Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Beatrix Zheng and Version 1 by Mario Štefanović.
Circulating tumor cells (CTCs) are intact cells separated from the primary tumor or metastases and released into the peripheral circulation. They were observed and discovered for the first time in 1869 in the blood of a patient with breast cancer [33]. CTCs mainly originate from solid tumors of epithelial origin (breast, prostate, colon, and lung). CTCs are nucleated and express epithelial cell adhesion molecules (EpCAM) and/or cytokeratins (CK) in the cytoplasm without coexpressing the common leukocyte antigen CD45. It is known today that there is significant heterogeneity in cell species and surface markers, which represents a challenge in isolating all clinically relevant subpopulations of CTCs.
- colorectal cancer
- liquid biopsy
- circulating tumor cells
1. Circulating Tumor Cells
The presence of circulating tumor cells (CTCs) is associated with a worse prognosis and a high probability of the occurrence of a metastatic process. However, adequate detection and characterization of CTCs can significantly contribute not only to the diagnosis of cancer, but also to the prediction of the outcome of the disease, the selection of the optimal therapy, and the monitoring of the therapeutic treatment (Figure 21).
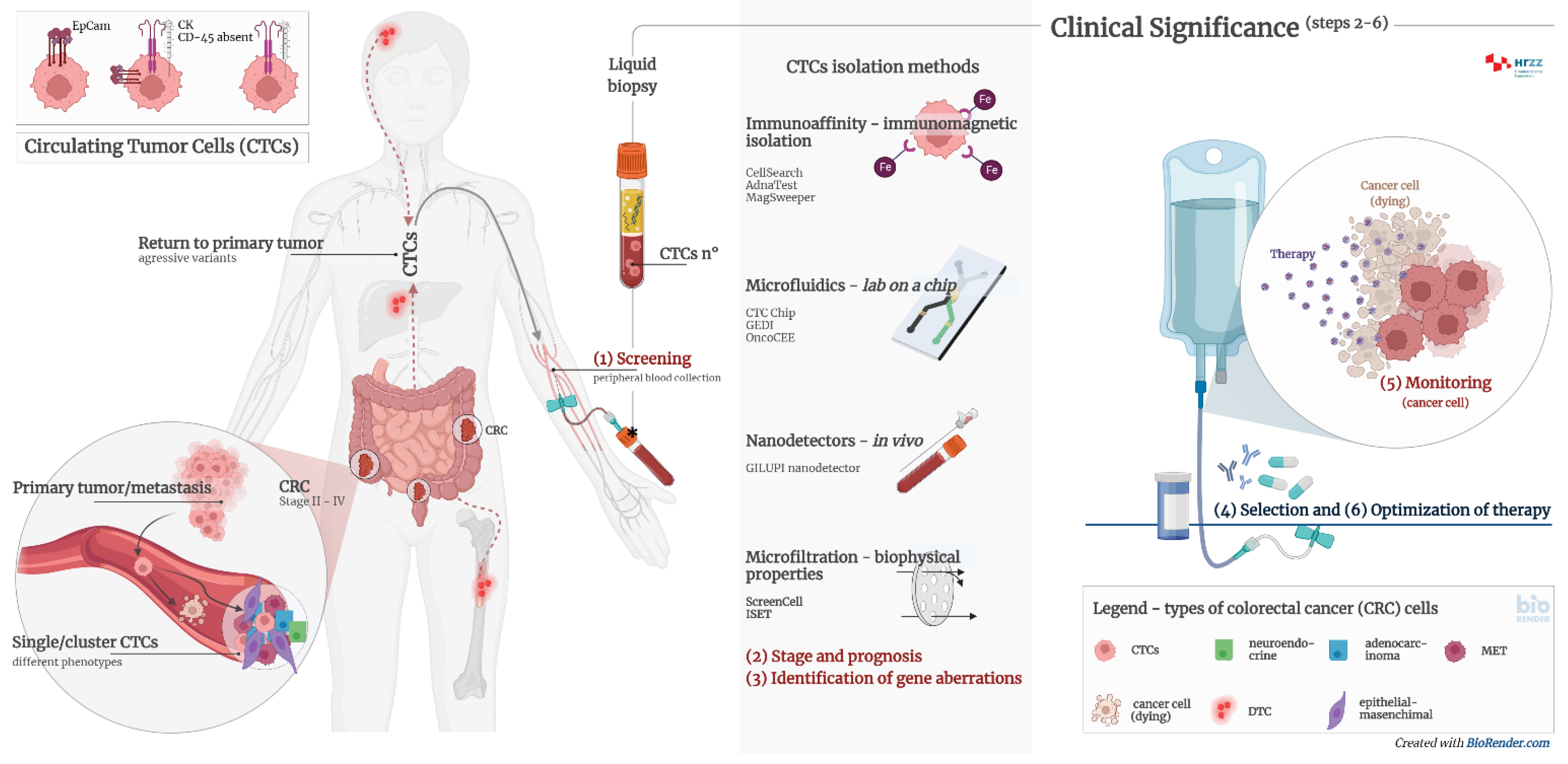
Figure 21. Clinical significance of CTCs and CTC clusters in diagnosis, monitoring, and optimization of therapy in cancer patients. Circulating tumour cells (CTCs) are proven to be prognostically relevant in cancer treatment in colorectal cancer (CRC). Adequate detection and characterization of CTCs can significantly contribute to cancer diagnosis, disease course prediction, therapy selection, and treatment monitoring. (1) Screening—detection of cancer patients by determining the number of CTCs and/or CTC clusters from peripheral blood; (2) stage and prognosis—detection of patients with an increased risk for the development of metastases by determining the number of CTCs and/or CTC clusters from peripheral blood; (3) identification of gene aberrations in CTCs important for therapy, such as mutations in KRAS, APC, EFGR, etc.; (4) selection of therapy—CTC cultivation and selection of effective pharmacotherapy; (5) monitoring—determining the number of CTCs and genome analysis to detect transcriptional changes; (6) optimization of therapy—monitoring the effectiveness of pharmacotherapy and early detection of disease remission by determining the number of CTCs and genome analysis.
The analysis of CTCs from blood can be used for the early detection of invasive cancer. Moreover, they have a prognostic significance in the monitoring of malignant disease or the response to chemotherapy. CTCs are sporadic cells with a frequency of one tumor cell per 5 × 106 leukocytes and 5 × 109 erythrocytes per mL of blood in patients with advanced cancer [34][1]. Numerous isolation technologies have been developed that enable the isolation and differentiation of CTCs from normal blood cells. Compared to blood cells, CTCs are more rigid, with a density of >1.077 g/mL, and larger in diameter than leukocytes, which have an average range of 8–11 μm. CTCs are relatively large cells, with an example of 30 μm in size in breast cancer patients [35][2].
