The use of wearable body sensors for health monitoring is a quickly growing field with the potential of offering a reliable means for clinical and remote health management. This includes both real-time monitoring and health trend monitoring with the aim to detect/predict health deterioration and also to act as a prevention tool. The aim of this systematic review was to provide a qualitative synthesis of studies using wearable body sensors for health monitoring. The synthesis and analysis have pointed out a number of shortcomings in prior research. Major shortcomings are demonstrated by the majority of the studies adopting an observational research design, too small sample sizes, poorly presented, and/or non-representative participant demographics (i.e., age, gender, patient/healthy). These aspects need to be considered in future research work.
- health monitoring
- IoT
- physical activity monitoring
- qualitative synthesis
- remote health management
- research shortcomings
- sensor systems
- user demography
- wearable body sensors
1. Introduction
The use of wearable body sensors for health monitoring as a means for supporting clinical and remote health monitoring in real-time and to provide health trend monitoring with the aim to predict/prevent health deterioration has the potential to lower the burden on the healthcare system and thereby reduce healthcare costs. The number of available wearable and wireless body sensors and systems are rapidly growing. Simultaneously, research on more energy-efficient and more accessible/smaller sensors for acquiring data as well as research on automatic data analysis of the Big Data, which the sensor-based systems are expected to generate, is being conducted. This advanced data analysis has the potential of generating personalized diagnoses and providing recommendations on treatments at a personalized level. While a promising area, we argue that the data collected for generating advanced data analysis algorithms need to come from participants representing the expected users of these systems.
This systematic review provides a qualitative synthesis of the articles retrieved on using wearable body sensors for health monitoring. We analyze the articles from many perspectives including author affiliations in countries, publication years, context of use, sensor category, research methodology, sample sizes, and participant demographics (i.e., age, gender, patient/healthy). This analysis has identified a number of shortcomings in prior research with respect to both sample size, but also to participant demographics where the latter strongly affects the validity of the results. These shortcomings need to be considered in future research, not only for understanding the user experience, but also to ensure that the advanced data analysis algorithms can reason on data which are representative and valid for the expected users of the systems.
2. Qualitative Synthesis
Inspired by Kekade et al.’s review from 2018 [[1]], we conducted a qualitative synthesis of the 73 included research articles. They were published between 2010 and 2019, i.e., spanning approx. 9.5 years, among which one article was published in 2010, two in 2011, seven in 2012, two in 2013, seven in 2014, twelve in 2015, nine in 2016, fourteen in 2017, fourteen in 2018 and five before April 24th 2019, see Figure 1. In average, 7.6 articles were published per year during the period 2010–2018. The authors of the 73 research articles were affiliated in 32 countries representing six continents (Africa, Asia, Australia, Europe, North America and South America). See Figure 2 and Figure 3 for further information on which countries authors are affiliated in and the number of publications per country with affiliated authors. The articles were sorted into the following article categories: Asthma/COPD, Cardiovascular diseases, Diabetes and nutrition, Gait and fall, Neurological diseases, Physical activity recognition, Rehabilitation, and Stress and sleep. All articles not directly related to any of the aforementioned article categories were sorted into an article category named Additional. Figure 4 depicts the category-wise distribution of the selected articles. Studying the distribution of articles related to health and physical activity monitoring respectively, it can be seen that 47 % of the articles were related to health (Asthma/COPD, Cardiovascular diseases, Diabetes and Nutrition, Neurological diseases, and Stress and sleep). As much as 39 % of the articles were related to physical activity monitoring (Gait and fall, Physical activity recognition, and Rehabilitation). It is unclear why such a large portion of the articles were related to physical activity monitoring. Possible reasons include that it is easier to monitor physical activity using sensors whereas measures relating to health, e.g., vital signs, need to be provided in a more timely manner.
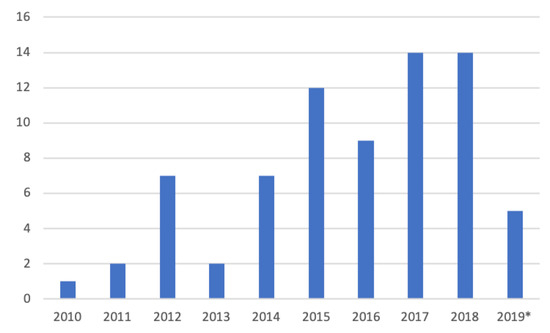
Figure 1. Number of articles per year. * only the articles published prior to 24 April 2019 are counted.
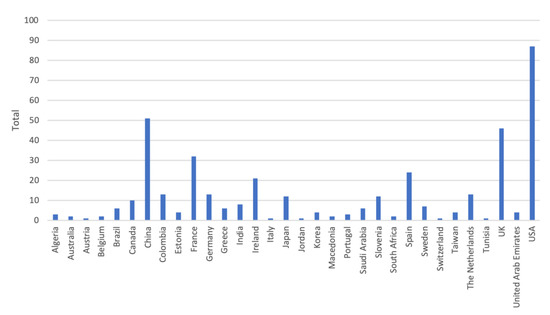
Figure 2. Number of authors affiliated in each country. Authors are calculated for each article, i.e., an author may be calculated more than once and in more than one country.
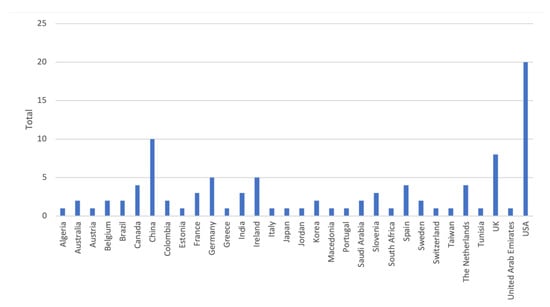
Figure 3. Number of articles per country. Papers with several authors may be counted for several countries.
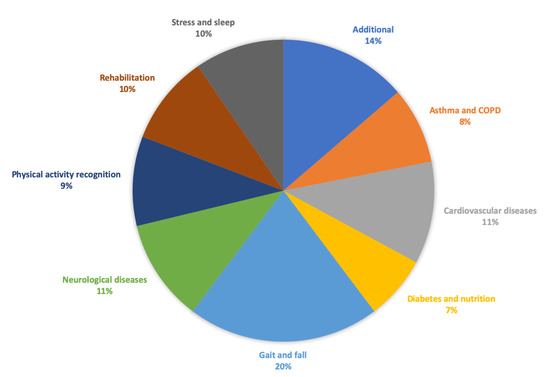
Figure 4. Category-wise distribution of the selected articles. Number of articles for Additional = 10, Asthma/COPD = 6, Cardiovascular diseases = 8, Diabetes and nutrition = 5, Gait and fall = 15, Neurological diseases = 8, Physical activity recognition = 7, Rehabilitation = 7, Stress and sleep = 7.
Sixty research articles reported on studies conducted with people at some level, these are reported in Table 1. We categorized the sensors according to the sensor categories used in [[1]]], namely, physical activity, vital signs, electrocardiography (ECG) and other. Studies reporting on devices measuring movement or activity were classified under the sensor category physical activity. Vital signs include the parameters: blood pressure (BP), body temperature (BT), respiratory rate (RR), heart rate (HR)/pulse, and peripheral oxygen saturation (SpO2). Studies measuring ECG were classified under ECG. Finally, studies using sensors for diabetes, swallowing, etc., or a combination of sensors from several sensor categories were classified under the sensor category other. The sensor categories physical activity and other include 23 studies each, vital signs includes three studies, and ECG includes ten studies reported upon in seven research articles.
