Multiple myeloma (MM) is a clonal plasma cell tumor originating from a post-mitotic lymphoid B-cell lineage. When two or more medications are combined to improve their therapeutic effects, this is referred to as drug synergism. For various diseases, including MM, appropriate drug combinations can reduce drug resistance or maximize efficacy. Bortezomib (BTZ), as a clinically used protease inhibitor, is a cornerstone of VRDelcade, Revlimid, and Dexamethasone (VRD). Flavonoids and non-flavonoid polyphenols are potential supportive therapies to address some of the challenges faced in BTZ treatment.
- multiple myeloma
- bortezomib
- flavonoids
- non-flavonoid polyphenols
1. Mechanisms of MM
1. Mechanisms of Multiple Myeloma
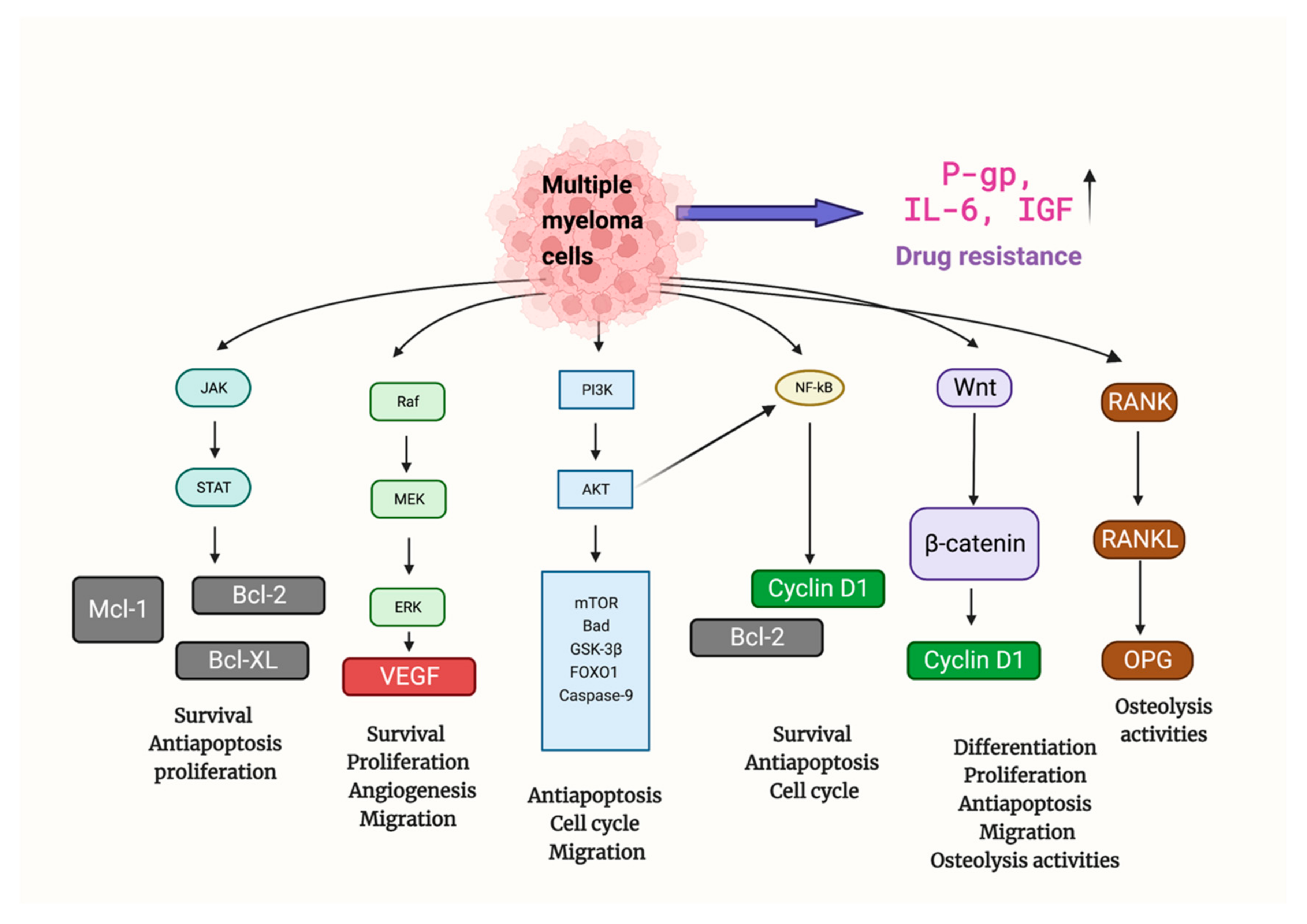
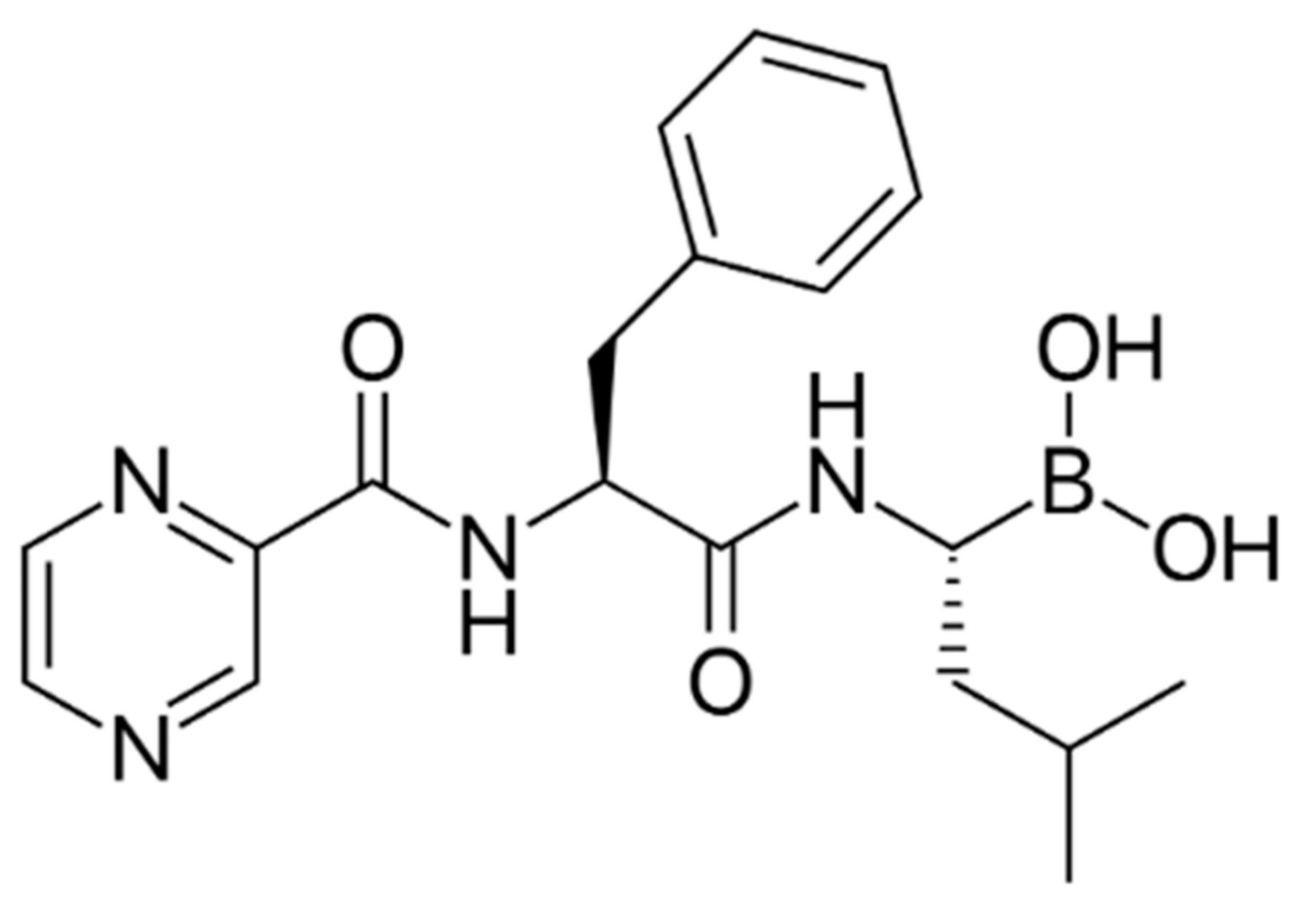
