Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Debanjana Debnath.
Due to the properties such as biodegradability, biocompatibility, non-toxicity, physiological inertness, and gel-forming properties, chitin has been found to have countless applications in different industries, e.g., food, cosmetic, pharmaceutical, manufacturing of synthetic materials, agriculture, and even electronics for the production of biosensors. It's important to understand the structure and formation of chitin.
- chitosan
- pathogen
- sustainable
1. Introduction
Global climatic changes are posing a threat to the security of the world’s food supply by adversely affecting plant growth, development, and yield in multiple directions, such as by causing abiotic stresses to plants and as well as encouraging and strengthening the biotic populations by increasing their resistance against conventional chemical management procedures. In this situation, the researchers are trying to identify some natural compounds or their derivatives which can be effectively established themselves as an ideal one to replace the chemicals against which the pathogens are growing resistance gradually. Chitosan, a chemically and physically diverse compound and long-chain polymer of N-acetyl-glucosamine and d-glucosamine derivative of chitin (second most prevalent polysaccharide after cellulose), was first discovered in 1859 by Rouget [1]. Due to its potential for usage in antiviral, antifungal, and antibacterial products, chitosan and its derivatives have gained attention in recent years. Other than chitosan, some other chitin-related compounds and chitin derivatives have also been identified as possible plant protection agents [2]. Chitosan, an aminoglucopyranan made up of N-acetyl-D-glucosamine (GlcNAc) and glucosamine (GlcN) residues, is currently being deemed essential due to its appealing features and biological activities [3,4][3][4]. An amino group and two hydroxyl groups make up the three reactive functional groups that make up chitosan.
According to Amine et al. 2021 chitin and its derivative chitosan are effective at boosting plant defence against pathogens in monocotyledons and dicotyledons [5]. Cell wall lignification, cytoplasmic acidification, membrane depolarization, changes in ion flux and protein phosphorylation, phytoalexin biosynthesis, production of reactive oxygen species, jasmonic acid biosynthesis, and activation of chitinase and glucanase are all targets of this increased plant defense [6].In addition to this, many dicot species have been observed to produce callose, proteinase inhibitors, and phytoalexin in response to chitosan that also have significant roles in plant defense [7]. In the current situation, the rising incidences of resistance, residue, and resurgence (3Rs) have facilitated the increased use of naturalytes in disease and pest control [8]. Consequently, chitosan, a byproduct of fungal and insect chitin, may be used as an autocidal agent to kill invasive disease causing pathogen.
2. Structure and Formation of Chitin
Chitin, a β (1,4)-linked homopolymer of N-acetylglucosamine, is a simple polysaccharide that is an important component of fungal cell wall [9]. It is an amino sugar biopolymer that forms elaborate structures such as insect cuticles and peritrophic membranes when combined with a range of proteins. This polymer is mostly used as a structural component, and it is similar to cellulose and collagen in plants and vertebrates, respectively [10]. 75% of the overall weight of shellfish including crab and shrimp generally discarded as waste among which 20-58% is made up of chitin [11].
Broadly, chitin is defined as a β-(1–4) linked linear cationic heteropolymer consisting of 2-acetamide-2-deoxy-D-glucopyranose (N–acetyl–D–glucosamine, GlcNAc) and randomly distributed units of 2-amino-2-deoxy-D-glucopyranose (D–glucosamine, GlcN) [12]. Due to the presence of the acetoamide groups in the trans position, chitin exhibits two important properties: a high degree of crystallinity and a lack of solubility in water and organic solvents [13].
X-ray diffraction analysis revealed that chitin generally occurs in three different crystalline forms, termed α-, β-, and γ-chitin [10], which mainly differ from each other in the degree of hydration, size of the unit cell, and the number of chitin chains per unit cell [14]. All the chains exhibit an anti-parallel orientation in the α form, whereas in the β form the chains are arranged in a parallel manner and in the γ form sets of two parallel strands alternate with single anti-parallel strands. All three crystalline forms are primarily found in the chitinous structures of insects. The α form is most prevalent in chitinous cuticles, whereas the β and γ forms are frequently found in cocoons [15,16][15][16].