Table 1. List of articles reporting on conducted studies. —indicates that information is missing.
|
Author, Year |
Ref. |
Article Category |
Research Design |
No. of Participants |
Sensor Category |
,[42],][43],][55],][59],][60]]]. Rather than excluding these from the tables, we indicate missing information with a “-”. However, we question the fact that all these studies were accepted for publication without providing any information on the participants.
Table 2. Demographic information on conducted studies. - indicates that information is missing.
|
Ref. |
Article Category |
No. of Participants |
Age Group |
Age Statistics |
Male |
Female |
Patient |
Healthy |
|||||||
|
Bonnevie et al. 2019 |
[[2]] |
Asthma/COPD |
Observational |
104 |
|||||||||||
|
Asthma/COPD |
104 | Vital signs | |||||||||||||
57–70 | 64 |
67 (64%) |
37 (36%) |
104 |
|||||||||||
|
5 |
50–66 | 5 |
|||||||||||||
62 | - | - |
5 |
Caulfield et al. 2014 |
|||||||||||
|
[[3]] |
Asthma/COPD |
Observational |
10 |
10 | |||||||||||
Asthma/COPD |
Physical activity |
||||||||||||||
61.5 ± 5.7 | 5 |
5 |
Estrada et al. 2016 |
[[4]] |
Asthma/COPD |
Observational |
1 |
Other |
|||||||
|
Katsaras et al. 2011 |
[[5]] |
Asthma/COPD |
Randomized control |
48 |
Other |
||||||||||
|
Naranjo-Hernández et al. 2018 |
Asthma/COPD |
Observational |
2 |
Vital signs |
|||||||||||
|
9 |
|||||||||||||||
10 |
Huang et al. 2014a |
Cardiovascular diseases |
- |
225 |
ECG |
||||||||||
|
Huang et al. 2014b |
[[8]] |
Cardiovascular diseases |
Case-control |
84 |
ECG |
||||||||||
|
Javaid et al. 2018 |
Cardiovascular diseases |
Observational |
60 |
Other |
|||||||||||
|
Li et al. 2019 |
[[10]] |
Cardiovascular diseases |
Observational |
16 |
Other |
||||||||||
- | - |
- |
- |
- |
Raad et al. 2015 |
||||||||||
|
[[11 | ] |
Cardiovascular diseases |
] |
- |
30 |
ECG |
|||||||||
Cardiovascular diseases |
30 |
20–23 |
- |
- |
- |
- |
- |
||||||||
|
2 |
- |
2 |
|||||||||||||
- | - |
- |
- |
2 |
- |
Simjanoska et al. 2018 |
[[12]] |
Cardiovascular diseases |
Observational |
16 |
|||||
|
[[12]] |
Cardiovascular diseases |
16 |
16–72 |
- |
ECG |
||||||||||
- | - | - |
- |
||||||||||||
3 | |||||||||||||||
3 |
25–27 |
- |
- |
- |
- |
- |
25 |
||||||||
|
25 |
20–73 |
- |
- |
- |
14 |
11 |
7 |
||||||||
|
7 | Dataset ECG | ||||||||||||||
20–74 | - |
- |
- |
- |
7 |
Susič and Stanič 2016 |
|||||||||
|
[[13]] |
Cardiovascular diseases |
- |
13 |
ECG |
|||||||||||
Cardiovascular diseases |
13 |
- |
50.6±9 |
8 |
5 |
- |
13 |
Al-Taee et al. 2015 |
[[14]] |
Diabetes and nutrition |
- |
22 |
Other |
||
|
[[14]] |
Diabetes and nutrition |
22 |
- |
- |
- |
- |
22 |
- |
Alshurafa et al. 2014 and Alshurafa et al. 2015 |
||||||
|
Diabetes and nutrition |
Diabetes and nutrition |
Observational |
10 |
10 |
Other |
||||||||||
20–40 |
20 |
||||||||||||||
- | 8 |
2 |
- |
- |
|||||||||||
|
20 |
20–40 |
- |
12 |
8 |
- |
- |
Dong and Biswas 2017 |
||||||||
|
[[17]] |
Diabetes and nutrition |
Diabetes and nutrition |
14 |
Observational |
- |
14 |
Other |
||||||||
9 | 5 |
- |
14 |
Onoue et al. 2017 |
|||||||||||
|
[[18] | ] |
Diabetes and nutrition |
Diabetes and nutrition |
Randomized control |
101 |
101 |
57.1±12.5 | Physical activity |
|||||||
56 | 45 | 101 |
- |
Atallah 2012 |
|||||||||||
|
[[ |
Gait and fall |
] |
Observational |
] |
34 |
Physical activity |
|||||||||
|
Gait and fall |
34 |
- |
28.22±12.77 |
21 |
13 |
- |
34 |
Godfrey et al. 2014 |
|||||||
|
[[20]] |
Gait and fall |
Gait and fall |
Observational |
24 |
Physical activity |
||||||||||
24 (12/12) |
20–40 |
32.5±4.8 |
7 |
5 |
- |
12 |
Lee et al. 2015 |
[[21]] |
Gait and fall |
Observational | |||||
|
Gait and fall |
- |
- |
11 |
Physical activity |
|||||||||||
65.0±8.8 | 5 | 7 |
- |
12 |
Liang et al. 2012 |
Gait and fall |
Observational |
8 |
Physical activity |
||||||
|
Liang et al. 2018 |
Gait and fall |
Observational |
18 |
Physical activity |
|||||||||||
|
Paiman et al. 2016 |
[[24]] |
Gait and fall |
Observational |
2 |
Other |
||||||||||
|
Tino et al. 2011 |
[[25]] |
Gait and fall |
Observational |
3 |
Other |
||||||||||
|
Williams et al. 2015 |
Gait and fall |
Observational |
5–6 |
Physical activity |
|||||||||||
|
Wu et al. 2013 |
Gait and fall |
Observational |
7 |
Physical activity |
|||||||||||
|
Wu et al. 2019 |
[[28]] |
Gait and fall |
Observational |
15 |
Physical activity |
||||||||||
|
Zhao et al. 2012 |
Gait and fall |
Observational |
8 |
Physical activity |
|||||||||||
|
Zhong et al. 2019 |
Gait and fall |
Observational |
Observational |
3 |
Other |
||||||||||
|
Doron et al. 2013 |
Physical activity recognition |
Observational |
65 |
Other |
|||||||||||
|
20 |
|||||||||||||||
|
Rednic et al. 2012 |
Physical activity recognition |
Observational |
17 |
Physical activity |
|||||||||||
|
Xu et al. 2014 |
Physical activity recognition |
Observational |
14 |
Other |
|||||||||||
|
Xu et al. 2016 |
|||||||||||||||
|
[[4]] |
Asthma/COPD |
1 |
- |
- |
1 |
1 |
[ | ||||||||
|
Asthma/COPD |
48 |
- |
- |
48 |
48 |
||||||||||
|
[[6]] |
Asthma/COPD |
||||||||||||||
|
[[21]] |
Gait and fall |
Physical activity recognition |
Observational |
4 |
Other |
||||||||||
2 |
3 |
Physical activity |
|||||||||||||
36 and 42 |
5 |
||||||||||||||
2 | 2 |
6 |
|||||||||||||
|
Argent et al. 2019 |
[[44]] |
Rehabilitation |
Observational |
15 |
Physical activity |
||||||||||
|
Banos et al. 2015 |
[[45]] |
Rehabilitation |
Observational |
10 |
Other |
||||||||||
|
Lee et al. 2018 |
Rehabilitation |
Case-control |
30 |
Physical activity |
|||||||||||
|
Timmermans et al. 2010 |
Rehabilitation |
Observational |
9 |
Physical activity |
|||||||||||
|
Whelan et al. 2017 |
Rawasdeh et al. 2017 |
Additional | |||||||||||||
|
9 |
55–76 |
64±6.6 |
6 |
3 |
9 |
2 |
|||||||||
|
[[7]] |
Cardiovascular diseases |
225 |
- |
- |
- |
- |
225 |
- |
|||||||
|
[[8]] |
Cardiovascular diseases |
84 |
- |
- |
- |
- |
1 group |
1 group |
|||||||
|
[[9]] |
Cardiovascular diseases |
60 |
- |
26.9±6.1 |
28 |
32 |
- |
60 |
|||||||
|
[[10]] |
Cardiovascular diseases |
16 |
56 |
Physical activity |
|||||||||||
- |
11 |
- |
27.6±4.3 |
11 |
- |
- |
11 |
||||||||
|
[[22]] |
Gait and fall |
8 |
- |
23±3.45 |
8 |
- |
- |
8 |
|||||||
|
[[23]] |
Gait and fall |
18 |
- |
25±3.24 |
12 |
6 |
- |
18 |
|||||||
|
[[24]] |
Gait and fall |
2 |
28 and 24 |
- |
1 |
