 981–990.
- Zhang, Y.; Gao, H.; Zhou, W.; Sun, S.; Zeng, Y.; Zhang, H.; Liang, L.; Xiao, X.; Song, J.; Ye, M.; et al. Targeting C-Met Receptor Tyrosine Kinase by the DNA Aptamer SL1 as a Potential Novel Therapeutic Option for Myeloma. J. Cell. Mol. Med. 2018, 22, 5978–5990.
- Ueki, R.; Sando, S. A DNA Aptamer to C-Met Inhibits Cancer Cell Migration. Chem. Commun. 2014, 50, 13131–13134.
- Boltz, A.; Piater, B.; Toleikis, L.; Guenther, R.; Kolmar, H.; Hock, B. Bi-Specific Aptamers Mediating Tumor Cell Lysis. J. Biol. Chem. 2011, 286, 21896–21905.
- Giudice, V.; Mensitieri, F.; Izzo, V.; Filippelli, A.; Selleri, C. Aptamers and Antisense Oligonucleotides for Diagnosis and Treatment of Hematological Diseases. Int. J. Mol. Sci. 2020, 21, 3252.
- Van De Donk, N.W.C.J.; Richardson, P.G.; Malavasi, F. CD38 Antibodies in Multiple Myeloma: Back to the Future. Blood 2018, 131, 13–29.
- Wen, J.; Tao, W.; Hao, S.; Iyer, S.P.; Zu, Y. A Unique Aptamer-Drug Conjugate for Targeted Therapy of Multiple Myeloma. Leukemia 2016, 30, 987–991.
- Roccaro, A.M.; Sacco, A.; Purschke, W.G.; Moschetta, M.; Buchner, K.; Maasch, C.; Zboralski, D.; Zöllner, S.; Vonhoff, S.; Mishima, Y.; et al. SDF-1 Inhibition Targets the Bone Marrow Niche for Cancer Therapy. Cell Rep. 2014, 9, 118–128.
- Waldschmidt, J.M.; Simon, A.; Wider, D.; Müller, S.J.; Follo, M.; Ihorst, G.; Decker, S.; Lorenz, J.; Chatterjee, M.; Azab, A.K.; et al. CXCL12 and CXCR7 Are Relevant Targets to Reverse Cell Adhesion-Mediated Drug Resistance in Multiple Myeloma. Br. J. Haematol. 2017, 179, 36–49.
- Hoellenriegel, J.; Zboralski, D.; Maasch, C.; Rosin, N.Y.; Wierda, W.G.; Keating, M.J.; Kruschinski, A.; Burger, J.A. The Spiegelmer NOX-A12, a Novel CXCL12 Inhibitor, Interferes with Chronic Lymphocytic Leukemia Cell Motility and Causes Chemosensitization. Blood 2014, 123, 1032–1039.
- Vater, A.; Klussmann, S. Turning Mirror-Image Oligonucleotides into Drugs: The Evolution of Spiegelmer® Therapeutics. Drug Discov. Today 2015, 20, 147–155.
- Ludwig, H.; Weisel, K.; Petrucci, M.T.; Leleu, X.; Cafro, A.M.; Laurent, G.; Zojer, N.; Foa, R.; Greil, R.; Yakoub-Agha, I.; et al. Final Results from the Phase IIa Study of the Anti-CXCL12 Spiegelmer® Olaptesed Pegol (NOX-A12) in Combination with Bortezomib and Dexamethasone in Patients with Multiple Myeloma. Blood 2014, 124, 2111.
- Yang, S.; Li, H.; Xu, L.; Deng, Z.; Han, W.; Liu, Y.; Jiang, W.; Zu, Y. Oligonucleotide Aptamer-Mediated Precision Therapy of Hematological Malignancies. Mol. Ther.-Nucleic Acids 2018, 13, 164–175.
- Bausch-Fluck, D.; Goldmann, U.; Müller, S.; van Oostrum, M.; Müller, M.; Schubert, O.T.; Wollscheid, B. The in Silico Human Surfaceome. Proc. Natl. Acad. Sci. USA 2018, 115, E10988–E10997.
- Rose, M.; Cardon, T.; Aboulouard, S.; Hajjaji, N.; Kobeissy, F.; Duhamel, M.; Fournier, I.; Salzet, M. Surfaceome Proteomic of Glioblastoma Revealed Potential Targets for Immunotherapy. Front. Immunol. 2021, 12, 1–11.
- Ferguson, I.D.; Patiño-Escobar, B.; Tuomivaara, S.T.; Lin, Y.-H.T.; Nix, M.A.; Leung, K.K.; Kasap, C.; Ramos, E.; Nieves Vasquez, W.; Talbot, A.; et al. The Surfaceome of Multiple Myeloma Cells Suggests Potential Immunotherapeutic Strategies and Protein Markers of Drug Resistance. Nat. Commun. 2022, 13, 4121.
- Yoon, S.; Rossi, J.J. Aptamers: Uptake Mechanisms and Intracellular Applications. Adv. Drug Deliv. Rev. 2018, 134, 22–35.
- Mondragón, E.; Maher, L.J. Anti-Transcription Factor RNA Aptamers as Potential Therapeutics. Nucleic Acid Ther. 2016, 26, 29–43.
More