Due to the properties such as biodegradability, biocompatibility, non-toxicity, physiological inertness, and gel-forming properties, chitin has been found to have countless applications in different industries, e.g., food, cosmetic, pharmaceutical, manufacturing of synthetic materials, agriculture, and even electronics for the production of biosensors [17,18][17][18].
In the first step, glycogen is converted by glycogen phosphorylase to glucose-1-P, which is either fed into glycolysis or used for trehalose synthesis [19]. Additionally, the enzyme trehalase can mobilise trehalose by hydrolyzing it to glucose. This is followed by the enzymes hexokinase, phosphoglucomutase, and glucose-6-P isomerase converting glucose to fructose-6-P. Finally, from fructose-6-P the chitin biosynthetic pathway branches off, with the first enzyme catalyzing this branch being glutamine-fructose-6-phosphate amidotransferase [10]. The reaction catalyzed by GFAT converts fructose-6-P into glucosamine-6-phosphate by transferring the ammonia from the co-substrate l-glutamine and isomerizing the resulting fructosimine-6-phosphate. Next, an acetyl group from coenzyme A is added by glucosamine-6-P acetyltransferase to obtain N-acetylglucosamine (GlcNAc)-6-P [20] (Figure 1), whose phosphate is then transferred from the C-6 to the C-1 position catalyzed by phosphoacetylglucosamine mutase. The resulting GlcNAc-1-P, finally, is uridinylated by UDP-GlcNAc pyrophosphorylase yielding UDP-GlcNAc which serves as a substrate for the chitin synthase, transferring the sugar moiety of UDP-GlcNAc to the growing chitin chain. Chitin is degraded by chitinases and N-acetylglucosaminidases yielding GlcNAc which can be reused for chitin biosynthesis [21].
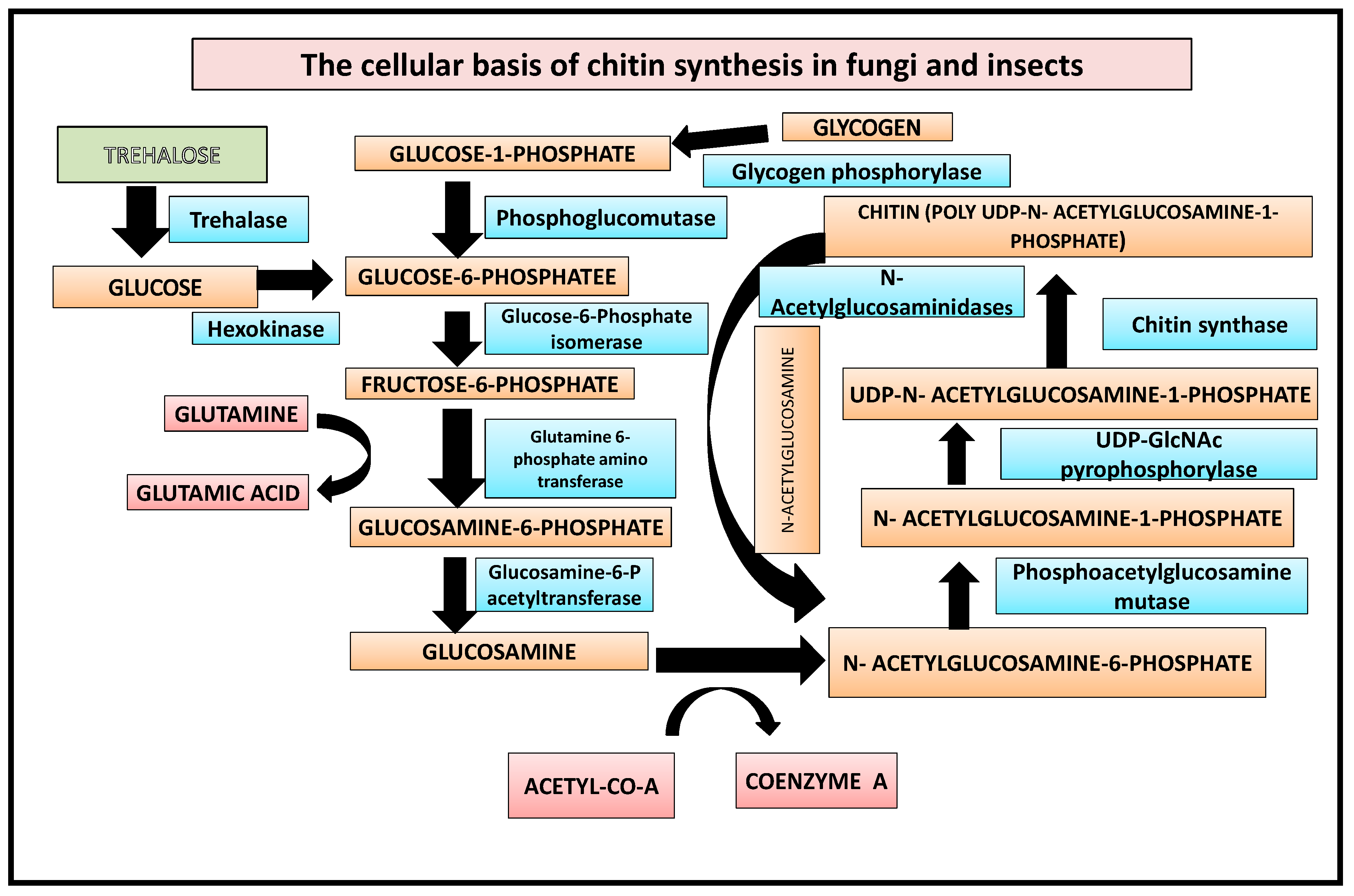
Figure 1.
Biochemical basis of chitin synthesis in fungi and insects.
Deacetylation and Hydrolysis of Chitin/Chitosan