1 |
- |
2 |
Giuberti et al. 2015 |
||||||
|
[[25]] |
Gait and fall |
3 |
40–70 |
- |
- |
- |
- |
- |
|||||||
|
[[26]] |
Gait and fall |
5–6 (1/5) |
27 |
- |
1 |
- |
- |
- |
|||||||
|
- |
21–36 |
27 |
4 |
1 |
- |
- |
[ | ||||||||
|
[[27]] |
Gait and fall |
Neurological diseases |
Observational |
24 |
Physical activity |
||||||||||
7 | - |
- |
- |
- |
- |
- |
|||||||||
|
[[28]] |
Gait and fall |
15 |
20–27 |
- |
- |
- |
- |
15 |
|||||||
|
[[29]] |
Gait and fall |
8 |
- |
28.5±4.3 |
- |
- |
- |
8 |
Gong et al. 2015, Gong et al. 2016 |
[[32 | |||||
|
[[30]] | ][33]] |
Gait and fall |
Neurological diseases |
56 (28/28) | Case-control |
- |
41 |
24.6±2.7 |
Physical activity |
||||||
14 | 14 | - |
28 |
Kuusik et al. 2018 |
Neurological diseases |
Observational | |||||||||
|
- |
>55 |
51 |
66.1±5.0 |
Physical activity |
|||||||||||
18 | 10 | - |
28 |
Sok et al. 2018 |
|||||||||||
|
[[31 | ] |
Neurological diseases |
] |
Observational |
Neurological diseases |
24 |
31–79 | 13 |
65.9±12.3 |
Physical activity |
|||||
17 | 7 |
24 |
- |
Stamate et al. 2017 and Stamate et al. 2018 |
|||||||||||
|
Neurological diseases |
Neurological diseases |
41 (28/13) |
Observational |
- |
12 |
40.5±9.4 |
Other |
||||||||
25% | 25% | 28 |
13 |
Castro et al. 2017 and Rodriguez et al. 2017 |
Physical activity recognition | ||||||||||
|
- |
- |
39.3±10.3 |
47% |
53% |
- |
- |
|||||||||
|
[[34]] |
Neurological diseases |
51 |
- |
- |
- |
- |
51 |
- |
|||||||
|
[[35]] |
Neurological diseases |
13 |
22–50 |
- |
9 |
4 |
13 |
- |
|||||||
|
Neurological diseases |
12 |
- |
- |
- |
- |
12 |
- |
||||||||
|
Physical activity recognition |
3 |
- |
- |
- |
- |
- |
- |
||||||||
|
[[40]] |
Physical activity recognition |
65 |
- |
- |
- |
- |
- |
- |
|||||||
|
20 |
- |
- |
- |
- |
- |
- |
|||||||||
|
[[41]] |
Physical activity recognition |
17 |
- |
- |
10 |
7 |
- |
- |
|||||||
|
[[42]] |
Physical activity recognition |
14 |
- |
- |
- |
- |
- |
- |
|||||||
|
[[43]] |
Physical activity recognition |
4 |
- |
- |
- |
- |
- |
- |
|||||||
|
3 |
- |
- |
- |
- |
- |
3 |
Rehabilitation |
||||||||
|
5 |
- |
- |
- |
- |
5 |
- |
|||||||||
|
6 |
- |
- |
3 |
3 |
- |
- |
Observational |
55 |
Physical activity |
||||||
|
[[44]] |
Rehabilitation |
15 |
- |
63±8.32 |
6 |
9 |
15 |
- |
Xu et al. 2017 |
[[49]] |
Rehabilitation |
||||
|
[[45 | Observational | ] |
6 |
Other |
|||||||||||
] |
Rehabilitation |
10 |
21–37 |
- |
8 |
2 |
- |
- |
Lin et al. 2012 |
||||||
|
[[46]] |
[[50]] |
Rehabilitation |
Stress and sleep |
Case-control |
18 (6/12) |
Physical activity |
|||||||||
20 |
- |
54.4±10.1 |
- |
- |
20 |
- |
Nakamura et al. 2017 |
[[51]] |
Stress and sleep |
Observational |
|||||
|
10 |
- |
4 |
Other |
||||||||||||
53.8±11.4 | - |
- |
- |
10 |
Parnandi and Gutierrez-Osuna 2017 |
||||||||||
|
[[47]] | ]][52] |
Rehabilitation |
Stress and sleep |
9 |
Randomized control |
- |
25 |
Other |
|||||||
60.7 | 5 |
4 |
9 |
- |
Uday et al. 2018 |
||||||||||
|
[[48 | Stress and sleep |
] |
Observational |
] |
10 |
Rehabilitation |
55 |
Other |
|||||||
- | 24.21±5.25 |
37 |
18 |
- |
55 |
Umemura et al. 2017 |
|||||||||
|
[[49]] |
[[54]] |
Stress and sleep |
Case-control |
54 |
Other |
||||||||||
Rehabilitation |
6 |
- |
72.5±6.0 |
3 |
3 |
- |
- |
Velicu et al. 2016 |
|||||||
|
[ | [ |
Stress and sleep |
Observational |
]] |
- |
Stress and sleep |
18 (6/12) |
- |
|||||||
19–22 overall | - |
5 |
1 |
- |
- |
Ayzenberg and Picard 2014 |
Additional |
Crossover |
|||||||
|
- |
- |
10 |
Other |
||||||||||||
- | 11 |
1 |
- |
- |
Pagán et al. 2016 |
[[57]] |
Additional |
Observational |
2 |
Other |
|||||
|
[[51]] |
Stress and sleep |
4 |
25–36 |
- |
4 |
- |
- |
4 |
Observational |
||||||
|
[[52 | 55 | ]] |
ECG |
||||||||||||
Stress and sleep |
25 |
19–33 |
- |
15 |
10 |
- |
- |
Seeger et al. 2012 |
|||||||
|
[[53]] |
Stress and sleep |
Additional |
10 |
- |
- |
- |
Other |
||||||||
- | - |
- |
- |
10 |
Wannenburg and Malekian 2015 |
||||||||||
|
[[ |
Additional |
] |
Observational |
] |
Stress and sleep |
54 (26/28) |
- |
4–8 |
Vital signs |
||||||
22 | - | - |
- |
Wu et al. 2018 |
Additional |
Observational |
20 |
ECG |
Similarly to Kekade et al. 2018 [[1]]], we also assessed the studies’ reporting of research design (Table 1), and the reported participant demography, i.e., number of participants, age, gender and the distribution of healthy participants and patients (Table 2). Many studies presented the participant demographics poorly, or not at all [[10],][27],[38],][39],][40]
54 | ||||||||
- | ||||||||
- | ||||||||
21 | ||||||||
- | ||||||||
- | ||||||||
- | ||||||||
- | ||||||||
[ | ||||||||
[ | ||||||||
] | ||||||||
] | ||||||||
Stress and sleep | ||||||||
- | ||||||||
- | ||||||||
- | ||||||||
- | ||||||||
- | ||||||||
- | - | |||||||
|
[[56]] |
Additional |
10 |
25–35 |
30.8±4.2 |
9 |
1 |
- |
10 |
|
[[57]] |
Additional |
2 |
- |
- |
- |
2 |
2 |
- |
|
[[58]] |
Additional |
55 |
18–22 |
- |
50% |
50% |
- |
- |
|
[[59]] |
Additional |
- |
- |
- |
- |
- |
- |
- |
|
[[60]] |
Additional |
4–8 (4/4) |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
- |
||
|
[[61]] |
Additional |
20 |
- |
- |
- |
- |
- |
- |
For completeness, the remaining 13 articles not listed in Table 1 and Table 2 were distributed over eight article categories: Asthma/COPD [[63]], Cardiovascular diseases [[64]]64], Gait and fall [[65][66][67]], Neurological diseases [[68]], Physical activity recognition [[69]], Rehabilitation [[70]], Stress and sleep [[71]], and Additional [[72][73][74][75]]. Six articles report on systems where studies are upcoming [[63][64][72][73][74][75]]. One of them [ [64]]] is a continuation of the study reported in [[13]]. Three articles report on studies using datasets [[66],[67],][69]]]. Two articles report on qualitative studies of observational and/or interview nature [[68],][70]]]. The continuation of the qualitative study [[70]] is reported upon in [[44]]. The evaluation in [ [65]]] is not clearly presented and the system developed in [ [71]]] uses wearable body sensors only to collect ground truth data for a contactless sleep monitoring system. Therefore, [ [71]]] was excluded from further qualitative analysis.
3. Discussion and Conclusions
