Brassicaceae family vegetables have an ample worldwide distribution, which can be found in all continents except Antarctica. One of the most striking features of this botanical family is the presence of several kinds of secondary metabolites with a distinctive taste, and also interesting bioactivities. The most deeply studied are the glucosinolates (GSL) and from their bioactive breakdown products, the isothiocyanates and the indoles. Moreover, these species are also rich and possess unique profiles of phenolic compounds, carotenoids, and other groups of less studied compounds such as phytoalexins, terpenes, phytosteroids, and tocopherols, here reviewed.
- Bioactive compounds, health-promoters, crucifers
Brassicaceae family vegetables have an ample worldwide distribution, which can be found in all continents except Antarctica [[1][2][3][4]]. One of the most striking features of this botanical family is the presence of several kinds of secondary metabolites with a distinctive taste, and also interesting bioactivities. The most deeply studied are the glucosinolates (GSL) and their breakdown products, isothiocyanates and indoles [[5][6][7]]. Moreover, these species are also rich and possess unique profiles of phenolic compounds, carotenoids, and other groups of less studied compounds such as phytoalexins, terpenes, phytosteroids, and tocopherols, here reviewed.
1. Phenolic Compounds
Phenolic compounds are a large class of plant secondary metabolites characterized by having at least one aromatic ring with one or more hydroxyl groups attached, showing a diversity of structures, ranging from rather simple and low molecular weight structures to complex polymeric compounds. More than 8000 phenolic compounds have been reported on plant kingdom [[8]]. Phenolic compounds are very important regarding the quality of plant-based foods since they are involved in flavor features (e.g., astringency), and they are responsible for the color of some fruits and vegetables and also because they serve as substrates for enzymatic deterioration [[9][10]]. Finally, phenolic compounds are considered to contribute to the health benefits associated with dietary consumption of Brassicaceae species such as antioxidant capacity, anticarcinogenic power, anti-aggregation activity, activation of detoxification enzymes, among others (Brassicaceae bioactive properties are reviewed and discussed in Section 3 of the present study). The most important phenolic compounds present in Brassicacea family vegetables are the flavonoids and the hydroxycinnamic acids [8]. Among flavonoids, the most important group corresponds to the flavonols. Quercetin, kaempferol, isorhamnetin, and cyanidin are the most representative flavonols in these species, but their qualitative and quantitative profiles vary significantly among species. For example, cauliflower main phenolic compounds are quercetin aglycon and catechins, but in white cabbage, the main compound are kaempferol glucosides and epicatechins [[11][12]]. The phenolic profile can also vary within the same plant species according to the plant organ being studied; for example, cruciferous sprouts can contain from 2 to 10 times more phenolic compound when compared with roots and inflorescences, which are the most common plant organ consumed [[13]]. Recently, Fusari et al. (2019) reported for the first time important levels of t-resveratrol -a p-terostilbene phenolic compound extensively reported as a potent antioxidant molecule- in several members of this botanical family, reaching, for example, 84 µg/g dry weight level in rocket leaves (Eruca sativa L.) [[12],[14]].
Another group of phenolic compounds frequently detected in Brassicaceae vegetables is the hydroxycinnamic acid group, which is characterized by the C6–C3 structure and can be found free or conjugated with sugars or with other acids. The most common in this species are ferulic acid, sinapic acid, caffeic acid, and p-coumaric acid, but as occur with flavonols, this varies significantly according to the plant species considered. For example, sinapic acid is the main hydroxycinnamic acid present in rocket salad but is absent in red cherry and daikon radishes [[11]]. Another example of this considerable variation among species is ferulic acid, which represents the main hydroxycinnamic acid for red cabbage but is absent in radish and rocket leaves [[12]]. In Figure 1, the chemical structure of the phenolic compounds usually present in Brassicaceae is shown.