Unfortunately, despite having so many advantages, there is very limited use of chitin due to resistance to different physical and chemical agents because of its highly ordered crystalline structure and its lack of solubility in water or any organic solvents. Chitosan, an N-deacetylated derivative of chitin that is soluble in aqueous solutions of both organic and inorganic acids, is frequently employed to get around this restriction [22]. This cationic polymer resembles chitin and also consists of β-(1–4) linked N–acetyl–D–glucosamine and the D–glucosamine residues [23]. Chitosan, which is produced industrially by hydrolyzing the amino acetyl groups of chitins, is less frequent in nature than chitin. It is also naturally present in the cell walls of filamentous fungi primarily belonging to the Zygomycetes class [24]. Carboxymethyl-chitin (CM-chitin), as a water-soluble anionic polymer, is the second most studied derivative of chitin after chitosan. Most of the chitin and chitosan biological properties are directly related to their physicochemical characteristics. These characteristics include degree of deacetylation, molecular mass, and the amount of moisture content [25].
Enzymatic degradation of chitin can be achieved by two different paths:
-
Chitin can be degraded by first being solubilized by deacetylation (Figure 2). This process is carried out by chitin deacetylases, and the derived substrate (chitosan) is hydrolysed by chitosanases [18,26].
-
The chitinolytic process requires direct hydrolysis of the beta-1,4 glycosidic bonds between the GlcNAc units by chitinases. Chitinases are produced by higher plants, which use the enzymes to defend themselves against pathogenic attacks by degrading chitin in the cell walls of fungi and bacteria [27]. Plant chitinases have molecular weights ranging from 25 to 40 kD and can be acidic or basic. Endochitinases and exochitinases are the two types of chitinases [28]. Chitinase genes from biocontrol fungi such as Trichoderma have significantly higher antifungal activity than comparable plant genes. These fungal genes encode for chitinolytic enzymes, which have higher antifungal activity similar to chemical fungicides [29].