In this systematic review, we provide a qualitative synthesis on retrieved articles on using wearable body sensors for health monitoring. The articles found were categorized as relating to: Asthma/COPD, Cardiovascular diseases, Diabetes and Nutrition, Gait and fall, Neurological diseases, Physical activity recognition, Rehabilitation, Stress and sleep, and Additional. Section 3 provided a qualitative synthesis of the studies with respect to research methodology and participant demography, i.e., number of participants, age, gender and the distribution of healthy participants and patients. Using this information, we have identified a number of shortcomings. Below follows a discussion on these shortcomings in relation to prior research.
There are many age-related health issues such as changing biological factors, the onset of illnesses which are often chronic and the decline of cognitive abilities. For example, “fall prediction is a challenging problem due to the combination of intrinsic and extrinsic fall risk factors that contribute to a fall. Intrinsic factors include age, fall history, mobility impairments, sleep disturbances, and neurological disorders", pp. 1 [[76]]76]. It is reported in [ [77]]77] that 35% of non-institutionalized adults had abnormal gait and that sleep disturbances are very common among older people. Further, chronic conditions affect physical activity levels, and activities such as rising from a chair is demanding for older people [ [77]]]. It is clear that the whole motion pattern changes with age and the onset of illnesses related to the human locomotor system. Yet, the majority of the studies focusing on gait and fall in this review were simulations that include none or few old participants. This shortcoming is also discussed in [[76]]], “It is evident that existing systems have mainly been tested in laboratory environments with controlled conditions and do not include frequent fallers and aging adults as test subjects.[.] future work should focus on longitudinal studies of fall detection and prediction systems in real-life conditions on a diverse group that includes frequent fallers, aging adults, and persons with neurological disorders.” p.8 [[76]]]. Not studying the sensor systems in real-life conditions affect the validity of the results since the performance is not studied in realistic conditions. The low number of studies with older people is also a shortcoming since age-related issues are not taken into consideration to a sufficient degree.
There are many differences between the two genders. As a first example, we want to mention the American Heart Association’s (AHA) scientific statement from 2016 [[78]] on acute myocardial infarction (AMI) in women. “Sex differences occur in the pathophysiology and clinical presentation of MI and affect treatment delays.”, p. 932 [[78]]]. Further, AHA reports that the same perfusion therapies are recommended despite the fact that the risk of bleeding or other complications is higher among women. Further, women are being under-treated with guideline recommendations. This results in increased readmission, re-infarction, and death rates during the first year after a myocardial infarction. Cardiac rehabilitation is also underused and under-prescribed among women [[78]]]. On the same lines, the results of a cohort study [[79]] with almost 5000 patientsμagμage > 65 who were admitted to 366 US hospitals in the period 2003–2009, has found that women are less likely to receive optimal care at discharge. Yet, only two of the studies retrieved within the category Cardiovascular diseases provide information on the participants’ gender. This is not the only shortcoming for studies on Cardiovascular diseases however. Several studies, or sub-studies, were conducted with very large age spans without the provision of a mean age. Others were conducted with young people or lacks information on age. Further, several works report on studies with healthy participants.
Hence, studies taking both genders into consideration, but also the age factor, are highly desired in the category Cardiovascular diseases. Not including information on gender and/or not considering gender/sex during data collection is a shortcoming regardless of the category to which a study belongs. It is argued in [[80]] that there are areas were specific data on women is lacking while specific data on men is missing in other areas.
Regitz-Zagrosek [[80]] outlines a number of differences between men and women. These include: women more frequently having anemia, women suffering from coronary artery disease in average ten years later than men, a higher frequency of boys having asthma in young ages while the frequency changes to young adulthood, diabetes increasing the risk for coronary heart disease more among women, and osteoporosis being more frequent in women but under-diagnosed in men. Osteoporosis disease is characterized by a decreased bone mass density and a disrupted normal trabecular architecture reducing bone strength [[81]]]. Therefore, Osteoporosis increases the risk of fractures after a fall but no symptoms of the disease are shown until a fracture occurs [[80]]. According to [[81]]], there are several factors relating to Osteoporosis which increases the risk of falling. These include the fear of falling, which increases the risk of falling [[82][83]]. In addition, [[81]] reports on studies discussing women with osteoporosis or low bone mass where fear of falling is associated with more falls [[84]]], and the confidence in balance is related to balance and mobility [[85]]]. Further, [[84]] reports that an increased thoracic kyphosis is associated with recent falls among women with Osteopororosis. I.e., women with thoracic kyphosis were more likely to have had a recent fall. Thoracic kyphosis is an abnormal convex curvature of the spine at chest height which is much more common among older women than men due to estrogen losses [[86]]. All these works [[81][82][83][84][85]] date from 2004-2011, hence it is astonishing that some articles retrieved within the article category Gait and fall have not reported information on gender and that some other articles were conducted solely with men. Hence, we argue that future studies in the categories discussed in this article must take gender into consideration. This shortcoming was also highlighted in [[1]]].