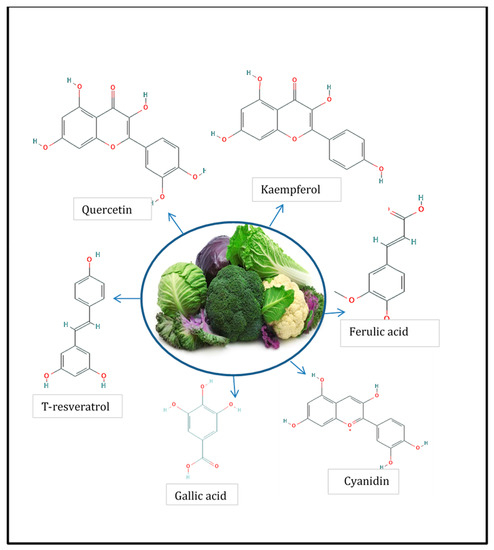
Figure 1. Phenolic compounds present in members of Brassicaceae [[12]].
Anthocyanins have also been reported in Brassicaceae vegetables. For example, the red or violet pigmentation of red cabbage, purple cauliflower, or red radishes is caused by anthocyanins. The major type of anthocyanins differs among species. While red radish contains mainly cyanidin and peonidin anthocyanins aclylated with aromatic acids [[15]], red cabbage and broccoli sprouts contain mainly cyaniding glucosides derivatives [[16]]. Besides, Lo Scalzo et al. (2008) [[17]] found that the p-coumaryl and feruloyl esterified forms of cyanidin-3-sophoroside-5-glucoside were predominant in cauliflower, while the sinapyl one was mostly present in red cabbage. In another study, Otsuki (2002) [[18]] found 12 different anthocyanins in radish roots, 6 of them corresponding to the pelargonidin derivatives. In red cabbage cultivars, the predominant anthocyanins resulted in being nonacylated cyanidin- glucosides [[19]]. Altogether, these results indicate that anthocyanins, as with other phenolic compounds, vary according to the vegetable species, the plant organ, and the cultivars of the single species considered.
2. Organosulfur Compounds
Among the organosulfur compounds accumulated by cruciferous vegetables, glucosinolates (GSL)-sulfur-containing glycosides- are the main secondary metabolites found. Their presence is evidenced whenever its tissue is disrupted, and their breakdown products are the principal responsible for the sharp and bitter-tasting flavors of these vegetables. There have been described in plant kingdom more than 120 different GSL, but in Brassicaceae, the amount reaches around 96, of which some are unique of some specific gender or species [[20]]. While genetic factors determining mainly the type of GSL, environmental factors influence the amount of them. The induction of GSL following abiotic or biotic stresses has frequently been described in order to increase the phytochemicals levels when GSL are hydrolyzed by myrosinase (thioglucoside glucohydrolase, EC 3.2.1.147) upon tissue disruption, numerous breakdown products are formed, including isothiocyanates (ITCs), thiocyanates, nitriles, ascorbigen, indoles, oxazolidine-2-thiones, and epithioalkanes depending upon different factors like pH, temperature, presence of myrosinase-interacting protein, and availability of ferrous ion [[21]]. Among the different GLS-degradation products formed, the most abundantly formed at physiological pH are the ITCs, which are also considered the main responsible for cruciferous foods bioactivity. Since ITCs are very unstable, their health benefits depend on numerous variables related to several factors, such as the initial GSL concentration, cooking processes, amount of vegetable intake, and human metabolism.
Because the GSL profile varies between different species and the hydrolysis conditions determine the identity and amount of ITC compounds formed, it is possible to find in the literature many ITC profiles for each species, and these profiles do not always coincide with each other. ITC profiles for each cruciferous vegetable have been extensively reviewed [[22][23][24][25]], and the reported information highly variable according to the extraction. It is also important to keep in mind that the majority of GLS will not always give rise to its ITC profile. For example, in radish, it has consistently been reported that the majority GLS is glucoraphasatin; however, the raphasatin levels found when the ITC profile was studied, were negligible or non-quantifiable because of the extraction conditions and the hydrolysis affecting the results, especially if the medium is polar or aqueous [[26]]. Furthermore, the ITC profile of each cruciferous species varies according to the cultivar or variety considered and also according to the plant tissue found [[27][28]]. For example, in rocket leaves, the main ITC generally detected corresponds to sativin [[29]], but in rocket seeds, the main ITC detected have been erucin and sulforaphane (SFN) [[30]]. Accordingly, in radish seeds and sprouts, the main ITC detected was sulforaphene according to several reports [[13],[27],[28],[31]] (Figure 2), but in radish roots, the chief ITC have been raphasatin and sulforaphene [[27],[32]]. In Figure 2, a graphical example of the ITC variation inside a single plant is schematized. The foregoing demonstrates that, in order to inform the ITC profile of a cruciferous vegetable, it is very important to consider, in addition to the species, the cultivation, the plant organ under study, and the extraction and detection techniques in order to make inferences and comparisons among studies.