- Chitin can be degraded by first being solubilized by deacetylation (Figure 2). This process is carried out by chitin deacetylases, and the derived substrate (chitosan) is hydrolysed by chitosanases [18][26].
- The chitinolytic process requires direct hydrolysis of the beta-1,4 glycosidic bonds between the GlcNAc units by chitinases. Chitinases are produced by higher plants, which use the enzymes to defend themselves against pathogenic attacks by degrading chitin in the cell walls of fungi and bacteria [27]. Plant chitinases have molecular weights ranging from 25 to 40 kD and can be acidic or basic. Endochitinases and exochitinases are the two types of chitinases [28]. Chitinase genes from biocontrol fungi such as Trichoderma have significantly higher antifungal activity than comparable plant genes. These fungal genes encode for chitinolytic enzymes, which have higher antifungal activity similar to chemical fungicides [29].
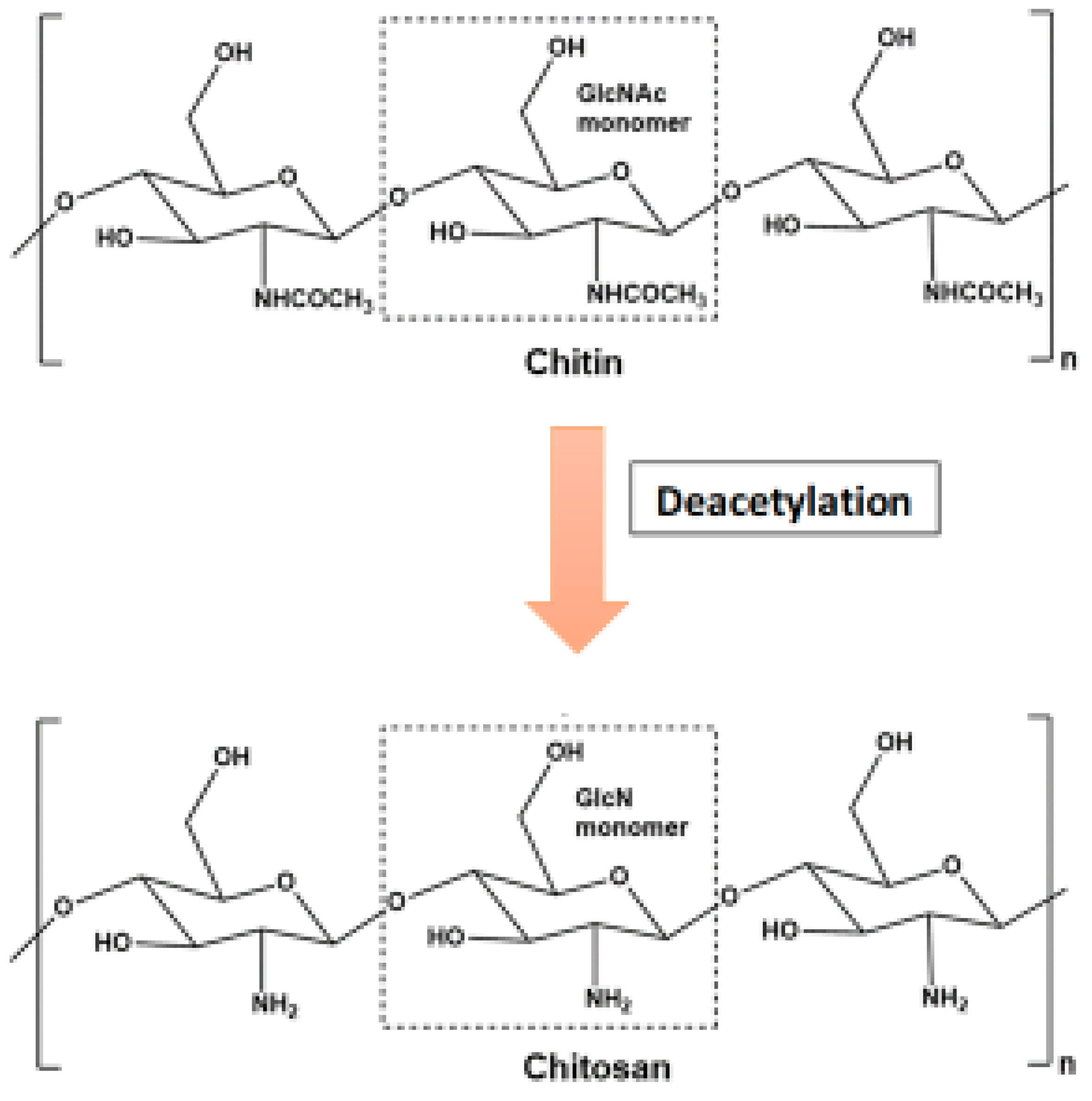
Figure 2.