Undoubtedly, healthy participants and patients differ in many aspects. Yet, only 65% of the studies overall reported this information. A positive example here is the fact that the studies reported upon in the category Asthma/COPD were conducted almost entirely with patients. This indicates that the results in this area are reliable. On the contrary, none of the studies within Gait and fall, or Stress and sleep have reported that the studies were conducted with patients. Also [[76][77]] have previously discussed the shortcoming of not conducting studies with patients in the category Gait and fall. Considering the research question for this review article, we question the fact that 35% of the retrieved articles lack information on whether the participants were healthy or patients. We argue that the use of healthy participants, or not providing this information, affect the validity of the study results. Future studies need to consider the inclusion of patients to a further extent.
Studying the sample size in the reported studies, 56% of the articles report on studies conducted with up to 20 participants, and only 20% of the articles report on studies conducted with 51 or more participants. The distribution of numbers vary between categories. The majority of the studies reported in the categories Asthma/COPD, Gait and fall, Physical activity recognition, Rehabilitation, and Stress and sleep were conducted with up to 20 participants. We find the overall low number of participants a shortcoming and recommend that future studies are conducted with larger study samples. However, taking demographic factors, i.e., age, gender and healthy/patient into consideration is highly needed prior to increasing the sample sizes in studies on health monitoring using wearable body sensors.
Funding
This research was conducted within the scope of the ESS-H+ (Embedded Sensor Systems for Health Plus). The project is funded by the Swedish Knowledge Foundation (project number: 20180158).
Citing
Please refer to the original publication in Sensors if citing this work: Annica Kristoffersson & Maria Lindén. A Systematic Review on the Use of Wearable Body Sensors for Health Monitoring: A Qualitative synthesis. Sensors 2020, 20(5), 1502; https://doi.org/10.3390/s20051502
References
- Shwetambara Kekade; Chung-Ho Hseieh; Mohaimenul Islam.; Suleman Atique; Abdulwahed Mohammed Khalfan; Yu-Chuan (Jack) Li; Shabbir Syed-Abdul; The usefulness and actual use of wearable devices among the elderly population. Computer Methods and Programs in Biomedicine 2018, 153, 137-159, 10.1016/j.cmpb.2017.10.008.
- Tristan Bonnevie; Francis-Edouard Gravier; Mark Elkins; Johan Dupuis; Guillaume Prieur; Yann Combret; Catherine Viacroze; David Debeaumont; Aurora Robleda-Quesada; Jean Quieffin; et al.Bouchra LamiaMaxime PatoutAntoine CuvelierJean-François MuirClement MedrinalCatherine Tardif People undertaking pulmonary rehabilitation are willing and able to provide accurate data via a remote pulse oximetry system: a multicentre observational study. Journal of Physiotherapy 2019, 65, 28-36, 10.1016/j.jphys.2018.11.002.
- Caulfield, B.; Kaljo, I.; Donnelly, S. Use of a consumer market activity monitoring and feedback device improves exercise capacity and activity levels in COPD. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 1765–1768.
- Estrada, L.; Torres, A.; Sarlabous, L.; Jané, R. Evaluating respiratory muscle activity using a wireless sensor platform. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 5769–5772.
- Katsaras, T.; Milsis, A.; Rizikari, M.; Saoulis, N.; Varoutaki, E.; Vontetsianos, A. The use of the “Healthwear” wearable system in chronic patients’ early hospital discharge: Control randomized clinical trial. In Proceedings of the 2011 5th International Symposium on Medical Information and Communication Technology, Montreux, Switzerland, 27–30 March 2011; pp. 143–146.
- David Naranjo-Hernández; Alejandro Talaminos-Barroso; Javier Reina-Tosina; Laura M. Roa; Gerardo Barbarov-Rostán; Pilar Cejudo-Ramos; Eduardo Márquez-Martín; Francisco Ortega-Ruiz; Smart Vest for Respiratory Rate Monitoring of COPD Patients Based on Non-Contact Capacitive Sensing. Sensors 2018, 18, 2144, 10.3390/s18072144.
- Anpeng Huang; Wenyao Xu; Zhinan Li; Linzhen Xie; Majid Sarrafzadeh; XiaoMing Li; Jason Cong; System Light-Loading Technology for mHealth: Manifold-Learning-Based Medical Data Cleansing and Clinical Trials in WE-CARE Project. IEEE Journal of Biomedical and Health Informatics 2013, 18, 1581-1589, 10.1109/jbhi.2013.2292576.
- Anpeng Huang; Chao Chen; Kaigui Bian; Xiaohui Duan; Min Chen; Hongqiao Gao; Chao Meng; Qian Zheng; Yingrui Zhang; BingLi Jiao; et al.Linzhen Xie WE-CARE: An Intelligent Mobile Telecardiology System to Enable mHealth Applications. IEEE Journal of Biomedical and Health Informatics 2013, 18, 693-702, 10.1109/jbhi.2013.2279136.
- Javaid, A.Q.; Chang, I.S.; Mihailidis, A. Ballistocardiogram Based Identity Recognition: Towards Zero-Effort Health Monitoring in an Internet-of-Things (IoT) Environment. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; Volume 2018, pp. 3326–3329.
- Yibin Li; Shengnan Li; Houbing Song; Bin Shao; Xiao Yang; Ning Deng; Noninvasive blood pressure estimation with peak delay of different pulse waves. International Journal of Distributed Sensor Networks 2019, 15, no, 10.1177/1550147719837877.
- Raad, M.W.; Sheltami, T.; Deriche, M. A Ubiquitous Telehealth System for the Elderly. In Internet of Things: User-Centric Iot, Pt I; Lecture Notes of the Institute for Computer Sciences Social Informatics and Telecommunications Engineering; Giaffreda, R., Vieriu, R.L., Pasher, E., Bendersky, G., Jara, A.J., Rodrigues, J., Dekel, E., Mandler, B., Eds.; Springer: Berlin, Germany, 2015; Volume 150, pp. 159–166
- Monika Simjanoska; Martin Gjoreski; Matjaž Gams; Ana Madevska Bogdanova; Non-Invasive Blood Pressure Estimation from ECG Using Machine Learning Techniques. Sensors 2018, 18, 1160, 10.3390/s18041160.
- Susič, T.P.; Stanič, U. Penetration of the ICT technology to the health care primary sector—Ljubljana PILOT. In Proceedings of the 2016 39th International Convention on Information and Communication Technology, Electronics and Microelectronics (MIPRO), Opatija, Croatia, 30 May–3 June 2016; pp. 436–441.
- Al-Taee, M.A.; Al-Nuaimy, W.; Al-Ataby, A.; Muhsin, Z.J.; Abood, S.N.; IEEE. Mobile Health Platform for Diabetes Management Based on the Internet-of-Things. In Proceedings of the 2015 IEEE Jordan Conference on Applied Electrical Engineering and Computing Technologies, New York, NY, USA, 3–5 November 2015
- Nabil Alshurafa; Haik Kalantarian; Mohammad Pourhomayoun; Shruti Sarin; Jason J. Liu; Majid Sarrafzadeh; Non-invasive monitoring of eating behavior using spectrogram analysis in a wearable necklace. 2014 IEEE Healthcare Innovation Conference (HIC) 2014, null, 71-74, 10.1109/hic.2014.7038877.
- Nabil Alshurafa; Haik Kalantarian; Mohammad Pourhomayoun; Jason J. Liu; Shruti Sarin; Behnam Shahbazi; Majid Sarrafzadeh; Recognition of Nutrition Intake Using Time-Frequency Decomposition in a Wearable Necklace Using a Piezoelectric Sensor. IEEE Sensors Journal 2015, 15, 3909-3916, 10.1109/jsen.2015.2402652.
