Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Beatrix Zheng and Version 1 by Natalia Mikhailovna Yudintceva.
Mesenchymal stem cells (MSCs) are attractive in various fields of regenerative medicine due to their therapeutic potential and complex unique properties. Basic stem cell research and the global COVID-19 pandemic have given impetus to the development of cell therapy for infectious diseases.
- mesenchymal stem cells
- extracellular vesicles
- infectious diseases
- COVID-19
- influenza
- HIV
- tuberculosis
- cholera
1. Viral Infectious Diseases
Over the past decades, a huge number of experimental and clinical studies have been devoted to the use of cell therapy in the treatment of oncological, cardiovascular, neurodegenerative, and other diseases [158,159,160][1][2][3]. Basic stem cell research and the global COVID-19 pandemic have given rise to the development of cell therapy for infectious diseases, which currently stands at 121 registered clinical trials [161][4].
1.1. COVID-19
Coronavirus and other respiratory viruses are the leading cause of morbidity and mortality in acute lung injury (ALI) and acute respiratory distress syndrome. Although scientific advances have enabled rapid progress in understanding pathogenesis and developing therapeutic agents, stem cell therapy has recently found numerous applications in the treatment of viral infections.
Severe forms of the disease caused by COVID-19 are accompanied by increased activation of the immune system, which, in addition to antiviral protection, leads to a side effect—damage to lung tissue and other organs. To date, several studies have proposed the use of MSCs for the treatment of pneumonia caused by COVID-19 [162,163,164,165][5][6][7][8]. MSCs have been shown to reduce inflammation and suppress viral infection [166][9]. In the ALI mouse model, it was shown that, due to the anti-inflammatory effect, MSCs improve lung function, synthesizing the keratinocytes growth factor (KGF), VEGF, and HGF to restore damaged epithelial cells and lung tissues. IDO, TGF-ß, and granulocyte-macrophage colony-stimulating factor (G-CSF) act on macrophages, neutrophils, and T-cells (Figure 21). The main mechanism of action is probably to reduce the secretion of inflammatory factors [167][10].
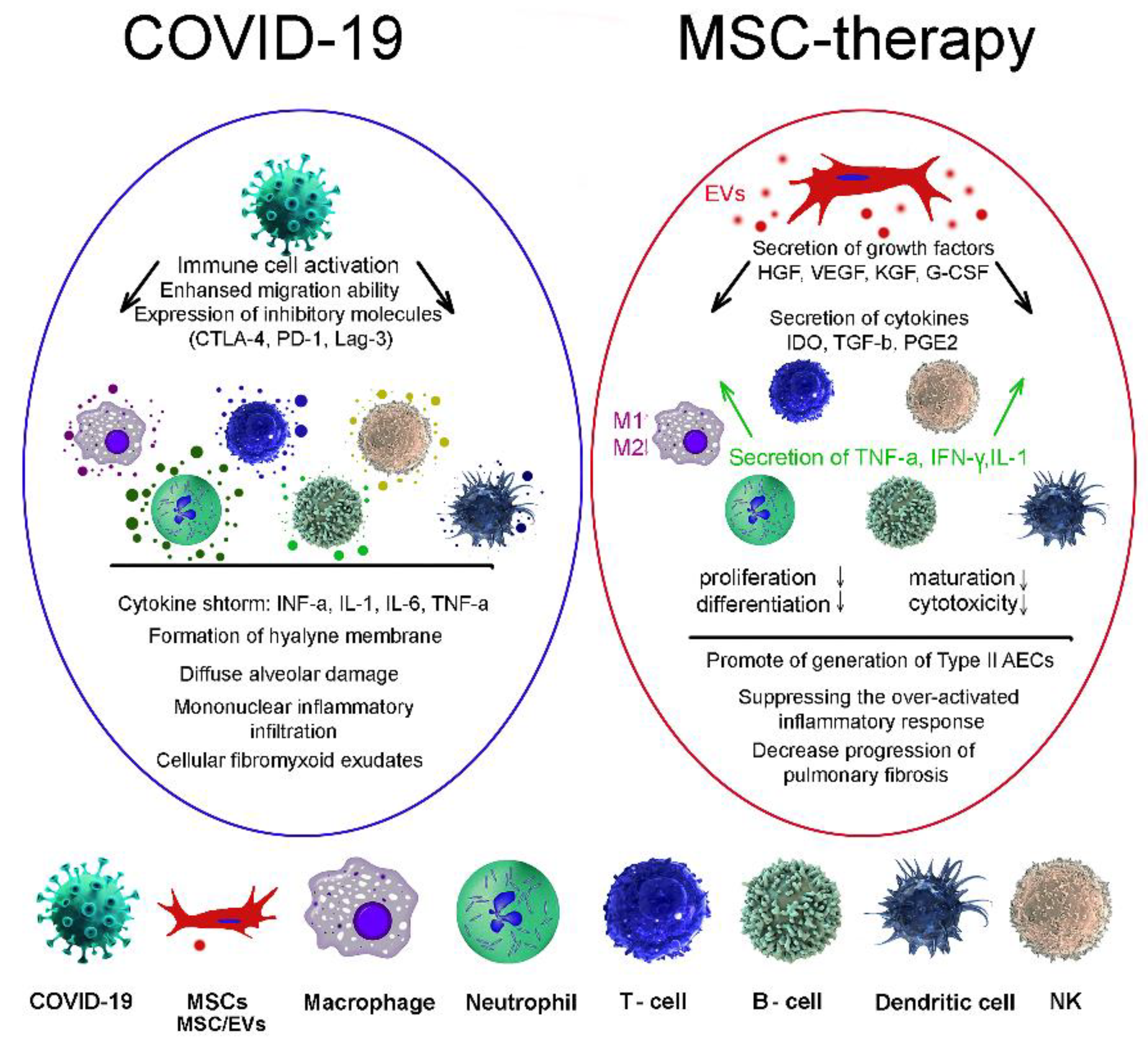
Figure 21. MSC therapies for treatment of coronavirus-induced lung injury. COVID-19, coronavirus; MSCs, mesenchymal stem/stromal cells; EVs, extracellular vesicles; macrophage; neutrophil; T-cell; B-cell; dendritic cell; NK, natural killer cell.