With the degradation of the tumor mass in the first stages of the metastatic cascade, the tumor cells are released and migrate to the distal parts of the body by lymphohematogenous dissemination. During circulation and colonization, various interactions with other cells can lead to changes in CTC phenotypes [36][3]. The majority of CTCs die, but 0.01% stay viable and have the potential to form metastases. They may be present as single cells or clusters of cells. Once localized in organs (bone marrow, liver, lung, or brain), they are called disseminated tumor cells (DTCs) [35][2]. DTCs and micrometastases can be in a dormant phase for years after the primary tumor resection. At some point, DTCs can proliferate under the influence of the tumor microenvironment, and the formation of metastases occurs. DTCs originating from metastases can recirculate through the blood, colonizing other organs and creating secondary metastases. Research suggests that DTCs can transform into CTCs and return to the primary tumor site, resulting in the formation of more aggressive variants of tumor metastases [22][4].
Noninvasive liquid biopsy opens up detection possibilities for very rare circulating cancer stem cells (CSCs). CSCs and CTCs are particularly interesting targets in basic research, but also in clinical studies, due to their multiple roles and involvement in tumorigenesis, cancer progression, and resistance to therapy [37,38,39][5][6][7]. However, it is currently difficult to use these cells in the clinical setting: firstly, due to the lack of fully validated biomarkers, and secondly, due to the nonunique method for their identification. In fact, there is still no specific single marker or combination of markers that represent these cell subpopulations and clearly distinguishes them from conventional cancer cells. Indeed, CSCs are known to constitute only a small percentage (0.05–1%) [40,41][8][9] of tumor cells. The difficulty of CTC analysis is cellular plasticity, especially the phenomenon of the epithelial–mesenchymal transition (EMT). The process of the epithelial–mesenchymal transition is the change from the epithelial to mesenchymal phenotype. The EMT is a complex process of cell dedifferentiation and increased cell mobility due to the reorganization of contact bonds and the loss of adhesion molecules. Although this mechanism primarily occurs in organogenesis and wound healing, it is associated with tumor dissemination and correlates with aggressiveness due to increased tumor cell migration. The opposite process is also possible (mesenchymal–epithelial transition), which is proposed to play a role in the transformation of dormant DTCs in organs to metastases. This can lead to the nondetection of the population of potentially aggressive tumor cells in the standard CTC diagnostic process, as it relies on epithelial markers [22][4]. New technologies that could separate different CTC phenotypes are needed, highly relevant, and essential [42][10].