- Bo Dong; Subir Biswas; Meal-time and duration monitoring using wearable sensors. Biomedical Signal Processing and Control 2017, 32, 97-109, 10.1016/j.bspc.2016.09.018.
- Takeshi Onoue; Motomitsu Goto; Tomoko Kobayashi; Takashi Tominaga; Masahiko Ando; Hiroyuki Honda; Yasuko Yoshida; Takahiro Tosaki; Hisashi Yokoi; Sawako Kato; et al.Shoichi MaruyamaHiroshi Arima Randomized controlled trial for assessment of Internet of Things system to guide intensive glucose control in diabetes outpatients: Nagoya Health Navigator Study protocol. Nagoya journal of medical science 2017, 79, 323-329, 10.18999/nagjms.79.3.323.
- Louis Atallah; Anatole Wiik; Gareth G. Jones; Benny P. L. Lo; Justin Cobb; Andrew A. Amis; Guang-Zhong Yang; Validation of an ear-worn sensor for gait monitoring using a force-plate instrumented treadmill. Gait & Posture 2011, 35, 674-676, 10.1016/j.gaitpost.2011.11.021.
- Godfrey, A.; Din, S.D.; Barry, G.; Mathers, J.C.; Rochester, L. Within trial validation and reliability of a single tri-axial accelerometer for gait assessment. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 5892–5895.
- Jung Keun Lee; Sn Robinovitch; Edward J. Park; Inertial Sensing-Based Pre-Impact Detection of Falls Involving Near-Fall Scenarios. IEEE Transactions on Neural Systems and Rehabilitation Engineering 2014, 23, 258-266, 10.1109/tnsre.2014.2357806.
- Liang, D.; Zhao, G.; Guo, Y.; Wang, L. Pre-impact & impact detection of falls using wireless Body Sensor Network. In Proceedings of the 2012 IEEE-EMBS International Conference on Biomedical and Health Informatics, Hong Kong, China, 5–7 January 2012; pp. 763–766
- Liang, S.; Chu, T.; Lin, D.; Ning, Y.; Li, H.; Zhao, G. Pre-impact Alarm System for Fall Detection Using MEMS Sensors and HMM-based SVM Classifier. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 4401–4405
- Charlotte Paiman; Daniel Lemus; Débora Short; Heike Vallery; Observing the State of Balance with a Single Upper-Body Sensor. Frontiers in Robotics and AI 2016, 3, 381, 10.3389/frobt.2016.00011.
- Tino, A.; Carvalho, M.; Preto, N.F.; McConville, K.M.V. Wireless vibrotactile feedback system for postural response improvement. In Proceedings of the 2011 33rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Boston, MA, USA, 30 August–3 September 2011; Volume 2011, pp. 5203–5206
- Williams, B.; Allen, B.; True, H.; Fell, N.; Levine, D.; Sartipi, M.; IEEE. A Real-time, Mobile Timed Up and Go System. In Proceedings of the 2015 IEEE 12th International Conference on Wearable and Implantable Body Sensor Networks (BSN), Cambridge, MA, USA, 9–12 June 2015
- Wu, X.; Wang, Y.; Chien, C.; Pottie, G. Self-calibration of sensor misplacement based on motion signatures. In Proceedings of the 2013 IEEE International Conference on Body Sensor Networks, Cambridge, MA, USA, 6–9 May 2013; pp. 1–5.
- Yinfeng Wu; Yiwen Su; Renjian Feng; Ning Yu; Xu Zang; Wearable-sensor-based pre-impact fall detection system with a hierarchical classifier. Measurement 2019, 140, 283-292, 10.1016/j.measurement.2019.04.002.
- Guoru Zhao; Zhanyong Mei; Ding Liang; Kamen Ivanov; Yanwei Guo; Yongfeng Wang; Lei Wang; Exploration and Implementation of a Pre-Impact Fall Recognition Method Based on an Inertial Body Sensor Network. Sensors 2012, 12, 15338-15355, 10.3390/s121115338.
- Runting Zhong; Pei-Luen Patrick Rau; Xinghui Yan; Gait Assessment of Younger and Older Adults with Portable Motion-Sensing Methods: A User Study. Mobile Information Systems 2019, 2019, 1-13, 10.1155/2019/1093514.
- Matteo Giuberti; Gianluigi Ferrari; Laura Contin; Veronica Cimolin; Corrado Azzaro; Giovanni Albani; Alessandro Mauro; Automatic UPDRS Evaluation in the Sit-to-Stand Task of Parkinsonians: Kinematic Analysis and Comparative Outlook on the Leg Agility Task. IEEE Journal of Biomedical and Health Informatics 2015, 19, 803–814, 10.1109/jbhi.2015.2425296.
- Jiaqi Gong; John Lach; Yanjun Qi; Myla D. Goldman; Causal analysis of inertial body sensors for enhancing gait assessment separability towards multiple sclerosis diagnosis. 2015 IEEE 12th International Conference on Wearable and Implantable Body Sensor Networks (BSN) 2015, null, 1-6, 10.1109/bsn.2015.7299400.
- Jiaqi Gong; Yanjun Qi; Myla D. Goldman; John Lach; Causality Analysis of Inertial Body Sensors for Multiple Sclerosis Diagnostic Enhancement. IEEE Journal of Biomedical and Health Informatics 2016, 20, 1273-1280, 10.1109/jbhi.2016.2589902.
- Kuusik, A.; Alam, M.M.; Kask, T.; Gross-Paju, K. Wearable m-assessment system for neurological disease patients. In Proceedings of the 2018 IEEE 4th World Forum on Internet of Things (WF-IoT), Singapore, Singapore, 5–8 February 2018; pp. 201–206.
- Pichleap Sok; Ting Xiao; Yohannes Azeze; Arun Jayaraman; Mark V. Albert; Activity Recognition for Incomplete Spinal Cord Injury Subjects Using Hidden Markov Models. IEEE Sensors Journal 2018, 18, 6369-6374, 10.1109/jsen.2018.2845749.
- C. Stamate; G.D. Magoulas; S. Kueppers; Effrosyni Nomikou; I. Daskalopoulos; M.U. Luchini; T. Moussouri; G. Roussos; Deep learning Parkinson's from smartphone data. 2017 IEEE International Conference on Pervasive Computing and Communications (PerCom) 2017, null, 31-40, 10.1109/percom.2017.7917848.
- C. Stamate; G.D. Magoulas; S. Kueppers; Effrosyni Nomikou; I. Daskalopoulos; A. Jha; J.S. Pons; J. Rothwell; M.U. Luchini; T. Moussouri; M. Iannone; George Roussos; The cloudUPDRS app: A medical device for the clinical assessment of Parkinson’s Disease. Pervasive and Mobile Computing 2018, 43, 146-166, 10.1016/j.pmcj.2017.12.005.
- Diego Castro; William Coral; Camilo Rodriguez; Jose Cabra; J. Colorado; Wearable-Based Human Activity Recognition Using an IoT Approach. Journal of Sensor and Actuator Networks 2017, 6, 28, 10.3390/jsan6040028.
- Acm-; ACM-78. ICGA Journal 1978, 1, 4-12, 10.3233/icg-1978-1102.
- Doron, M.; Bastian, T.; Maire, A.; Dugas, J.; Perrin, E.; Gris, F.; Guillemaud, R.; Deschamps, T.; Bianchi, P.; Caritu, Y.; et al. Estimation of physical activity monitored during the day-to-day life by an autonomous wearable device (SVELTE project). In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 4629–4632
- Rednic, R.; Gaura, E.; Brusey, J.; Kemp, J. Wearable posture recognition systems: Factors affecting performance. In Proceedings of the 2012 IEEE-EMBS International Conference on Biomedical and Health Informatics, Hong Kong, China, 5–7 January 2012; pp. 200–203.