2. Bortezomib in MM Therapy
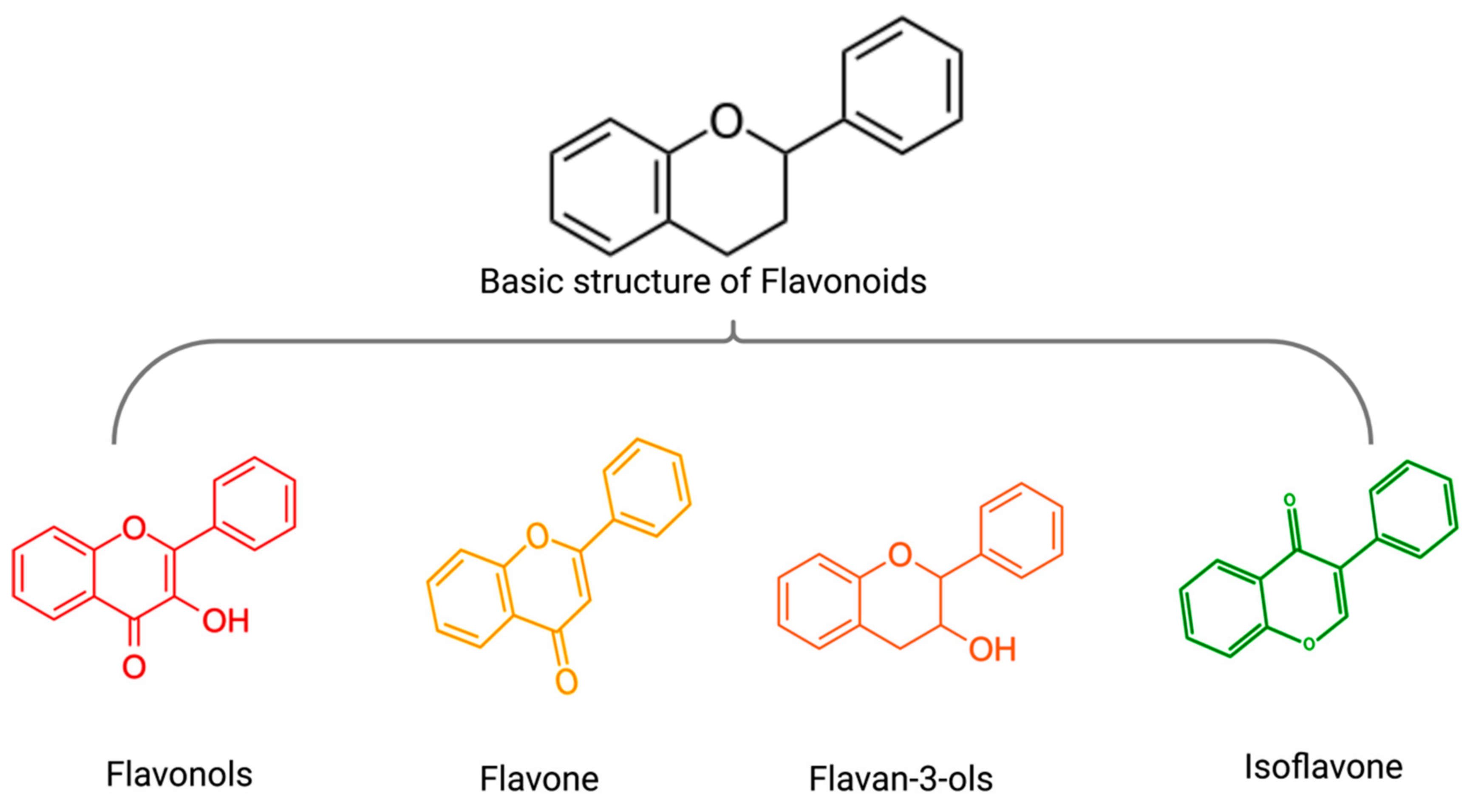
3. Flavonoids and Non-Flavonoid Polyphenols in MM Therapy
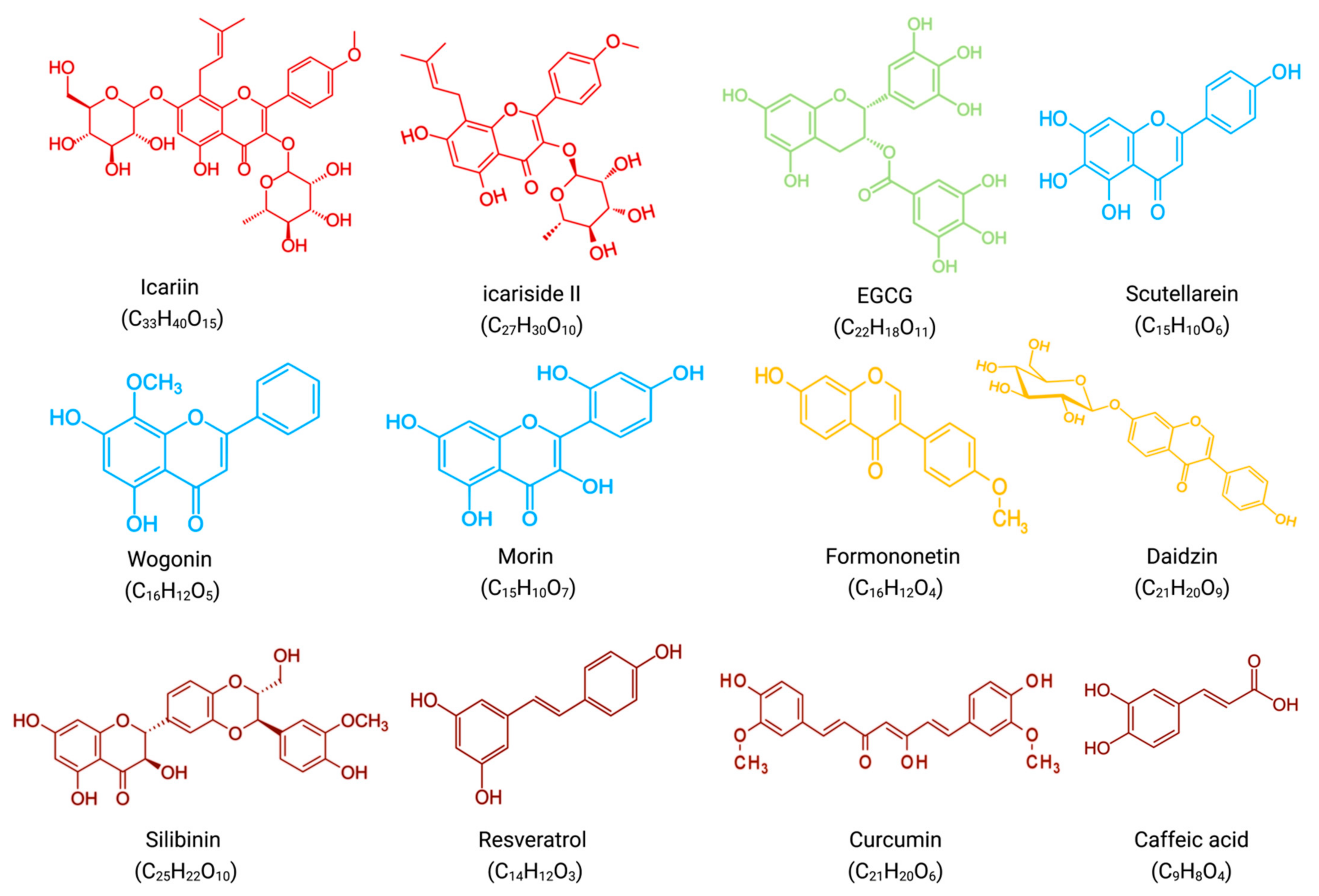
4. Synergistic Effects of Flavonoids and Bortezomib in Anti-MM
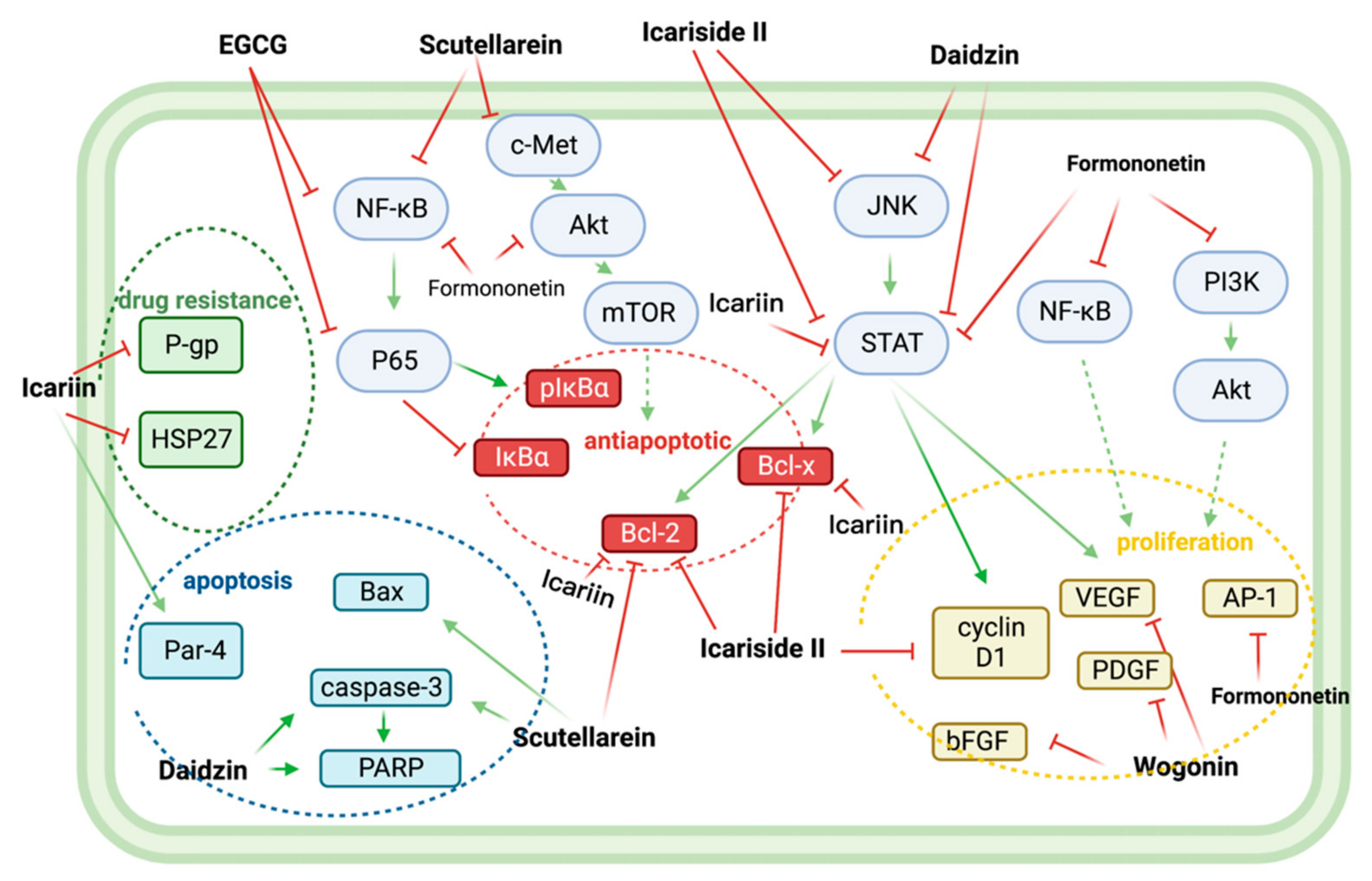
5. Synergistic Effects of Non-Flavonoid Polyphenols and Bortezomib in Anti-MM
In combination with low concentrations of BTZ, silibinin increased the cytotoxic effect of BTZ by increasing the expression of activated caspases, which promoted apoptosis [32][22].