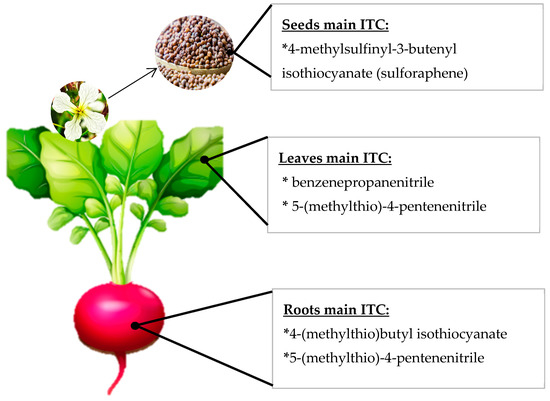
Figure 2. Schematic representation of different isothiocyanate (ITC) profiles in several radish plant organs.
3. Carotenoids
Carotenoids are highly pigmented phytochemicals that possess a C40 backbone structure and are classified as symmetrical tetraterpenes. They are produced in many plants and microorganisms, and they can be yellow, orange, or red pigments. Some carotenoids such as β-carotene and α-carotene and β-cryptoxanthin have provitamin A activity since they act as precursors of vitamin A and, therefore, acquires an important function as a human health promoter. These compounds have been extensively studied for their health-enhancing properties and also for their biological functions as attractants to pollinators, as photoprotection pigments, and as light‐harvesting pigments. Similarly, to phenolic compounds, the accumulation of carotenoids in cruciferous is regulated by the environment, tissue type, and developmental stage [[33][34]]. These groups of bioactive compounds have not been deeply characterized for Brassicaceae species, but it has been reported that the predominant ones are β-carotene and luteolin, but variable amounts of zeaxanthin, cryptoxanthin, neoxanthin, and violaxanthin have also been detected [[35][36][37]]. Other reports have found that carotenoid-containing cruciferous vegetables include kale (Brassica oleracea L. convar. Acephala var. sabellica), brussels sprouts, broccoli, cauliflower, red cabbage, white cabbage, pakchoi (Brassica rapa subsp. chinensis), and kohlrabi (Brassica oleracea var. ganglyoides) [[38][39]]. Kale is considered the richest cruciferous source of this compound, surpassing cabbage in about 40 times [35]. Among these, kale stands out for its high contents, not only within the cruciferous but also when compared to other vegetables, resulting in one of the main dietary source of carotenoids [[35],[40][41]]. Kale main carotenoids have been proposed to be zeaxantin and luteolin [[40]] but important differences were found among several kale cultivars [[42]]. Carotenoid profile among Brassicaceae species varies greatly; for example, broccoli contains mainly β-carotene and luteolin [[43]], cabbage contains mainly luteolin, followed by β-carotene, zeaxantin, and α-carotene [[44]].
4. Other Terpenes Present in Brassicaceae Vegetables
Other naturally occurring terpenes compounds that can be found in Brassicaceae vegetables are tocotrienols and tocopherols. According to Podsedeck (2007) [[45]], the descending order of total tocopherols and tocotrienol content in Brassica vegetables is as follows: broccoli > broccoli sprouts > cabbage. Furtheremore, Kurilich et al. (1999) [[46]] reported that kale was the best source among other Brassicaceae vegetables of α -tocopherol and γ-tocopherol. Beside Brassicaceae vegetables, these compounds have also been reported in oils and cereals [[47]]. These phytochemicals have been extensively researched due to its anticarcinogenic properties [[48][49]]. Another bioactivity extensively reported in these lipid-soluble compounds is the antioxidant activity through hydrogen atom transference [[50]].
Phytosterols are another important terpene subclass. It has been reported to possess anti-inflammatory, anti-neoplastic, anti-pyretic, and immune-modulating activity. Also, it has been reported that phytosterols reduce serum or plasma total cholesterol and low-density lipoprotein (LDL) cholesterol [[51]]. Among cruciferous vegetables, Brassica napus L., known as rapeseed, is the most abundant natural source of phytosterols, reaching levels of up to 9.79 gr/kg oil [[52]]. Another rich source of phytosterols among cruciferous vegetables is Brassica Juncea, of which, according to the cultivar analyzed, different compositions and levels of phytosterol can be detected [[53]].