- James Y. Xu; Hua-I. Chang; Chieh Chien; William J. Kaiser; Gregory J. Pottie; Context-driven, Prescription-Based Personal Activity Classification: Methodology, Architecture, and End-to-End Implementation. IEEE Journal of Biomedical and Health Informatics 2013, 18, 1015-1025, 10.1109/jbhi.2013.2282812.
- Xu, J.Y.; Wang, Y.; Barrett, M.; Dobkin, B.; Pottie, G.J.; Kaiser, W.J.; Personalized multilayer maily life profiling through context enabled activity classification and motion reconstruction: An integrated system approach. IEEE J. Biomed. Health Inform. 2016, 20, 177–188.
- Rob Argent; Patrick Slevin; Antonio Bevilacqua; Maurice Neligan; Ailish Daly; Brian Caulfield; Wearable Sensor-Based Exercise Biofeedback for Orthopaedic Rehabilitation: A Mixed Methods User Evaluation of a Prototype System. Sensors 2019, 19, 432, 10.3390/s19020432.
- Oresti Banos; Jose A. Moral-Munoz; Ignacio Diaz-Reyes; Manuel Arroyo-Morales; Miguel Damas; Francisco Herrera; Choong Seon Hong; Sungyoung Lee; Hector Pomares; Ignacio Rojas; et al.Claudia Villalonga mDurance: A Novel Mobile Health System to Support Trunk Endurance Assessment. Sensors 2015, 15, 13159-13183, 10.3390/s150613159.
- Sunghoon Ivan Lee; Catherine Adans-Dester; Matteo Grimaldi; Ariel V. Dowling; Peter C. Horak; Randie Black-Schaffer; Paolo Bonato; Joseph T. Gwin; Enabling Stroke Rehabilitation in Home and Community Settings: A Wearable Sensor-Based Approach for Upper-Limb Motor Training. IEEE Journal of Translational Engineering in Health and Medicine 2018, 6, 1-11, 10.1109/JTEHM.2018.2829208.
- Annick A. A. Timmermans; Henk A. M. Seelen; Richard P. J. Geers; Privender K. Saini; Stefan Winter; Juergen Te Vrugt; Herman Kingma; Sensor-Based Arm Skill Training in Chronic Stroke Patients: Results on Treatment Outcome, Patient Motivation, and System Usability. IEEE Transactions on Neural Systems and Rehabilitation Engineering 2010, 18, 284-292, 10.1109/tnsre.2010.2047608.
- Martin O’Reilly; Tomas Ward; Eamonn Delahunt; Brian Caulfield; Darragh F. Whelan; Technology in Rehabilitation: Comparing Personalised and Global Classification Methodologies in Evaluating the Squat Exercise with Wearable IMUs. Methods of Information in Medicine 2017, 56, 361-369, 10.3414/me16-01-0141.
- Xu, J.K.; Lee, U.H.; Bao, T.; Huang, Y.J.; Sienko, K.H.; Shull, P.B.; IEEE. Wearable sensing and haptic feedback research platform for gait retraining. In Proceedings of the 2017 IEEE 14th International Conference on Wearable and Implantable Body Sensor Networks, Eindhoven, The Netherlands, 9–12 May 2017; pp. 125–128
- Lin, C.; Gamble, J.; Yang, Y.; Wang, J. Estimating the influence of chronotype and social zeitgebers on circadian rhythms using an accelerometer-based sensor network. In Proceedings of the 2012 IEEE-EMBS International Conference on Biomedical and Health Informatics, Hong Kong, China, 5–7 January 2012; pp. 285–288.
- Takashi Nakamura; Valentin Goverdovsky; Mary J. Morrell; Danilo P. Mandic; Automatic Sleep Monitoring Using Ear-EEG. IEEE Journal of Translational Engineering in Health and Medicine 2017, 5, 1-8, 10.1109/JTEHM.2017.2702558.
- Avinash Parnandi; Ricardo Gutierrez-Osuna; Physiological Modalities for Relaxation Skill Transfer in Biofeedback Games. IEEE Journal of Biomedical and Health Informatics 2015, 21, 361-371, 10.1109/JBHI.2015.2511665.
- Uday, S.; Jyotsna, C.; Amudha, J.; IEEE. Detection of Stress using Wearable Sensors in IoT Platform. In Proceedings of the 2018 Second International Conference on Inventive Communication and Computational Technologies (ICICCT), Coimbatore, India, 20–21 April 2018; pp. 492–498
- Umemura, G.S.; Pinho, J.P.; Furtado, F.; Gonçalves, B.S.B.; Fomer-Cordero, A. Comparison of sleep parameters assessed by actigraphy of healthy young adults from a small town and a megalopolis in an emerging country. In Proceedings of the 2017 IEEE Healthcare Innovations and Point of Care Technologies (HI-POCT), Bethesda, MD, USA, 6–8 November 2017; pp. 200–203
- Velicu, O.R.; Madrid, N.M.; Seepold, R.; IEEE. Experimental sleep phases monitoring. In Proceedings of the 2016 3rd IEEE Embs International Conference on Biomedical and Health Informatics, Las Vegas, NV, USA, 24–27 February 2016; pp. 625–628
- Yadid Ayzenberg; Rosalind W. Picard; FEEL: A System for Frequent Event and Electrodermal Activity Labeling. IEEE Journal of Biomedical and Health Informatics 2013, 18, 266-277, 10.1109/jbhi.2013.2278213.
- Pagán, J.; Risco-Martín, J.L.; Moya, J.M.; Ayala, J.L. Grammatical Evolutionary Techniques for Prompt Migraine Prediction. In Proceedings of the Genetic and Evolutionary Computation Conference, Denver, CO, USA, 20–24 July 2016; pp. 973–98
- Rawashdeh, M.; Al-Qurishi, M.; Al-Rakhami, M.; Al-Quraishi, M.S. A multimedia cloud-based framework for constant monitoring on obese patients. In Proceedings of the 2017 IEEE International Conference on Multimedia & Expo Workshops (ICMEW), Hong Kong, China, 10–14 July 2017; pp. 139–144
- Seeger, C.; Buchmann, A.; Van Laerhoven, K. An Event-based BSN Middleware That Supports Seamless Switching Between Sensor Configurations. In Proceedings of the 2nd ACM SIGHIT International Health Informatics Symposium, Miami, Fl, USA, 28–30 January 2012; pp. 503–512
- Johan Wannenburg; Reza Malekian; Wannenburg J.; Malekian R.; Body Sensor Network for Mobile Health Monitoring, a Diagnosis and Anticipating System. IEEE Sensors Journal 2015, 15, 6839-6852, 10.1109/jsen.2015.2464773.
- Wanqing Wu; Sandeep Pirbhulal; Arun Kumar Sangaiah; S.C. Mukhopadhyay; Guanglin Li; Optimization of signal quality over comfortability of textile electrodes for ECG monitoring in fog computing based medical applications. Future Generation Computer Systems 2018, 86, 515-526, 10.1016/j.future.2018.04.024.
- Caulfield, B.; Kaljo, I.; Donnelly, S. Use of a consumer market activity monitoring and feedback device improves exercise capacity and activity levels in COPD. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 1765–1768
- Buonocore, C.M.; Rocchio, R.A.; Roman, A.; King, C.E.; Sarrafzadeh, M. Wireless Sensor-Dependent Ecological Momentary Assessment for Pediatric Asthma mHealth Applications. In Proceedings of the Second IEEE/ACM International Conference on Connected Health: Applications, Systems and Engineering Technologies, Philadelphia, PA, USA, 17–19 July 2017; pp. 137–146.