In vitro, resveratrol induced apoptosis in MM144 cells by upregulating the Fas/CD95 signaling pathway and caspase-8 and caspase-10. Moreover, when used in combination, resveratrol, and BTZ highly enhanced U266 cell apoptosis [37][23].
Curcumin enhances the apoptotic effect of BTZ on MM cells by regulating multiple signaling pathways. The bone marrow microenvironment, in which bone marrow stromal cells (BMSCs) interact with MM, influences the survival and growth of MM cells. In vitro, curcumin inhibited the activation of JAK/STAT and MAPK pathways in U266 cells after treatment with BMSCs cell supernatant. Additionally, curcumin and BTZ co-treatment efficiently prevented IL-6-induced STAT3 and Erk phosphorylation, increased PARP cleavage, and decreased pro-caspase-3 levels. Through these mechanisms mentioned above, this combination inhibited the growth of U266 cells and promoted apoptosis [38][24].
References
- Hu, J.; Hu, W.-X. Targeting signaling pathways in multiple myeloma: Pathogenesis and implication for treatments. Cancer Lett. 2018, 414, 214–221.
- Anderson, K.C.; Carrasco, R.D. Pathogenesis of Myeloma. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 249–274.
- Robak, P.; Drozdz, I.; Szemraj, J.; Robak, T. Drug resistance in multiple myeloma. Cancer Treat. Rev. 2018, 70, 199–208.
- Gandolfi, S.; Laubach, J.P.; Hideshima, T.; Chauhan, D.; Anderson, K.C.; Richardson, P.G. The proteasome and proteasome inhibitors in multiple myeloma. Cancer Metastasis Rev. 2017, 36, 561–584.
- Scott, K.; Hayden, P.J.; Will, A.; Wheatley, K.; Coyne, I. Bortezomib for the treatment of multiple myeloma. Cochrane Database Syst. Rev. 2016, 4, CD010816.
- Tan, C.R.C.; Abdul-Majeed, S.; Cael, B.; Barta, S.K. Clinical Pharmacokinetics and Pharmacodynamics of Bortezomib. Clin. Pharmacokinet. 2018, 58, 157–168.
- Thibaudeau, T.A.; Smith, D.M. A Practical Review of Proteasome Pharmacology. Pharmacol. Rev. 2019, 71, 170–197.
- Manasanch, E.E.; Orlowski, R.Z. Proteasome inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 2017, 14, 417–433.
- Tzanova, M.; Atanasov, V.; Yaneva, Z.; Ivanova, D.; Dinev, T. Selectivity of Current Extraction Techniques for Flavonoids from Plant Materials. Processes 2020, 8, 1222.
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273.
- Li, Z.Y.; Li, Z.J.; Chen, X.; Huang, X.R.; Fang, Z.Q.; Zhang, J.G.; Fang, J. Icaritin Reverses Multidrug Resistance of Multiple Myeloma Cell Line KM3/BTZ. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2017, 25, 1690–1695.
- Kim, S.-H.; Ahn, K.S.; Jeong, S.-J.; Kwon, T.-R.; Jung, J.H.; Yun, S.-M.; Han, I.; Lee, S.-G.; Kim, D.K.; Kang, M.; et al. Janus activated kinase 2/signal transducer and activator of transcription 3 pathway mediates icariside II-induced apoptosis in U266 multiple myeloma cells. Eur. J. Pharmacol. 2011, 654, 10–16.
- Jung, Y.Y.; Lee, J.H.; Nam, D.; Narula, A.S.; Namjoshi, O.A.; Blough, B.E.; Um, J.-Y.; Sethi, G.; Ahn, K.S. Anti-myeloma Effects of Icariin Are Mediated Through the Attenuation of JAK/STAT3-Dependent Signaling Cascade. Front. Pharmacol. 2018, 9, 531.