5. Phytoalexins
These groups of compounds were described initially in 1940 and are considered phenolic-related compounds with highly diverse chemical structures and several bioactivities, including anti-cancer properties. They possess low molecular weight and are thought to serve as an important defense mechanism for the plant [[54][55]]. Brassicaceae members containing phytoalexins present an indolic ring with C3 substitutions with N and S atoms, which confers a unique structure among other vegetables. The proposed biosynthetic pathway for phytoalexins formation includes brassinin formations from GSL; thereafter, other related phytoalexins are produced from brassinin. Klein and Sattely (2017) [[55]] reported that over 30 compounds arise from oxidative tailoring and rearrangement of Brassinin. Among edible cruciferous, phytoalexins have been reported in Brassica napus [[56]], Brassica oleracea [[57]], Brassica Juncea [[58]], Sinapsis alba [[59]], Wasabi japonica [[60]], and in Raphanus sativus [[61]].
6. Alkaloids
Alkaloids are secondary metabolites of plants synthesized from amino acids. These nitrogen compounds have been reported in several Brassicaceae species, including Capsella bursa pastoris, Lepidium cartilacineum, Nasturtium montanum, and Raphanus sativus, among others [[62]]. Among edible Brassicaceae alkaloid compounds have also been reported in a cabbage cultivar collection [[63]], in cabbage seeds by Mohammed (2014) [[64]], in cauliflower leaves [[65]], in broccoli florets [[66]]. and red cabbage florets [[67]]. Besides, a screening of tropane alkaloids—a class of alkaloids typically found in Solanaceae vegetables—in 43 different Brassicaceae species reveal that 18 of them presented alkaloid of different structures, and the authors proposed that alkaloid compounds are typical secondary cruciferous metabolites [[68]].
As described above, the Brassicacea family is characterized by the presence of GSL and the isothiocyanates that are exclusive of this family. Also, Brassicacea stands out because they possess phytochemicals of multiple chemical groups, being these species’ excellent sources of bioactive compounds that have been studied throughout history. Currently, modern analytical techniques allow us to expand the knowledge about new compounds and metabolites. Consequently, it is possible to understand that each species, each Brassica-derived product, is themselves a mixture of multiple components. For this reason, an exhaustive determination of their phytochemical profiles must be made in each case.
References
- Catalina Vargas-Rincón; Gina Sánchez-León; Pedro Jiménez-Morales; La Producción de Metabolitos Secundarios en la Familia Brassicaceae. Revista Facultad de Ciencias Básicas 2014, 9, 282, 10.18359/rfcb.388.
- Branca, F.; Argento, S.; Alessandro, T. Assessing genetic reserves in Sicily (Italy): The Brassica wild relatives case study. In Agrobiodiversity Conservation: Securing the Diversity of Crop Wild Relatives and Landraces; Maxted, N., Ehsan Dulloo, M., Ford-Lloyd, B.V., Frese, L., Iriondo, J.M., Pinheiro de Carvalho, M.A.A., Eds.; CABI: Wallingford, Oxfordshire, UK, 2012; pp. 52–58. [Google Scholar]
- Pinheiro de Carvalho, M.Â. Agrobiodiversity Conservation: Securing the Diversity of Crop Wild Relatives and Landraces; CABI: Wallingford, Oxfordshire, UK, 2012.
- Ferdinando Branca; G.L. Chiarenza; L. Ragusa; Sergio Argento; MORPHOLOGICAL CHARACTERIZATION OF THE ECPGR WILD BRASSICA SPECIES COLLECTION. Acta Horticulturae 2013, null, 157-163, 10.17660/actahortic.2013.1005.15.
- Sergio Argento; Maria Grazia Melilli; Ferdinando Branca; Enhancing Greenhouse Tomato-Crop Productivity by Using Brassica macrocarpa Guss. Leaves for Controlling Root-Knot Nematodes. Agronomy 2019, 9, 820, 10.3390/agronomy9120820.