Clinical intravenous administration of MSCs has shown an increase in the number of peripheral lymphocytes, hyperactivation of some types of T-cells as well as a decrease in the level of C-reactive protein [168][11]. A major factor in organ damage in severe COVID-19 cases is the cytokine storm. Due to their strong immunomodulatory ability, MSCs not only suppress the cytokine storm, but also promote the activation of the endogenous regenerative mechanism [169][12]. At the same time, MSC-EVs play an important role in the implementation of intercellular communication, since they are able to enter the bloodstream, pass through it for long distances and pass through histohematic barriers [170,171][13][14].
Several clinical studies have demonstrated the ability of MSC-EVs to reduce the level of inflammatory factors and increase immunity in various forms of COVID-19 (NCT04384445, USA; NCT04276987, China; NCT04491240, Russia). Two clinical trials are currently underway: one study group (NCT04276987) is investigating the efficacy of inhaled treatment of COVID-19 pneumonia using EVs derived from A-MSCs, and the second one (NCT04313647) is evaluating their safety and tolerability in healthy volunteers.
Due to their specific structure, various drugs can be introduced into MSC-EVs to use them as delivery systems [172][15] and one of the tools in the treatment of viral infection [173][16]. In addition, compared with other types of treatment, such as monoclonal antibody therapy, the economic costs of obtaining and using MSC-EV are significantly lower, which is important when using this method during a pandemic [28][17]. Ongoing clinical trials highlight the potential benefits of using both MSCs and MSC-EVs for the treatment of patients with COVID-19. However, further studies to evaluate and confirm their efficacy and safety are needed.
1.2. Flu
Due to the fact that infectious diseases of the respiratory organs caused by various viruses can occur like the common cold but also have severe acute respiratory syndromes [174][18], it is rather difficult to determine the specific agents involved in the infection [175][19]. Currently, influenza therapy mainly includes antibacterial and antiviral drugs.
Several studies on animal models infected with the influenza virus have shown a positive effect of the use of MSCs of various tissue origins [176,177,178,179,180][20][21][22][23][24]. Cocultivation of BM-MSCs with H5N1 virus-infected AECs inhibits their permeability under in vitro conditions. Possible mechanisms for this are related to the secretion of angiopoietin-1 (Ang1) and KGF by BM-MSCs [178][22]. In vivo experiments demonstrate that BM-MSCs have a significant anti-inflammatory effect by increasing the number of macrophages and releasing various cytokines and interleukins: IL-1 beta, IL-4, IL-6, IL-8, and IL-17 [180,181][24][25]. Similar anti-inflammatory effects have been shown using another model of lung injury caused by the H9N2 virus [179][23]. Intravenous injection of a suspension of BM-MSCs into virus-infected mice significantly attenuates virus-induced lung inflammation by reducing the levels of chemokines (GM-CSF, MCP-1, KC, MIP-1α, and MIG) and proinflammatory factors IL-1 alpha, IL-6, TNF-alpha, and IFN-gamma. Using an in vitro model of lung injury caused by the H5N1 virus, human UC-MSCs, through the secretion of Ang1 and HGF, had the same anti-inflammatory effect as BM-MSCs [182][26]. In one clinical study in patients with lung injury caused by the H7N9 influenza virus, the use of MSCs did not cause side effects and significantly increased their survival [183][27]. Despite the data indicating the therapeutic effect of MSCs in various preclinical models of lung injury, some studies have shown that the use of a suspension of MSCs with an antiviral drug was ineffective [184,185][28][29]. In addition, when using cell therapy, it is necessary to take into account the condition of the donor and recipient. It has been shown that when MSCs are administered to a patient with ongoing disease, cells can become infected with the influenza virus, and transplantation of BM-MSCs from influenza virus-infected donors, in turn, can also transmit the infection to recipients. Thus, when using cell therapy in the treatment of pulmonary influenza, it is imperative to take into account these factors and observe safety.
1.3. AIDS
The human immunodeficiency virus (HIV) is caused by a retrovirus of the lentivirus genus. It affects cells of the immune system that have CD4 receptors on their surface: T-helpers, monocytes, macrophages, Langerhans cells, dendritic cells, and microglial cells. As a result, the work of the immune system is inhibited and the syndrome of acquired immune deficiency (AIDS) develops, the patient’s body loses the ability to defend itself against infections and tumors. Despite the fact that the first case of AIDS was discovered almost 27 years ago, it is still not possible to effectively control the AIDS pandemic [186][30]. Of the 35 million people living with HIV infection, a fraction survives thanks to antiretroviral therapy, but in the absence of it, death occurs on average 9–11 years after infection. There are currently three known cases of a cure for the virus. In the medical literature, they appear under the names “Berlin”, “London”, and “Sao Paulo” patients [187,188][31][32].