- Depolli, M.; Avbelj, V.; Trobec, R.; Kališnik, J.M.; Tadej, K.; Susič, A.P.; Stanič, U.; Semeja, A.; PCARD platform for mhealth monitoring. Informatica 2016, 40, 117–123.
- Mohammed Ghazal; Yasmina Al Khalil; Fatemeh Jalil Dehbozorgi; Marah Talal Alhalabi; An integrated caregiver-focused mHealth framework for elderly care. 2015 IEEE 11th International Conference on Wireless and Mobile Computing, Networking and Communications (WiMob) 2015, null, 238-245, 10.1109/wimob.2015.7347967.
- Anita Ramachandran; Adarsh R.; Piyush Pahwa; Anupama K. R.; Machine Learning-Based Techniques for Fall Detection in Geriatric Healthcare Systems. 2018 9th International Conference on Information Technology in Medicine and Education (ITME) 2018, null, 232-237, 10.1109/itme.2018.00059.
- Elhocine Boutellaa; Oussama Kerdjidj; Khalida Ghanem; Covariance matrix based fall detection from multiple wearable sensors.. Journal of Biomedical Informatics 2019, 94, 103189, 10.1016/j.jbi.2019.103189.
- Mevludin Memedi; Gaki Tshering; Martin Fogelberg; Ilir Jusufi; Ella Kolkowska; Gunnar Klein; An Interface for IoT: Feeding Back Health-Related Data to Parkinson’s Disease Patients. Journal of Sensor and Actuator Networks 2018, 7, 14, 10.3390/jsan7010014.
- Chelsea Dobbins; Reza Rawassizadeh; Elaheh Momeni; Detecting physical activity within lifelogs towards preventing obesity and aiding ambient assisted living. Neurocomputing 2017, 230, 110-132, 10.1016/j.neucom.2016.02.088.
- Rob Argent; Patrick Slevin; Antonio Bevilacqua; Maurice Neligan; Ailish Daly; Brian Caulfield; Clinician perceptions of a prototype wearable exercise biofeedback system for orthopaedic rehabilitation: a qualitative exploration. BMJ Open 2018, 8, e026326, 10.1136/bmjopen-2018-026326.
- Zhuang, Y.; Song, C.; Wang, A.; Lin, F.; Li, Y.; Gu, C.; Li, C.; Xu, W. SleepSense: Non-invasive sleep event recognition using an electromagnetic probe. In Proceedings of the 2015 IEEE 12th International Conference on Wearable and Implantable Body Sensor Networks (BSN), Cambridge, MA, USA, 9–12 June 2015
- Tlija Amira; Istrate Dan; Bennani Az-eddine; Hoai Huong Ngo; Gattoufi Said; Wegrzyn-Wolska Katarzyna; Monitoring chronic disease at home using connected devices. 2018 13th Annual Conference on System of Systems Engineering (SoSE) 2018, null, 400-407, 10.1109/sysose.2018.8428754.
- R. Cortinas; J. M. Gonzaga; A. R. Green; A. M. Saulenas; B. F. Busha; TCNJ Athlete Tracker. 2015 41st Annual Northeast Biomedical Engineering Conference (NEBEC) 2015, null, 1-2, 10.1109/nebec.2015.7117126.
- Timm Hormann; Marc Hesse; Michael Adams; Ulrich Rückert; A software assistant for user-centric calibration of a wireless body sensor. 2016 IEEE 13th International Conference on Wearable and Implantable Body Sensor Networks (BSN) 2016, null, 183-188, 10.1109/bsn.2016.7516256.
- Lisanne Warmerdam; Heleen Riper; Michel Mca Klein; Pepijn Van Den Ven; Artur Rocha; Mario Ricardo Henriques; Eric Tousset; Hugo Silva; Gerhard Andersson; Pim Cuijpers; Innovative ICT solutions to improve treatment outcomes for depression: the ICT4Depression project.. Studies in health technology and informatics 2012, 181, , null.
- Ramesh Rajagopalan; Irene Litvan; Tzyy-Ping Jung; Fall Prediction and Prevention Systems: Recent Trends, Challenges, and Future Research Directions. Sensors 2017, 17, 2509, 10.3390/s17112509.
- Salvatore Tedesco; John Barton; Brendan O’Flynn; A Review of Activity Trackers for Senior Citizens: Research Perspectives, Commercial Landscape and the Role of the Insurance Industry. Sensors 2017, 17, 1277, 10.3390/s17061277.
- Laxmi S. Mehta; Theresa M. Beckie; Holli A. Devon; Cindy L. Grines; Harlan M. Krumholz; Michelle N. Johnson; Kathryn J. Lindley; Viola Vaccarino; Tracy Y. Wang; Karol E. Watson; et al.Nanette K. Wenger Acute Myocardial Infarction in Women. Circulation 2016, 133, 916-947, 10.1161/cir.0000000000000351.
- Shanshan Li; Gregg C. Fonarow; Kenneth J. Mukamal; Li Liang; Phillip J. Schulte; Eric E. Smith; Adam D. Devore; Adrian F. Hernandez; Eric D. Peterson; Deepak L. Bhatt; et al. Sex and Race/Ethnicity–Related Disparities in Care and Outcomes After Hospitalization for Coronary Artery Disease Among Older Adults. Circulation: Cardiovascular Quality and Outcomes 2016, 9, S36-S44, 10.1161/circoutcomes.115.002621.
- Regitz-Zagrosek, V.; Sex and gender differences in health. Science & Society Series on Sex and Science. EMBO Rep. 2012, 13, 596–603.
- Smulders, E.; van Lankveld, W.; Laan, R.; Duysens, J.; Weerdesteyn, V.; Does osteoporosis predispose falls? a study on obstacle avoidance and balance confidence. BMC Musculoskelet. Disord. 2011, 12, 1.
- Alice C Scheffer; Marieke J. Schuurmans; Nynke Van Dijk; Truus Van Der Hooft; Sophia E De Rooij; Fear of falling: measurement strategy, prevalence, risk factors and consequences among older persons. Age And Ageing 2008, 37, 19-24, 10.1093/ageing/afm169.
- Kim Delbaere; Geert Crombez; Guy Vanderstraeten; Tine Willems; D Cambier; Fear-related avoidance of activities, falls and physical frailty. A prospective community-based cohort study. Age And Ageing 2004, 33, 368-373, 10.1093/ageing/afh106.
- Cathy M. Arnold; Angela J. Busch; Candice L. Schachter; Elizabeth Harrison; Wojciech Olszynski; The Relationship of Intrinsic Fall Risk Factors to a Recent History of Falling in Older Women With Osteoporosis. Journal of Orthopaedic & Sports Physical Therapy 2005, 35, 452-460, 10.2519/jospt.2005.35.7.452.
- Teresa Liu-Ambrose; K. M. Khan; Meghan G. Donaldson; Janice J. Eng; Stephen R. Lord; Heather A. McKay; Falls-related self-efficacy is independently associated with balance and mobility in older women with low bone mass.. The Journals of Gerontology: Series A 2006, 61, 832-838, 10.1093/gerona/61.8.832.
- A Patient’s Guide to Adult Kyphosis. Available online: https://www.umms.org/ummc/health-services/orthopedics/services/spine/patient-guides/adult-kyphosis (accessed on 16 January 2020)