2. Diagnostic Significance of Circulating Tumor Cells
Developing new methods that enable the early diagnosis and follow-up for patients in all stages of the disease is crucial for reducing mortality and improving the outcome of CRC. The main objectives of research on CTCs as multifunctional biomarkers include: (1) identification of therapeutic targets, (2) stratification of oncological patients and monitoring of therapy in real-time (predictive significance), (3) a risk assessment for metastatic relapse or disease progression (prognostic significance), and (4) understanding the tumor biology and mechanisms of resistance to therapy [13,22][4][11].
Molecular characterization of CTCs provides a noninvasive approach to tumor genotypic and phenotypic characteristics. Treatment decisions are empirically defined by tumor histology after biopsy, which is sometimes performed after the initial treatment decision. Characterizing tumors from CTCs using a simple blood test could enable more effective treatment choices and reduce the incidence of unnecessary toxicity and side effects in patients receiving chemotherapy and immunotherapy [43][12]. Detection of mutations based on CTCs in the blood provides information that could significantly accelerate the discovery of a therapeutic target. Compared to tissue biopsy, a shorter turnaround time (TAT) of molecular analysis leads to faster therapeutic intervention and better disease outcomes. Since the tumor mutational profile affects the choice of therapy, research has been performed on colorectal cancer [44][13], lung cancer [45][14], breast cancer, and melanoma [46,47][15][16]. Table 1 shows the clinical significance of CTC determination in patients with CRC. The table also shows values such as the proportion of patients with positive CTC findings and the clinical usefulness of the determination [48,49[17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34],50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65],
Table 1.
Clinical significance of CTCs in colorectal cancers.
| Number of Patients | Detection Method | CTC No. (%) | Clinical Significance | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| 34 | Multiplex PCR | 20 (59) | Therapy alignment and monitoring; CTCs could predict chemotherapy response; moreover, EGFR status of CTCs could predict the likelihood of targeted therapy response. | [48] | [17] | |||
| 30 | Density gradient centrifugation, CK20 qRT-PCR and immunomagnetic CTC number determination | 30 (100) | CTC number reflects the chemotherapeutic sensitivity of CRC patients. Microscopic CTC single-cell, doublet, and cluster numbers were found in correlation with CK20 qRT-PCR results. | [49] | [18] | |||
| 40 | CELLection Dynabeads | ® | 27 (68) | Therapy alignment and monitoring. Significant shorter progression-free survival (PFS) was found in patients with CTCs positive for the expression of ALDH1, survivin and MRP5. | [50] | [19] | ||
| 467 | CellSearch | 467 (100) | Therapy alignment and monitoring; CTC count provides additional information to CT imaging for early recurrence monitoring. | [51] | [20] | |||
| 141 | RT-PCR | 141 (100) | Therapy alignment and monitoring; CTC persistence after surgical resection was a significant marker for early recurrence. | [52] | [21] | |||
| 14 | CellSearch | 14; 4 (29) after chemotherapy |
Therapy alignment and monitoring; CTC-negative patients after chemotherapy had significantly better treatment response. | [53] | [22] | |||
| 42 | CellSearch | 22 (52.3) | Patients with CTCs ≥3/7.5 mL may benefit from the intensive 4-drug regimen (irinotecan, oxaliplatin, and tegafur-uracil with leucovorin and cetuximab). | [54] | [23] | |||
| 61 | CellSearch | 27 (44.3) | CTC heterozygosity and heterogeneity exist in KRAS status among CTCs within all patients and between CTCs and tumor tissues. | [55] | [24] | |||