Recently, a new strategy for the treatment of HIV and AIDS using stem cells, in particular BM-MSCs, has emerged [189][33]. According to the data, circulating replicative HIV remains the most serious threat to effective AIDS therapy. The main therapy strategy is aimed at reducing the number of replicating virus particles. As a result of its application, the destruction of HIV circulating in the blood occurs with the help of erythrocytes integrated with the CD4 receptor and chemokine receptors, which selectively bind circulating HIV particles [190,191,192,193][34][35][36][37].
One of the most interesting studies focused on the use of MSCs to increase antiviral immune activity and minimize the amount of virus. It has been shown that the administration of MSCs, even in the absence of antiviral drugs, can enhance the host’s antiviral response due to the restoration of lymphoid follicles and mucosal immunity, all of which become the target of the virus at an early stage [194][38]. The results of scientific and clinical studies provide an appropriate scientific basis for the future use of MSCs in the treatment of HIV and other infectious diseases. Researchers are still developing comprehensive and effective treatments for AIDS and related conditions.
Cell-based therapies initially were reserved to the most severely affected patients with viral infectious diseases (COVID-19, flu, and AIDS) and most clinical trials were also focused on them.
The application of cell and vesicles therapy in most of the clinical trials resulted in symptomatic relief and treatment success. However, in order to ensure the widespread clinical implementation of MSC-based therapy, there are many challenges that need to be resolved (stages of the disease, clinical indicators, gender and age of patients, the source and age of MSCs, etc.).
2. Bacterial Infectious Diseases
2.1. Tuberculosis
Tuberculosis (TB) is one of the 10 leading causes of death worldwide. According to WHO data for 2021, over 9.9 million people worldwide became infected and about 1.3 million people died from TB [218][39]. The emergence of the COVID-19 pandemic has severely disrupted global TB prevention and control [219,220][40][41]. Nearly half a million people suffer from the rifampin-resistant TB strain, of which 78% are multidrug-resistant. In this regard, the actual direction is the search for fundamentally new approaches in the treatment of resistant TB, among which a certain place is occupied by MSC therapy.
Once in the lower respiratory tract, mycobacteria (Mycobacterium tuberculosis (µTb)) are mainly absorbed by macrophages. In this case, the resulting inflammatory reaction causes a large number of immune cells (monocytes, dendritic cells, neutrophils, and T-lymphocytes) to be attracted to the infected area, resulting in the formation of tuberculous granuloma (TG), which is a pathological sign of TB [221,222][42][43]. TG formation is a key event in preventing the spread of infection, and the period during which µTb are able to avoid the host’s immune response and remain dormant can be decades [223][44]. Numerous studies show that MSCs are involved in the formation and development of TG. It was found using CD29 as a marker that the cells are in a cluster with acid-resistant bacteria and are distributed in the TG area. In the pathogenesis of TB, MSCs, on the one hand, are able to inhibit the T-cell response through the synthesis of nitric oxide (NO) and, thereby, reduce the immune response, and on the other hand, NO itself can inhibit the growth of µTb and limit their proliferation within TG. Thus, it can be assumed that the formation of TG is associated precisely with this mechanism [224][45]. It has been shown that MSCs are able to regulate and limit the growth of µTb [225][46] using scavenger receptors for this [226,227][47][48]. Whether MSCs can influence the growth of µTb in any other way is still a question that still needs further study.
It has been shown that MSCs are natural host cells of latent µTb infection. In addition, recent research found that MSCs exist in the lungs and extrapulmonary tuberculosis granuloma. After infection with MSCs, the metabolic activity of µTb in cells becomes low, and, thus, they gradually acquire resistance to antituberculosis drugs [228,229][49][50]. Thus, along with immune cells, MSCs can not only provide a niche for dormant µTb but also limit their growth to a certain extent and participate in the emergence and development of TB.
The incidence of TB largely depends not on primary or secondary infection but on the reactivation of the dormant form of TB against the background of the emerging immunodeficiency [230][51]. In this regard, in recent years, therapy methods aimed at increasing infection control, reducing inflammation by modulating the immune response, and reducing tissue damage have become widespread [231][52]. Immunomodulatory properties and the ability to replace or repair damaged tissues make MSCs ideal candidates for the treatment of both pulmonary and extrapulmonary TB [232][53]. A number of studies have shown that the therapeutic potential of MSCs is associated with the antibacterial activity of cells directed against various pathogens (Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus pneumonia) through the secretion of antimicrobial peptides [233,234,235][54][55][56]. However, it is not yet known whether MSCs affect µTb growth in the same way.
MSCs and MSC-EVs have a wide range of immunomodulatory effects on various cells of the immune system: they promote the function of regulatory T cells (Treg and Th2) [236[57][58],237], inhibit the release of IFN-gamma, regulate the balance of Th1/Th2 [238][59], promote polarization of macrophages from M1 to M2 by expression of IDO and activation of CD39 and CD73/adenosine signaling pathways [237,238[58][59][60],239], and inhibit activation and promote B cell transformation [240,241,242][61][62][63]. In addition, MSCs are able to regulate the survival of the alveolar epithelium by secreting factors KGF and HGF that protect cells from apoptosis [243][64].
Previously, wthe researchers showed that intravenous administration of MSCs results in accumulation and retention of MSCs in µTb-affected rabbit kidney tissue, due to which the cells are able to reduce the level of the inflammatory response and enhance the process of tissue repair [244][65]. A decrease in the level of expression and synthesis of hydroxyproline, collagen Types I and III leads to a decrease in fibrosis, restoration of damage, and prevention of pulmonary edema [245,246,247][66][67][68].