Conversion of chitosan from chitin through deacetylation.
Despite having many significant similarities in the molecular structures of chitin and chitosan, the physicochemical characteristics of both the biopolymers and the reactions are often surprisingly distinct [18]. Both polymers have the same reactive hydroxyl and amino groups in different molecular ratios, but the lower crystallinity of chitosan makes it more accessible to reagents [12]. Perhaps the most important and crucial difference between chitin and chitosan in terms of their applications is based on their degree of deacetylation and their solubility. Unlike chitin, chitosan has a below pKa (pH~6.5) in most acidic aqueous solutions such as acetic acid, formic acid, lactic acid, citric acid, and other solvents such as dimethylsulfoxide and p-toluenesulfonic acid [30].
References
- Ghule, M.R.; Ramteke, P.K.; Ramteke, S.D.; Kodre, P.S.; Langote, A.; Gaikwad, A.V.; Holkar, S.K.; Jambhekar, H. Impact of chitosan seed treatment of fenugreek for management of root rot disease caused by Fusarium solani under in vitro and in vivo conditions. 3 Biotech 2021, 11, 290.
- Sun, R.; Liu, C.; Zhang, H.; Wang, Q. Benzoylurea Chitin Synthesis Inhibitors. J. Agric. Food Chem. 2015, 63, 6847–6865.
- Jayakumar, R.; Prabaharan, M.; Kumar, P.T.S.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337.
- Prasad, R.; Chandrika, K.; Godbole, V. A novel chitosan biopolymer based Trichoderma delivery system: Storage stability, persistence and bio efficacy against seed and soil borne diseases of oilseed crops. Microbiol. Res. 2020, 237, 126487.
- Amine, R.; Tarek, C.; Hassane, E.; Noureddine, E.H.; Khadija, O. Chemical Proprieties of Biopolymers (Chitin/Chitosan) and their Synergic Effects with Endophytic Bacillus Species: Unlimited Applications in Agriculture. Molecules 2021, 26, 1117.
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11.
- Hassan, O.; Chang, T. Chitosan for Eco-friendly Control of Plant Disease. Asian J. Plant Pathol. 2017, 11, 53–70.
- Jones, M.; Kujundzic, M.; John, S.; Bismarck, A. Crab vs. Mushroom: A Review of Crustacean and Fungal Chitin in Wound Treatment. Mar. Drugs 2020, 18, 64.
- Chen, J.-K.; Shen, C.-R.; Liu, C.-L. N-Acetylglucosamine: Production and Applications. Mar. Drugs 2010, 8, 2493–2516.
- Merzendorfer, H. The cellular basis of chitin synthesis in fungi and insects: Common principles and differences. Eur. J. Cell Biol. 2011, 90, 759–769.