| 66 | CanPatrol Multiplex mRNA-ISH | 57 (86.4) | CTC count ≥6/5 mL was associated with decreased PFS and OS. LGR5 expression in CTCs may serve as a marker for CRC metastasis. | [56] | [25] | |||
| 138 | ISET device-CTCBIOPSY | 63 (45.7) | Postcurative resection CTC count > 1/2.5 mL was associated with shorter 3-year RFS rate. | [57] | [26] | |||
| 91 | CanPatrol mRNA-ISH |
51 CTC (56.0); 46 mCTC (50.5) | Mesenchymal CTC count ≥1/5 mL and COX-2 expression in mCTCs were associated with distance metastasis. | [58] | [27] | |||
| 34 | Microfluidic chips | 34 (100) | Therapy alignment and monitoring; comparison of mutational status of CTCs, ctDNA, and primary tumor tissue revealed great heterogeneity. | [59] | [28] | |||
| 130 | MACS | 67 (51.54) | Postoperative CTC count ≥2/3.2 mL in non-mCRC was associated with decreased RFS. | [60] | [29] | |||
| 106 | MACS | 100 (94) | HAI/target therapy with drugs selected by liquid biopsy precision oncotherapy is a safe and efficacious alternative therapeutic strategy for unresectable colorectal liver metastases patients. | [61] | [30] | |||
| 21 | ScreenCell | ® | 21 (100) | Isolation of CTCs by size (as a label-free technique with subsequent immunofluorescence labeling) gives a very high detection rate. | [62] | [31] | ||
| 21 | CK20 RT-qPCR | 15 (71.4) | The CK20 RT-qPCR method gives a relatively high detection rate. | [62] | [31] | |||
| 21 | NYONE | ® | 11 (52.4) | Application of a semiautomated microscopic approach with NYONE | ® | , an examiner-independent procedure for CTC detection. | [62] | [31] |
| 50 | CellSearch | 46 (92) | CTC counts ≥3/7.5 mL at baseline and day 21 after initiation of regorafenib were associated with decreased PFS and OS. Patients had significantly increased EGFR expression at day 21 and/or PD compared to baseline. | [63] | [32] | |||
| 589 | CellSearch | 241 (41) | Baseline CTC counts ≥3/7.5 mL were associated with clinical or pathologic features associated with poor prognosis. | [64] | [33] | |||
| 7 | ScreenCell | ® | , Immunofluorescence Staining | 7 (100) | Promising test for the future isolation and characterization of different CTC subtypes, including clusters. | [65] | [34] |
References
- De Wit, S.; van Dalum, G.; Terstappen, L.W.M.M. Detection of Circulating Tumor Cells. Scientifica 2014, 2014, 1–11.
- Zhe, X.; Cher, M.L.; Bonfil, R.D. Circulating Tumor Cells: Finding the Needle in the Haystack. Am. J. Cancer Res. 2011, 1, 740–751.
- Lozar, T.; Gersak, K.; Cemazar, M.; Kuhar, C.G.; Jesenko, T. The Biology and Clinical Potential of Circulating Tumor Cells. Radiol. Oncol. 2019, 53, 131–147.
- Alix-Panabières, C.; Pantel, K. Circulating Tumor Cells: Liquid Biopsy of Cancer. Clin. Chem. 2013, 59, 110–118.
- Marzagalli, M.; Fontana, F.; Raimondi, M.; Limonta, P. Cancer Stem Cells—Key Players in Tumor Relapse. Cancers 2021, 13, 376.
- Meng, S.; Tripathy, D.; Frenkel, E.P.; Shete, S.; Naftalis, E.Z.; Huth, J.F.; Beitsch, P.D.; Leitch, M.; Hoover, S.; Euhus, D.; et al. Circulating tumor cells in patients with breast cancer dormancy. Clin. Cancer Res. 2004, 10, 8152–8162.
- Aramini, B.; Masciale, V.; Arienti, C.; Dominici, M.; Stella, F.; Martinelli, G.; Fabbri, F. Cancer Stem Cells (CSCs), Circulating Tumor Cells (CTCs) and Their Interplay with Cancer Associated Fibroblasts (CAFs): A New World of Targets and Treatments. Cancers 2022, 14, 2408.
- Yu, Z.; Pestell, T.G.; Lisanti, M.P.; Pestell, R.G. Cancer stem cells. Int. J. Biochem. Cell Biol. 2012, 44, 2144–2151.
- Yu, Y.; Ramena, G.; Elble, R.C. The role of cancer stem cells in relapse of solid tumors. Front. Biosci. 2012, 4, 1528–1541.
- Ferreira, M.M.; Ramani, V.C.; Jeffrey, S.S. Circulating Tumor Cell Technologies. Mol. Oncol. 2016, 10, 374–394.
- Hu, M.; Wang, Z.; Wu, Z.; Ding, P.; Pei, R.; Wang, Q.; Xing, C. Circulating Tumor Cells in Colorectal Cancer in the Era of Precision Medicine. J. Mol. Med. 2022, 100, 197–213.