- F. Branca; L. Ragusa; A. Tribulato; R. Lo Scalzo; V. Picchi; S. Argento; THE GLUCOSINOLATES AND VARIATION OF ANTIOXIDANT COMPOUNDS IN SEEDS AND SPROUTS OF BROCCOLI (BRASSICA OLERACEA L. VAR. ITALIC) AND ROCKET (ERUCA SATIVA L.) IN RELATION TO TEMPERATURE AND GERMINATIVE STAGE. Acta Horticulturae 2013, null, 271-277, 10.17660/actahortic.2013.1005.30.
- Stefania Galletti; Manuela Bagatta; Ferdinando Branca; Sergio Argento; Gina Rosalinda De Nicola; Stefano Cianchetta; Renato Iori; Paolino Ninfali; Isatis canescensis a rich source of glucobrassicin and other health-promoting compounds. Journal of the Science of Food and Agriculture 2014, 95, 158-164, 10.1002/jsfa.6697.
- María Elena Cartea; Marta Francisco; Pilar Soengas; Pablo Velasco; Phenolic Compounds in Brassica Vegetables. Molecules 2010, 16, 251-280, 10.3390/molecules16010251.
- Elena Hurtado-Fernández; Maria Gomez Romero; Alegria Carrasco Pancorbo; Alberto Fernandez Gutierrez; Application and potential of capillary electroseparation methods to determine antioxidant phenolic compounds from plant food material. Journal of Pharmaceutical and Biomedical Analysis 2010, 53, 1130-1160, 10.1016/j.jpba.2010.07.028.
- Todaro, A.; Cavallaro, R.; Argento, S.; Branca, F.; Spagna, G. Study and Characterization of Polyphenol Oxidase from Eggplant (Solanum melongena L.). J. Agric. Food Chem. 2011, 59, 11244–11248.
- Zhifeng Li; Hui Wen Lee; Xu Liang; Ng Liang; Qi Wang; Dejian Huang; Choon Nam Ong; Profiling of Phenolic Compounds and Antioxidant Activity of 12 Cruciferous Vegetables. Molecules 2018, 23, 1139, 10.3390/molecules23051139.
- Fusari, C.; Beretta, H.; Locatelli, D.; Nazareno, M.; Camargo, A. Seasonal isothiocyanates variation and market availability of Brassicaceae species consumed in Mendoza. Rev. Fac. Cienc. Agrar. 2019, 51, 403–408.
- Nieves Baenas; Isabel Gómez-Jodar; Diego A. Moreno; Cristina García-Viguera; Paula M. Periago; Broccoli and radish sprouts are safe and rich in bioactive phytochemicals. Postharvest Biology and Technology 2017, 127, 60-67, 10.1016/j.postharvbio.2017.01.010.
- Bianke Loedolff; Jolene Brooks; Maria A. Stander; Shaun Peters; Jens Kossmann; High light bio-fortification stimulates de novo synthesis of resveratrol in Diplotaxis tenuifolia (wild rocket) micro-greens. Functional Foods in Health and Disease 2017, 7, 859, 10.31989/ffhd.v7i11.380.
- Riccardo Matera; Simone Gabbanini; Gina Rosalinda De Nicola; Renato Iori; Gianna Petrillo; Luca Valgimigli; Identification and analysis of isothiocyanates and new acylated anthocyanins in the juice of Raphanus sativus cv. Sango sprouts. Food Chemistry 2012, 133, 563-572, 10.1016/j.foodchem.2012.01.050.
- Diego A. Moreno; Santiago Pérez-Balibrea; Federico Ferreres; Ángel Gil-Izquierdo; Cristina García-Viguera; Acylated anthocyanins in broccoli sprouts. Food Chemistry 2010, 123, 358-363, 10.1016/j.foodchem.2010.04.044.
- Roberto Lo Scalzo; A. Genna; Ferdinando Branca; M. Chedin; H. Chassaigne; Anthocyanin composition of cauliflower (Brassica oleracea L. var. botrytis) and cabbage (B. oleracea L. var. capitata) and its stability in relation to thermal treatments. Food Chemistry 2008, 107, 136-144, 10.1016/j.foodchem.2007.07.072.
- Takashi Otsuki; Hiroshi Matsufuji; Mitsuharu Takeda; Masatake Toyoda; Yukihiro Goda; Acylated anthocyanins from red radish (Raphanus sativus L.).. Phytochemistry 2002, 60, 79-87, 10.1016/s0031-9422(02)00063-8.