- Wang, Q.; Li, J.; Gu, J.; Huang, B.; Zhao, Y.; Zheng, D.; Ding, Y.; Zeng, L. Potentiation of (−)-epigallocatechin-3-gallate-induced apoptosis by bortezomib in multiple myeloma cells. Acta Biochim. et Biophys. Sin. 2009, 41, 1018–1026.
- Shi, L.; Wu, Y.; Lv, D.L.; Feng, L. Scutellarein selectively targets multiple myeloma cells by increasing mitochondrial superoxide production and activating intrinsic apoptosis pathway. Biomed. Pharmacother. 2018, 109, 2109–2118.
- Fu, R.; Chen, Y.; Wang, X.-P.; An, T.; Tao, L.; Zhou, Y.-X.; Huang, Y.-J.; Chen, B.-A.; Li, Z.-Y.; You, Q.-D.; et al. Wogonin inhibits multiple myeloma-stimulated angiogenesis via c-Myc/VHL/HIF-1α signaling axis. Oncotarget 2015, 7, 5715–5727.
- Gupta, S.C.; Phromnoi, K.; Aggarwal, B.B. Morin inhibits STAT3 tyrosine 705 phosphorylation in tumor cells through activation of protein tyrosine phosphatase SHP1. Biochem. Pharmacol. 2012, 85, 898–912.
- Kim, C.; Lee, S.-G.; Yang, W.M.; Arfuso, F.; Um, J.-Y.; Kumar, A.P.; Bian, J.; Sethi, G.; Ahn, K.S. Formononetin-induced oxidative stress abrogates the activation of STAT3/5 signaling axis and suppresses the tumor growth in multiple myeloma preclinical model. Cancer Lett. 2018, 431, 123–141.
- Kim, C.; Lee, J.H.; Ko, J.-H.; Chinnathambi, A.; Alharbi, S.A.; Shair, O.H.; Sethi, G.; Ahn, K.S. Formononetin Regulates Multiple Oncogenic Signaling Cascades and Enhances Sensitivity to Bortezomib in a Multiple Myeloma Mouse Model. Biomolecules 2019, 9, 262.
- Yang, M.H.; Jung, S.H.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Sethi, G.; Ahn, K.S. Attenuation of STAT3 Signaling Cascade by Daidzin Can Enhance the Apoptotic Potential of Bortezomib against Multiple Myeloma. Biomolecules 2019, 10, 23.
- Tibullo, D.; Caporarello, N.; Giallongo, C.; Anfuso, C.D.; Genovese, C.; Arlotta, C.; Puglisi, F.; Parrinello, N.L.; Bramanti, V.; Romano, A.; et al. Antiproliferative and Antiangiogenic Effects of Punica granatum Juice (PGJ) in Multiple Myeloma (MM). Nutrients 2016, 8, 611.
- Geraldes, C.; Goncalves, A.; Alves, R.; Roque, A.; Manuel, J.; Costa, N.; Sarmento-Ribeiro, A. New Targeted Drugs in Multiple Myeloma Therapy—in Vitro Studies. Clin. Lymphoma Myeloma Leuk. 2015, 15, e254.
- Reis-Sobreiro, M.; Gajate, C.; Mollinedo, F. Involvement of mitochondria and recruitment of Fas/CD95 signaling in lipid rafts in resveratrol-mediated antimyeloma and antileukemia actions. Oncogene 2009, 28, 3221–3234.
- Park, J.; Ayyappan, V.; Bae, E.-K.; Lee, C.; Kim, B.-S.; Kim, B.K.; Lee, Y.-Y.; Ahn, K.-S.; Yoon, S.-S. Curcumin in combination with bortezomib synergistically induced apoptosis in human multiple myeloma U266 cells. Mol. Oncol. 2008, 2, 317–326.
- Altayli, E.; Koru, Ö.; ÖNGÜRÜ, Ö.; Ide, T.; Acikel, C.; Sarper, M.; Elci, M.P.; Sağkan, R.I.; Astarci, E.; Tok, D.; et al. An in vitro and in vivo investigation of the cytotoxic effects of caffeic acid (3,4-dihydroxycinnamic acid) phenethyl ester and bortezomib in multiple myeloma cells. Turk. J. Med. Sci. 2015, 45, 38–46.