A number of studies have shown that, at low concentrations, MSCs can inhibit the activation of lymphocytes [248][69]. Thus, the ratio of the number of MSCs and immune cells can be a turning point for inhibiting or activating the immune response. Overall, the results obtained with MSCs in vivo are encouraging, but the safety and efficacy of MSCs in the treatment of TB remains to be confirmed.
2.2. Cholera
Vibrio cholerae (VCh) is the causative agent of cholera, which is commonly associated with a high infection rate, mortality, and a major public health problem in many parts of the world [249,250][70][71]. According to WHO data, each year there are from 1.3 to 4 million cases of cholera, and 100,000 to 130,000 deaths worldwide due to cholera per year. The emergence of multidrug-resistant VCh strains in developing countries is of great concern [251,252][72][73]. The high mortality rate and the lack of effective antimicrobials necessitate the development of new effective approaches for the treatment of drug-resistant strains. Various vaccines have been developed (Dukoral, Shanhol, and Euvichol), but none provide complete long-term protection and are not approved for use in children under 1 year of age. Inflammation caused by the interaction of Vibrio cholerae with epithelial cells is considered as the main cause of the spread of bacteria in the gastrointestinal tract and the progression of its consequences. One of the effective therapeutic approaches to treatment is to reduce the level of inflammatory cytokines caused by VCh infection.
MSCs exert their antibacterial properties through the synthesis of compounds such as antimicrobial peptides (hCAP18/LL-37), which control the growth and reproduction of bacteria. One study using a neonatal mouse model showed the immunomodulatory effect of a medium conditioned with MSCs supplemented with LPS (lipopolysaccharide necessary to protect the body from VCh) [253][74], a decrease in the level of the inflammatory response and the induction of the production of vibriocidal antibodies that protect against VCh. In addition, MSCs have been shown to be effective in the treatment of bacterial sepsis [254,255][75][76].
A-MSCs show dual effects on inflammatory response and epithelial barrier integrity by reduction of bacterial attachment and increasing bacterial internalization. On the one hand, A-MSCs reduce bacterial adhesion and colony formation by secreting various antimicrobial peptides (including IDO, and TIMP). A decrease in the rate of bacterial adhesion, in turn, leads to a decrease in the expression of chloratoxin and an increase in the secretion of IL-6, which has a positive effect on maintaining the integrity of the epithelial barrier. On the other hand, increased bacterial internalization by cells stimulates the inflammatory reactions. An increase in the level of expression of the proinflammatory genes TNF-alpha, IL-1beta, and IL-8 leads to an increase in the level of cytokines, induction of apoptosis, and degradation of the tight junction between epithelial cells. Thus, A-MSCs are able to exert different effects on the inflammatory response and the integrity of the epithelial barrier by reducing bacterial adhesion and enhancing bacterial internalization. The probable reason for this effect is the high level of MSC expression of matrix metalloproteinases and tissue inhibitor of proteinases (TIMP), as well as other antibacterial peptides [256][77]. Therefore, it is recommended that future studies focus on the protective effects of MSCs’ secretome.
It can be assumed that the reduction of bacterial internalization may also become an appropriate therapeutic approach to limit the inflammatory reactions caused by VCh, while it is more efficient to use MSC-EVs as a therapeutic agent instead of intact cells.
Currently, the evaluation of the effectiveness of cell therapy for bacterial infectious diseases is carried out mainly in vitro and in vivo conditions. There are few clinical studies on this topic (Table 41).
Table 41.
MSC- and MSC-EV-based clinical trials of the bacterial infection diseases.
| Study Title | Abbreviation | Bacterial Diseases | Status | Country | Description | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Effectivity of Local Implantation of the Mesenchymal Stem Cell on Vertebral Bone Defect Due to Mycobaterium Tuberculosis Infection (Clinical Trial) | MSCs | Extrapulmonary tuberculosis | Phase 2 | Indonesia | 3 × 10 | 7 | cells/kg diluted in 2 mL 0.9% NaCl intravenously | [257] | [78] |
| 2 | Systemic Transplantation of Autologous Mesenchymal Stem Cells of the Bone Marrow in the Treatment of Patients With Multidrug-Resistant Pulmonary Tuberculosis | MSCs | Tuberculosis; multidrug resistant, extensive drug resistant | Completed | Russia | Not stated | [258] | [79] | ||
| 3 | Autologous Mesenchymal Stromal Cell Infusion as Adjunct Treatment in Patients With Multidrug and Extensively Drug-Resistant Tuberculosis: An Open-Label Phase 1 Safety Trial. | BM-MSCs | Tuberculosis; multidrug resistant, extensive drug resistant | Phase 1 | Belarus | 1 × 10 | 7 | cells/kg diluted in saline | [259] | [80] |
| 4 | Effectiveness of a Novel Cellular Therapy to Treat Multidrug-Resistant Tuberculosis. | BM-MSCs | Tuberculosis; multidrug resistant, extensive drug resistant | Phase 1 | Belarus | 1 × 10 | 7 | cells/kg diluted in saline | [260] | [81] |
MSC, mesenchymal stem cells; BM-MSC, bone marrow-derived mesenchymal stem cells.
References
- Jiang, D.; Muschhammer, J.; Qi, Y.; Kügler, A.; de Vries, J.C.; Saffarzadeh, M.; Sindrilaru, A.; Beken, S.V.; Wlaschek, M.; Kluth, M.A.; et al. Suppression of neutrophil-mediated tissue damage—A novel skill of mesenchymal stem cells. Stem Cells 2016, 3, 2393–2406.