- Krebs, M.G.; Hou, J.-M.; Ward, T.H.; Blackhall, F.H.; Dive, C. Circulating Tumour Cells: Their Utility in Cancer Management and Predicting Outcomes. Ther. Adv. Med. Oncol. 2010, 2, 351–365.
- Kong, S.L.; Liu, X.; Suhaimi, N.-A.M.; Koh, K.J.H.; Hu, M.; Lee, D.Y.S.; Cima, I.; Phyo, W.M.; Lee, E.X.W.; Tai, J.A.; et al. Molecular Characterization of Circulating Colorectal Tumor Cells Defines Genetic Signatures for Individualized Cancer Care. Oncotarget 2017, 8, 68026–68037.
- Marchetti, A.; Del Grammastro, M.; Felicioni, L.; Malatesta, S.; Filice, G.; Centi, I.; De Pas, T.; Santoro, A.; Chella, A.; Brandes, A.A.; et al. Assessment of EGFR Mutations in Circulating Tumor Cell Preparations from NSCLC Patients by Next Generation Sequencing: Toward a Real-Time Liquid Biopsy for Treatment. PLoS ONE 2014, 9, e103883.
- Wechsler, J.; Benali-Furet, N.; Ye, F.; Avril, M.-F.; Boitier, F.; Carlotti, A.; Clauser, E.; North, M.-O.; Paraiso, I.; Cayre, Y.E. Analysis of BRAF Mutations in Circulating Tumor Cells Selected by Size from Patients with Melanoma and Comparision to the Primary Tumor. J. Clin. Oncol. 2012, 30, e21014.
- CELLSEARCH®|Home. Available online: https://www.cellsearchctc.com/ (accessed on 29 August 2022).
- Lankiewicz, S.; Zimmermann, S.; Hollmann, C.; Hillemann, T.; Greten, T.F. Circulating Tumour Cells as a Predictive Factor for Response to Systemic Chemotherapy in Patients with Advanced Colorectal Cancer. Mol. Oncol. 2008, 2, 349–355.
- Molnar, B.; Floro, L.; Sipos, F.; Toth, B.; Sreter, L.; Tulassay, Z. Elevation in Peripheral Blood Circulating Tumor Cell Number Correlates with Macroscopic Progression in UICC Stage IV Colorectal Cancer Patients. Dis. Markers 2008, 24, 141–150.
- Gazzaniga, P.; Gradilone, A.; Petracca, A.; Nicolazzo, C.; Raimondi, C.; Iacovelli, R.; Naso, G.; Cortesi, E. Molecular Markers in Circulating Tumour Cells from Metastatic Colorectal Cancer Patients. J. Cell. Mol. Med. 2010, 14, 2073–2077.
- Tol, J.; Koopman, M.; Miller, M.C.; Tibbe, A.; Cats, A.; Creemers, G.J.M.; Vos, A.H.; Nagtegaal, I.D.; Terstappen, L.W.M.M.; Punt, C.J.A. Circulating Tumour Cells Early Predict Progression-Free and Overall Survival in Advanced Colorectal Cancer Patients Treated with Chemotherapy and Targeted Agents. Ann. Oncol. 2010, 21, 1006–1012.
- Lu, C.-Y.; Uen, Y.-H.; Tsai, H.-L.; Chuang, S.-C.; Hou, M.-F.; Wu, D.-C.; Hank Juo, S.-H.; Lin, S.-R.; Wang, J.-Y. Molecular Detection of Persistent Postoperative Circulating Tumour Cells in Stages II and III Colon Cancer Patients via Multiple Blood Sampling: Prognostic Significance of Detection for Early Relapse. Br. J. Cancer 2011, 104, 1178–1184.
- Neki, K.; Kawahara, H.; Watanabe, K.; Toyama, Y.; Akiba, T.; Yanaga, K. Usefulness of Circulating Tumor Cells after Preliminary Chemotherapy for Prediction of Response to Further Anticancer Therapy in Patients with Initially Unresectable Metastatic Colorectal Cancer. Anticancer Res. 2013, 33, 1769–1772.
- Krebs, M.G.; Renehan, A.G.; Backen, A.; Gollins, S.; Chau, I.; Hasan, J.; Valle, J.W.; Morris, K.; Beech, J.; Ashcroft, L.; et al. Circulating Tumor Cell Enumeration in a Phase II Trial of a Four-Drug Regimen in Advanced Colorectal Cancer. Clin. Colorectal Cancer 2015, 14, 115–122.e2.