- Wieslaw Wiczkowski; Dorota Szawara-Nowak; Joanna Topolska; Red cabbage anthocyanins: Profile, isolation, identification, and antioxidant activity. Food Research International 2013, 51, 303-309, 10.1016/j.foodres.2012.12.015.
- Anjum, N.A.; Gill, S.S.; Ahmad, I.; Pacheco, M.; Duarte, A.C.; Umar, S.; Khan, N.A.; Pereira, M.E. The Plant Family Brassicaceae: An Introduction. In The Plant Family Brassicaceae: Contribution Towards Phytoremediation; Anjum, N.A., Ahmad, I., Pereira, M.E., Duarte, A.C., Umar, S., Khan, N.A., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 1–33.
- Luke Bell; Hanis Nadia Yahya; Omobolanle Oluwadamilola Oloyede; Lisa Methven; Carol Wagstaff; Changes in rocket salad phytochemicals within the commercial supply chain: Glucosinolates, isothiocyanates, amino acids and bacterial load increase significantly after processing. Food Chemistry 2017, 221, 521-534, 10.1016/j.foodchem.2016.11.154.
- Ibrahim Guillermo Castro-Torres; Víctor Alberto Castro-Torres; Minerva Hernández-Lozano; Elia Brosla Naranjo-Rodríguez; Miguel Ángel Domínguez-Ortiz; Glucosinolates and metabolism. Glucosinolates: Properties, Recovery, and Applications 2020, null, 107-141, 10.1016/b978-0-12-816493-8.00004-4.
- Jed W. Fahey; Amy T Zalcmann; Paul Talalay; Corrigendum to “The chemical diversity and distribution of glucosinolates and isothiocyanates among plants” [Phytochemistry 56 (2001) 5–51]. Phytochemistry 2002, 59, 237, 10.1016/s0031-9422(01)00419-8.
- Richard Mithen; Matthijs Dekker; Ruud Verkerk; Sylvie Rabot; Ian Johnson; The nutritional significance, biosynthesis and bioavailability of glucosinolates in human foods. Journal of the Science of Food and Agriculture 2000, 80, 967-984, 10.1002/(sici)1097-0010(20000515)80:7<967::aid-jsfa597>3.3.co;2-m.
- Mohammed Sani Jaafaru; Nurul Ashikin Abd Karim; Enas Mohamed Eliaser; Patrick Rollin; Emanuela Mazzon; Ahmad Faizal Abdull Razis; Protective Effect of Glucosinolates Hydrolytic Products in Neurodegenerative Diseases (NDDs). Nutrients 2018, 10, 580, 10.3390/nu10050580.
- Nieves Baenas; Stefanie Piegholdt; Anke Schloesser; Diego A. Moreno; Cristina García-Viguera; Gerald Rimbach; Anika E. Wagner; Metabolic Activity of Radish Sprouts Derived Isothiocyanates in Drosophila melanogaster. International Journal of Molecular Sciences 2016, 17, 251, 10.3390/ijms17020251.
- Blažević, I.; Mastelić, J. Glucosinolate degradation products and other bound and free volatiles in the leaves and roots of radish (Raphanus sativus L.). Food Chem. 2009, 113, 96–102. [Google Scholar] [CrossRef]
- Gibum Yi; Sooyeon Lim; Won Byoung Chae; Jeong Eun Park; Hye Rang Park; Eun Jin Lee; Jin Hoe Huh; Root Glucosinolate Profiles for Screening of Radish (Raphanus sativusL.) Genetic Resources. Journal of Agricultural and Food Chemistry 2015, 64, 61-70, 10.1021/acs.jafc.5b04575.
- Fechner, J.; Kaufmann, M.; Herz, C.; Eisenschmidt, D.; Lamy, E.; Kroh, L.W.; Hanschen, F.S.; The major glucosinolate hydrolysis product in rocket (Eruca sativa L.), sativin, is 1,3-thiazepane-2-thione: Elucidation of structure, bioactivity, and stability compared to other rocket isothiocyanates. Food Chem. 2018, 261, 57–65.