- Aly, R.M. Current state of stem cell-based therapies: An overview. Stem Cell Investig. 2020, 7, 8.
- Chari, S.; Nguyen, A.; Saxe, J. Stem cells in the clinic. Cell Stem Cell 2018, 22, 781–782.
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and safety issues of stem cell-based therapy. Int. J. Med. Sci. 2018, 15, 36–45.
- Wang, L.T.; Liu, K.J.; Sytwu, H.K.; Yen, M.L.; Yen, B.L. Advances in mesenchymal stem cell therapy for immune and inflammatory diseases: Use of cell-free products and human pluripotent stem cell-derived mesenchymal stem cells. Stem Cells Transl. Med. 2021, 10, 1288–1303.
- Metcalfe, S.M. Mesenchymal stem cells and management of COVID-19 pneumonia. Med. Drug Discov. 2020, 5, 100019.
- Gentile, P.; Sterodimas, A. Adipose-derived stromal stem cells (ASCs) as a new regenerative immediate therapy combating coronavirus (COVID-19)-induced pneumonia. Expert Opin. Biol. Ther. 2020, 20, 711–716.
- Leng, Z.; Zhu, R.; Hou, W.; Feng, Y.; Yang, Y.; Han, Q.; Zhao, R.C. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020, 11, 216.
- Golchin, A.; Seyedjafari, E.; Ardeshirylajimi, A. Mesenchymal stem cell therapy for COVID-19: Present or future. Stem Cell Rev. Rep. 2020, 16, 427–433.
- Ji, F.; Li, L.; Li, Z.; Jin, Y.; Liu, W. Mesenchymal stem cells as a potential treatment for critically ill patients with coronavirus disease Stem Cells Transl. Med. 2020, 9, 813–814.
- Zhao, L.; Hu, C.; Zhang, P.; Jiang, H.; Chen, J. Preconditioning strategies for improving the survival rate and paracrine ability of mesenchymal stem cells in acute kidney injury. J. Cell Mol. Med. 2019, 23, 720–730.
- Shu, L.; Niu, C.; Li, R.; Huang, T.; Wang, Y.; Huang, M.; Ji, N.; Zheng, Y.; Chen, X.; Shi, L.; et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 361.
- Zhang, K.; Chen, S.; Sun, H.; Wang, L.; Li, H.; Zhao, J.; Zhang, C.; Li, N.; Guo, Z.; Han, Z.; et al. In vivo two-photon microscopy reveals the contribution of Sox9+ cell to kidney regeneration in a mouse model with extracellular vesicle treatment. J. Biol. Chem. 2020, 295, 12203–12213.
- Eirin, A.; Zhu, X.Y.; Puranik, A.S.; Tang, H.; McGurren, K.A.; van Wijnen, A.J.; Lerman, A.; Lerman, L.O. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017, 92, 114–124.
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156.
- Gupta, P.S.; Krishnakumar, V.; Sharma, Y.; Dinda, A.K.; Mohanty, S. Mesenchymal stem cell derived exosomes: A nano platform for therapeutics and drug delivery in combating COVID-19. Stem Cell Rev. Rep. 2020, 1, 33–43.
- Caplan, A.I. Mesenchymal stem cells: Time to change the name! Stem Cells Transl. Med. 2017, 6, 1445–1451.
- Baker, S.C. Coronaviruses: From common colds to severe acute respiratory syndrome. Pediatr. Infect. Dis. J. 2004, 23, 1049–1050.
- Sloots, T.P.; Whiley, D.M.; Lambert, S.B.; Nissena, M.D. Emerging respiratory agents: New viruses for old diseases? J. Clin. Virol. 2008, 42, 233–243.
- Khatri, M.; Richardson, L.A.; Meulia, T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res. Ther. 2018, 9, 17.
- Du, J.; Li, H.; Lian, J.; Zhu, X.; Qiao, L.; Lin, J. Stem cell therapy: A potential approach for treatment of influenza virus and coronavirus-induced acute lung injury. Stem Cell Res. Ther. 2020, 11, 192.
- Chan, M.C.; Kuok, D.I.; Leung, C.Y.; Hui, K.P.; Valkenburg, S.A.; Lau, E.H.; Peiris, J.M. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2016, 113, 3621–3626.
- Li, Y.; Xu, J.; Shi, W.; Chen, C.; Shao, Y.; Zhu, L.; Lu, W.; Hanet, X. Mesenchymal stromal cell treatment prevents H9N2 avian influenza virus-induced acute lung injury in mice. Stem Cell Res. Ther. 2016, 7, 159.
- Loy, H.; Kuok, D.I.T.; Hui, K.P.Y.; Choi, M.H.L.; Yuen, W.; Nicholls, J.M.; Peiris, J.S.M.; Chan, M.C.W. Therapeutic implications of human umbilical cord mesenchymal stromal cells in attenuating influenza A (H5N1) virusassociated acute lung injury. J. Infect. Dis. 2019, 219, 186–196.
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal stem cell immunomodulation: Mechanisms and therapeutic potential. Trends Pharmacol. Sci. 2020, 41, 653–664.
- Suzdaltseva, Y.; Goryunov, K.; Silina, E.; Manturova, N.; Stupin, V.; Kiselev, S.L. Equilibrium among inflammatory factors determines human MSC-mediated immunosuppressive effect. Cells 2022, 11, 1210.