- Wang, S.L.; Chang, W.T. Purification and characterization of two bifunctional chitinases/lysozymes extracellularly produced by Pseudomonas aeruginosa K-187 in a shrimp and crab shell powder medium. Appl. Environ. Microbiol. 1997, 63, 380–386.
- Mourya, V.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051.
- Synowiecki, J.; Al-Khateeb, N.A. Production, Properties, and Some New Applications of Chitin and Its Derivatives. Crit. Rev. Food Sci. Nutr. 2003, 43, 145–171.
- Kramer, K.J.; Koga, D. Insect chitin: Physical state, synthesis, degradation and metabolic regulation. Insect Biochem. 1986, 16, 851–877.
- Merzendorfer, H.; Zimoch, L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 2003, 206, 4393–4412.
- Hu, X.; Chen, L.; Xiang, X.; Yang, R.; Yu, S.; Wu, X. Proteomic analysis of peritrophic membrane (PM) from the midgut of fifth-instar larvae, Bombyx mori. Mol. Biol. Rep. 2012, 39, 3427–3434.
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632.
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic Modifications of Chitin, Chitosan, and Chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243.
- Arrese, E.L.; Soulages, J.L. Insect Fat Body: Energy, Metabolism, and Regulation. Annu. Rev. Èntomol. 2010, 55, 207–225.
- Salama, D.M.; El-Aziz, M.A.; Rizk, F.A.; Elwahed, M.A. Applications of nanotechnology on vegetable crops. Chemosphere 2021, 266, 129026.
- Suresh, P.V.; Kumar, P.K.A. Enhanced degradation of α-chitin materials prepared from shrimp processing byproduct and production of N-acetyl-d-glucosamine by thermoactive chitinases from soil mesophilic fungi. Biogeochemistry 2012, 23, 597–607.
- Brasselet, C.; Pierre, G.; Dubessay, P.; Dols-Lafargue, M.; Coulon, J.; Maupeu, J.; Vallet-Courbin, A.; de Baynast, H.; Doco, T.; Michaud, P.; et al. Modification of Chitosan for the Generation of Functional Derivatives. Appl. Sci. 2019, 9, 1321.
- Mourya, V.K.; Inamdar, N.N.; Choudhari, Y.M. Chitooligosaccharides: Synthesis, characterization and applications. Polym. Sci. Ser. A 2011, 53, 583–612.
- Chatterjee, S.; Adhya, M.; Guha, A.; Chatterjee, B. Chitosan from Mucor rouxii: Production and physico-chemical characterization. Process Biochem. 2005, 40, 395–400.
- LogithKumar, R.; KeshavNarayan, A.; Dhivya, S.; Chawla, A.; Saravanan, S.; Selvamurugan, N. A review of chitosan and its derivatives in bone tissue engineering. Carbohydr. Polym. 2016, 151, 172–188.
- Arnold, N.D.; Brück, W.M.; Garbe, D.; Brück, T.B. Enzymatic Modification of Native Chitin and Conversion to Specialty Chemical Products. Mar. Drugs 2020, 18, 93.
- Sharma, N.; Sharma, K.; Gaur, R.; Gupta, V. Role of Chitinase in Plant Defense. Asian J. Biochem. 2011, 6, 29–37.
- Rathore, A.S.; Gupta, R.D. Chitinases from Bacteria to Human: Properties, Applications, and Future Perspectives. Enzym. Res. 2015, 2015, 791907.
- Loc, N.H.; Huy, N.D.; Quang, H.T.; Lan, T.T.; Ha, T.T.T. Characterisation and antifungal activity of extracellular chitinase from a biocontrol fungus, Trichoderma asperellum PQ34. Mycology 2019, 11, 38–48.
- Roy, J.C.; Salaun, F.; Giraud, S.; Ferri, A.; Chen, G.; Guan, J. Solubility of chitin: Solvents, solution behaviors and their related mechanisms. In Solubility of Polysaccharides; Xu, Z., Ed.; Intech Open: London, UK, 2017.
More