- Kondo, Y.; Hayashi, K.; Kawakami, K.; Miwa, Y.; Hayashi, H.; Yamamoto, M. KRAS Mutation Analysis of Single Circulating Tumor Cells from Patients with Metastatic Colorectal Cancer. BMC Cancer 2017, 17, 311.
- Wang, W.; Wan, L.; Wu, S.; Yang, J.; Zhou, Y.; Liu, F.; Wu, Z.; Cheng, Y. Mesenchymal Marker and LGR5 Expression Levels in Circulating Tumor Cells Correlate with Colorectal Cancer Prognosis. Cell. Oncol. 2018, 41, 495–504.
- Yang, C.; Shi, D.; Wang, S.; Wei, C.; Zhang, C.; Xiong, B. Prognostic Value of Pre- and Post-Operative Circulating Tumor Cells Detection in Colorectal Cancer Patients Treated with Curative Resection: A Prospective Cohort Study Based on ISET Device. Cancer Manag. Res. 2018, 10, 4135–4144.
- Cai, J.; Huang, L.; Huang, J.; Kang, L.; Lin, H.; Huang, P.; Zhu, P.; Wang, J.; Dong, J.; Wang, L.; et al. Associations between the cyclooxygenase-2 Expression in Circulating Tumor Cells and the Clinicopathological Features of Patients with Colorectal Cancer. J. Cell. Biochem. 2019, 120, 4935–4941.
- Takeda, K.; Yamada, T.; Takahashi, G.; Iwai, T.; Ueda, K.; Kuriyama, S.; Koizumi, M.; Matsuda, A.; Shinji, S.; Ohta, R.; et al. Analysis of Colorectal Cancer-related Mutations by Liquid Biopsy: Utility of Circulating Cell-free DNA and Circulating Tumor. Cells Cancer Sci. 2019, 110, 3497–3509.
- Wang, D.; Yang, Y.; Jin, L.; Wang, J.; Zhao, X.; Wu, G.; Zhang, J.; Kou, T.; Yao, H.; Zhang, Z. Prognostic Models Based on Postoperative Circulating Tumor Cells Can Predict Poor Tumor Recurrence-Free Survival in Patients with Stage II-III Colorectal Cancer. J. Cancer 2019, 10, 4552–4563.
- Guadagni, S.; Clementi, M.; Mackay, A.R.; Ricevuto, E.; Fiorentini, G.; Sarti, D.; Palumbo, P.; Apostolou, P.; Papasotiriou, I.; Masedu, F.; et al. Real-Life Multidisciplinary Treatment for Unresectable Colorectal Cancer Liver Metastases Including Hepatic Artery Infusion with Chemo-Filtration and Liquid Biopsy Precision Oncotherapy: Observational Cohort Study. J. Cancer Res. Clin. Oncol. 2020, 146, 1273–1290.
- Hendricks, A.; Brandt, B.; Geisen, R.; Dall, K.; Röder, C.; Schafmayer, C.; Becker, T.; Hinz, S.; Sebens, S. Isolation and Enumeration of CTC in Colorectal Cancer Patients: Introduction of a Novel Cell Imaging Approach and Comparison to Cellular and Molecular Detection Techniques. Cancers 2020, 12, 2643.
- Matsusaka, S.; Hann, D.L.; Ning, Y.; Yang, D.; Cao, S.; Berger, M.D.; Miyamoto, Y.; Suenaga, M.; Dan, S.; Mashima, T.; et al. Epidermal Growth Factor Receptor mRNA Expression: A Potential Molecular Escape Mechanism from Regorafenib. Cancer Sci. 2020, 111, 441–450.
- Sastre, J.; Orden, V.d.l.; Martínez, A.; Bando, I.; Balbín, M.; Bellosillo, B.; Palanca, S.; Peligros Gomez, M.I.; Mediero, B.; Llovet, P.; et al. Association Between Baseline Circulating Tumor Cells, Molecular Tumor Profiling, and Clinical Characteristics in a Large Cohort of Chemo-Naïve Metastatic Colorectal Cancer Patients Prospectively Collected. Clin. Colorectal Cancer 2020, 19, e110–e116.
- Francescangeli, F.; Magri, V.; De Angelis, M.L.; De Renzi, G.; Gandini, O.; Zeuner, A.; Gazzaniga, P.; Nicolazzo, C. Sequential Isolation and Characterization of Single CTCs and Large CTC Clusters in Metastatic Colorectal Cancer Patients. Cancers 2021, 13, 6362.
- Fabisiewicz, A.; Grzybowska, E. CTC Clusters in Cancer Progression and Metastasis. Med. Oncol. 2017, 34, 12.
More