- Chen, J.; Hu, C.; Chen, L.; Tang, L.; Zhu, Y.; Xu, X.; Chen, L.; Gao, H.; Lu, X.; Yu, L.; et al. Clinical study of mesenchymal stem cell treatment for acute respiratory distress syndrome induced by epidemic influenza A (H7N9) infection: A hint for COVID-19 treatment. Engineering 2020, 6, 1153–1161.
- Gotts, J.E.; Abbott, J.; Matthay, M.A. Influenza causes prolonged disruption of the alveolar-capillary barrier in mice unresponsive to mesenchymal stem cell therapy. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 307, L395–L406.
- Darwish, I.; Banner, D.; Mubareka, S.; Kim, H.; Besla, R.; Kelvin, D.J.; Kain, K.C.; Liles, W.C. Mesenchymal stromal (stem) cell therapy fails to improve outcomes in experimental severe influenza. PLoS ONE 2013, 8, e71761.
- Taylor, B.S.; Sobieszczyk, M.E.; McCutchan, F.E.; Hammer, S.M. The challenge of HIV-1 subtype diversity. N. Engl. J. Med. 2008, 358, 1590–1602.
- Cohen, J. Has a second person with HIV been cured? Sci. Mag. 2019, 363, 1021.
- Cohen, J. An intriguing—But far from proven—HIV cure in the ‘São Paulo Patient’. Sci. Mag. 2020.
- Kitchen, S.G.; Zack, J.A. Stem cell-based approaches to treating HIV infection. Curr. Opin. HIV AIDS 2011, 6, 68–73.
- Kandula, U.R.; Wake, A. Promising stem cell therapy in the management of HIV and AIDS: A narrative review. Biol. Targets Ther. 2022, 16, 89–105.
- Khalid, K.; Padda, J.; Fernando, R.W.; Mehta, K.A.; Almanie, A.H.; Hennawi, H.A.; Padda, S.; Cooper, A.C.; Jean-Charles, G. Stem cell therapy and its significance in HIV infection. Cureus 2021, 13, e17507.
- Allers, K.; Hutter, G.; Hofmann, J.; Loddenkemper, C.; Rieger, K.; Thiel, E.; Schneider, T. Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation. Blood 2011, 117, 2791–2799.
- Fackler, O.T.; Murooka, T.T.; Imle, A.; Mempel, T.R. Adding new dimensions: Towards an integrative understanding of HIV-1 spread. Nat. Rev. Microbiol. 2014, 12, 563–574.
- Weber, M.G.; Walters-Laird, C.J.; Kol, A.; Rocha, C.S.; Hirao, L.A.; Mende, A.; Balan, B.; Arredondo, J.; Elizaldi, S.R.; Iyer, S.S.; et al. Gut germinal center regeneration and enhanced antiviral immunity by mesenchymal stem/stromal cells in SIV infection. JCI Insight 2021, 6, e149033.
- Eveni, J.; Filipo, K.; Garfin, A.M.C.; Geocaniga-Gaviola, D.M.; Huot, C.; Iavro, E.; Ismail, K.; Itogo, N.; Kako, H.; Kal, M.; et al. Global Tuberculosis Report 2021; World Health Organization: Geneva, Switzerland, 2021; pp. 1–57.
- Can Sarinoglu, R.; Sili, U.; Eryuksel, E.; Olgun Yildizeli, S.; Cimsit, C.; Karahasan Yagci, A. Tuberculosis and COVID-19: An overlapping situation during pandemic. J. Infect. Dev. Ctries 2020, 14, 721–725.
- Lange, C.; Dheda, K.; Chesov, D.; Mandalakas, A.M.; Udwadia, Z.; Horsburgh, C.R.J. Management of drug-resistant tuberculosis. Lancet 2019, 394, 953–966.
- Cohen, S.B.; Gern, B.H.; Delahaye, J.L.; Adams, K.N.; Plumlee, C.R.; Winkler, J.K.; Sherman, D.R.; Gerner, M.Y.; Urdahl, K.B. Alveolar macrophages provide an early mycobacterium tuberculosis niche and initiate dissemination. Cell Host Microbe 2018, 24, 439–446.e4.
- Orme, I.M.; Basaraba, R.J. The Formation of the granuloma in tuberculosis infection. Semin. Immunol. 2014, 26, 601–609.
- Sandor, M.; Weinstock, J.V.; Wynn, T.A. Granulomas in Schistosome and Mycobacterial Infections: A Model of Local Immune Responses. Trends Immunol. 2003, 24, 44–52.
- Raghuvanshi, S.; Sharma, P.; Singh, S.; Van Kaer, L.; Das, G. Mycobacterium tuberculosis evades host immunity by recruiting mesenchymal stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 21653–21658.
- Schwartz, Y.S.; Belogorodtsev, S.N.; Filimonov, P.N.; Cherednichenko, A.G.; Pustylnikov, S.V.; Krasnov, V.A. BCG infection in mice is promoted by naive mesenchymal stromal cells (MSC) and suppressed by Poly(A:U)-conditioned MSC. Tuberculosis 2016, 101, 130–136.
- Khan, A.; Mann, L.; Papanna, R.; Lyu, M.A.; Singh, C.R.; Olson, S. Mesenchymal stem cells internalize mycobacterium tuberculosis through scavenger receptors and restrict bacterial growth through autophagy. Sci. Rep. 2017, 7, 15010.
- Alquraini, A.; Khoury, J.E. Scavenger receptors. Curr. Biol. 2020, 30, R790–R795.
- Jain, N.; Kalam, H.; Singh, L.; Sharma, V.; Kedia, S.; Das, P.; Ahuja, V.; Kumar, D. Mesenchymal stem cells offer a drug-tolerant and immune-privileged niche to mycobacterium tuberculosis. Nat. Commun. 2020, 11, 3062.
- Singh, V.K.; Mishra, A.; Bark, S.; Mani, A.; Subbian, S.; Hunter, R.L.; Jagannath, C.; Khan, A. Human mesenchymal stem cell based intracellular dormancy model of mycobacterium tuberculosis. Microbes Infect. 2020, 22, 423–431.
- Shamputa, I.C.; Van Deun, A.; Salim, M.A.; Hossain, M.A.; Fissette, K.; de Rijk, P.; Rigouts, L.; Portaels, F. Endogenous reactivation and true treatment failure as causes of recurrent tuberculosis in a high incidence setting with a low HIV infection. Trop. Med. Int. Health 2007, 12, 700–708.
- Tsenova, L.; Singhal, A. Effects of host-directed therapies on the pathology of tuberculosis. J. Pathol. 2020, 250, 636–646.
- Yudintceva, N.M.; Bogolyubova, I.O.; Muraviov, A.N.; Sheykhov, M.G.; Vinogradova, T.I.; Sokolovich, E.G.; Samusenko, I.A.; Shevtsov, M.A. Application of the allogenic mesenchymal stem cells in the therapy of the bladder tuberculosis. J. Tissue Eng. Regen. Med. 2018, 12, e1580–e1593.
- Harman, R.M.; Yang, S.; He, M.K.; Van de Walle, G.R. Antimicrobial peptides secreted by equine mesenchymal stromal cells inhibit the growth of bacteria commonly found in skin wounds. Stem Cell Res. Ther. 2017, 8, 157.
- Chow, L.; Johnson, V.; Impastato, R.; Coy, J.; Strumpf, A.; Dow, S. Antibacterial activity of human mesenchymal stem cells mediated directly by constitutively secreted factors and indirectly by activation of innate immune effector cells. Stem Cells Transl. Med. 2020, 9, 235–249.
- Sutton, M.T.; Fletcher, D.; Ghosh, S.K.; Weinberg, A.; van Heeckeren, R.; Kaur, S.; Sadeghi, Z.; Hijaz, A.; Reese, J.; Lazarus, H.M.; et al. Antimicrobial properties of mesenchymal stem cells: Therapeutic potential for cystic fibrosis infection, and treatment. Stem Cells Int. 2016, 2016, 5303048.
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Ann. Rev. Cell Dev. Biol. 2014, 30, 255–289.
- Favaro, E.; Carpanetto, A.; Lamorte, S.; Fusco, A.; Caorsi, C.; Deregibus, M.C.; Bruno, S.; Amoroso, A.; Giovarelli, M.; Port, M.; et al. Human Mesenchymal Stem Cell-Derived Microvesicles Modulate T Cell Response to Islet Antigen Glutamic Acid Decarboxylase in Patients With Type 1 Diabetes. Diabetologia 2014, 57, 1664–1673.
- Li, P.; Zhao, Y.; Ge, L. Therapeutic Effects of Human Gingiva-Derived Mesenchymal Stromal Cells on Murine Contact Hypersensitivity via Prostaglandin E2-EP3 Signaling. Stem Cell Res. Ther. 2016, 7, 103.
- Huang, F.; Chen, M.; Chen, W.; Gu, J.; Yuan, J.; Xue, Y.; Dang, J.; Su, W.; Wang, J.; Zadeh, H.H.; et al. Human Gingiva-Derived Mesenchymal Stem Cells Inhibit Xeno-Graft-Versus-Host Disease via CD39-CD73-Adenosine and IDO Signals. Front. Immunol. 2017, 8, 68.
- Zhang, X.; Huang, F.; Li, W.; Dang, J.L.; Yuan, J.; Wang, J.; Zeng, D.-L.; Sun, C.-X.; Liu, Y.-Y.; Ao, Q.; et al. Human Gingiva-Derived Mesenchymal Stem Cells Modulate Monocytes/Macrophages and Alleviate Atherosclerosis. Front. Immunol. 2018, 9, 878.
- Ren, W.; Hou, J.; Yang, C.; Wang, H.; Wu, S.; Wu, Y.; Zhao, X.; Lu, C. Extracellular Vesicles Secreted by Hypoxia Pre-Challenged Mesenchymal Stem Cells Promote non-Small Cell Lung Cancer Cell Growth and Mobility as Well as Macrophage M2 Polarization via miR-21-5p Delivery. J. Exp. Clin. Cancer Res. 2019, 38, 62.
- Peng, Y.; Chen, X.; Liu, Q.; Zhang, X.; Huang, K.; Liu, L.; Li, H.; Zhou, M.; Huang, F.; Fan, Z.; et al. Mesenchymal Stromal Cells Infusions Improve Refractory Chronic Graft Versus Host Disease Through an Increase of CD5+ Regulatory B Cells Producing Interleukin 10. Leukemia 2015, 29, 636–646.
- Budoni, M.; Fierabracci, A.; Luciano, R.; Petrini, S.; Di Ciommo, V.; Muraca, M. The Immunosuppressive Effect of Mesenchymal Stromal Cells on B Lymphocytes is Mediated by Membrane Vesicles. Cell Transplant. 2013, 22, 369–379.
- Bernard, O.; Jeny, F.; Uzunhan, Y.; Dondi, E.; Terfous, R.; Label, R.; Sutton, A.; Larghero, J.; Vanneaux, V.; Nunes, H.; et al. Mesenchymal Stem Cells Reduce Hypoxia-Induced Apoptosis in Alveolar Epithelial Cells by Modulating HIF and ROS Hypoxic Signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L360–L371.
- Yudintceva, N.; Mikhailova, N.; Bobkov, D.; Yakovleva, L.; Nikolaev, B.; Krasavina, D.; Muraviov, A.; Vinogradova, T.; Yablonskiy, P.; Samusenko, I.; et al. Evaluation of the biodistribution of mesenchymal stem cells in a pre-clinical renal tuberculosis model by non-linear magnetic response measurements. Front. Phys. 2021, 9, 198.
- Chen, S.; Cui, G.; Peng, C.; Lavin, M.F.; Sun, X.; Zhang, E. Transplantation of Adipose-Derived Mesenchymal Stem Cells Attenuates Pulmonary Fibrosis of Silicosis via Anti-Inflammatory and Anti-Apoptosis Effects in Rats. Stem Cell Res. Ther. 2018, 9, 110.
- Zhu, Y.G.; Feng, X.M.; Abbott, J.; Fang, X.H.; Hao, Q.; Monsel, A.; Qu, J.; Matthay, M.A.; Lee, J.W. Human Mesenchymal Stem Cell Microvesicles for Treatment of Escherichia Coli Endotoxin-Induced Acute Lung Injury in Mice. Stem Cells 2014, 3, 116–125.
- Li, X.; Wang, Y.; An, G.; Liang, D.; Zhu, Z.; Lian, X.; Niu, P.; Guo, C.; Tian, L. Bone Marrow Mesenchymal Stem Cells Attenuate Silica-Induced Pulmonary Fibrosis via Paracrine Mechanisms. Toxicol. Lett. 2017, 270, 96–107.
- Poggi, A.; Zocchi, M.R. Immunomodulatory properties of mesenchymal stromal cells: Still unresolved “Yin and Yang”. Curr. Stem Cell Res. Ther. 2019, 14, 344–350.
- Sack, D.A.; Sack, R.B.; Chaignat, C.L. Getting serious about cholera. N. Engl. J. Med. 2006, 355, 649–651.
- Bishop, A.L.; Camilli, A. Vibrio cholerae: Lessons for mucosal vaccine design. Expert Rev. Vaccines 2011, 10, 79–94.
- Bhattacharya, D.; Dey, S.; Roy, S.; Parande, M.V.; Telsang, M.; Seema, M.H.; Parande, A.V.; Mantur, B.G. Multidrug-resistant Vibrio cholerae O1 was responsible for a cholera outbreak in 2013 in Bagalkot, North Karnataka. Jpn. J. Infect. Dis. 2015, 68, 347–350.
- Bhattacharya, D.; Sayi, D.S.; Thamizhmani, R.; Bhattacharjee, H.; Bharadwaj, A.P.; Roy, A.; Sugunan, A.P. Emergence of multidrug-resistant Vibrio cholerae O1 biotype El Tor in Port Blair, India. Am. J. Trop. Med. Hyg. 2012, 86, 1015–1017.
- Chatterjee, S.N.; Chaudhuri, K. Lipopolysaccharides of Vibrio cholerae: III. Biological functions. Biochim. Biophys. Acta 2006, 1762, 1–16.
- Saeedi, P.; Halabian, R.; Fooladi, A.I. Antimicrobial effects of mesenchymal stem cells primed by modified LPS on bacterial clearance in sepsis. J. Cell Physiol. 2019, 234, 4970–4986.
- Moulazadeh, A.; Soudi, S.; Bakhshi, B. Immunomodulatory effects of adipose-derived mesenchymal stem cells on epithelial cells function in response to Vibrio cholera in a co-culture model. Iran. J. Allergy Asthma Immunol. 2021, 20, 550–562.
- Clinicaltrials.gov. Effectivity of Local Implantation of the Mesenchymal Stem Cell on Vertebral Bone Defect Due to Mycobaterium Tuberculosis Infection (Clinical Trial) Clinicaltrials.gov, Identifier: NCT04493918. Available online: https://clinicaltrials.gov/ct2/show/NCT04493918 (accessed on 3 August 2020).
- Erokhin, V.V.; Vasil’eva, I.A.; Konopliannikov, A.G.; Chukanov, V.I.; Tsyb, A.F.; Bagdasarian, T.R.; Danilenko, A.A.; Lepekhina, L.A.; Kal’sina, S.S.; Semenkova, I.V.; et al. Systemic transplantation of autologous mesenchymal stem cells of the bone marrow in the treatment of patients with multidrug-resistant pulmonary tuberculosis. Probl. Tuberk. Bolezn. Legk. 2008, 10, 3–6.
- Skrahin, A.; Ahmed, R.K.; Ferrara, G.; Rane, L.; Poiret, T.; Isaikina, Y.; Skrahina, A.; Zumla, A.; Maeurer, M.J. Autologous mesenchymal stromal cell infusion as adjunct treatment in patients with multidrug and extensively drug-resistant tuberculosis: An open-label Phase 1 Safety Trial. Lancet Respir. Med. 2014, 2, 108–122.
- Skrahin, A.; Jenkins, H.E.; Hurevich, H.; Solodovnikova, V.; Isaikina, Y.; Klimuk, D.; Rohava, Z.; Skrahina, A. Effectiveness of a Novel Cellular Therapy to Treat Multidrug-Resistant Tuberculosis. Int. J. Mycobacteriol. 2016, 5, 23.
More