- P. Franco; S. Spinozzi; Eleonora Pagnotta; Luca Lazzeri; L. Ugolini; C. Camborata; Aldo Roda; Development of a liquid chromatography–electrospray ionization–tandem mass spectrometry method for the simultaneous analysis of intact glucosinolates and isothiocyanates in Brassicaceae seeds and functional foods. Journal of Chromatography A 2016, 1428, 154-161, 10.1016/j.chroma.2015.09.001.
- Kuang, P.; Song, D.; Yuan, Q.; Yi, R.; Lv, X.; Liang, H.; Separation and purification of sulforaphene from radish seeds using macroporous resin and preparative high-performance liquid chromatography. Food Chem. 2013, 136, 342–347.
- Jae-Won Kim; Mi-Bo Kim; Sang-Bin Lim; Formation and Stabilization of Raphasatin and Sulforaphene from Radish Roots by Endogenous Enzymolysis. Preventive Nutrition and Food Science 2015, 20, 119-125, 10.3746/pnf.2015.20.2.119.
- Bianyun Yu; Derek J. Lydiate; Lester W. Young; Ulrike A. Schäfer; Abdelali Hannoufa; Enhancing the carotenoid content of Brassica napus seeds by downregulating lycopene epsilon cyclase. Transgenic Research 2007, 17, 573-585, 10.1007/s11248-007-9131-x.
- Katja Frede; Monika Schreiner; Susanne Baldermann; Light quality-induced changes of carotenoid composition in pak choi Brassica rapa ssp. chinensis. Journal of Photochemistry and Photobiology B: Biology 2019, 193, 18-30, 10.1016/j.jphotobiol.2019.02.001.
- Jessica Nilsson; Kerstin Olsson; Gabriele Engqvist; Jimmy Ekvall; Marie Olsson; Margareta Nyman; B. Åkesson; Variation in the content of glucosinolates, hydroxycinnamic acids, carotenoids, total antioxidant capacity and low-molecular-weight carbohydrates inBrassica vegetables. Journal of the Science of Food and Agriculture 2006, 86, 528-538, 10.1002/jsfa.2355.
- Zhang, B.; Liu, C.; Wang, Y.; Yao, X.; Wang, F.; Wu, J.; King, G.J.; Liu, K. Disruption of a Carotenoid Cleavage Dioxygenase 4 gene converts flower colour from white to yellow in Brassica species. New Phytol. 2015, 206, 1513–1526. [Google Scholar] [CrossRef] [PubMed]
- Ivette Guzman; Gad G. Yousef; Allan F. Brown; Simultaneous Extraction and Quantitation of Carotenoids, Chlorophylls, and Tocopherols in Brassica Vegetables. Journal of Agricultural and Food Chemistry 2012, 60, 7238-7244, 10.1021/jf302475d.
- Park, W.T.; Kim, J.K.; Park, S.; Lee, S.-W.; Li, X.; Kim, Y.B.; Uddin, M.R.; Park, N.I.; Kim, S.-J.; Park, S.U. Metabolic Profiling of Glucosinolates, Anthocyanins, Carotenoids, and Other Secondary Metabolites in Kohlrabi (Brassica oleracea var. gongylodes). J. Agric. Food Chem. 2012, 60, 8111–8116.
- Jin-Hyuk Chun; Na-Hyung Kim; Mi-Suk Seo; Mina Jin; Sang Un Park; Mariadhas Valan Arasu; Jung Bong Kim; Naif Abdullah Al-Dhabi; Molecular characterization of glucosinolates and carotenoid biosynthetic genes in Chinese cabbage ( Brassica rapa L. ssp. pekinensis ). Saudi Journal of Biological Sciences 2018, 25, 71-82, 10.1016/j.sjbs.2016.04.004.
- Rachel P. Walsh; Hannah Bartlett; Frank Eperjesi; Variation in Carotenoid Content of Kale and Other Vegetables: A Review of Pre- and Post-harvest Effects. Journal of Agricultural and Food Chemistry 2015, 63, 9677-9682, 10.1021/acs.jafc.5b03691.
- El-Sayed M. Abdel-Aal; Humayoun Akhtar; K. Zaheer; Rashida Ali; El-Sayed M. Abdel-Aal; Dietary Sources of Lutein and Zeaxanthin Carotenoids and Their Role in Eye Health. Nutrients 2013, 5, 1169-1185, 10.3390/nu5041169.
- Vera Mageney; Susanne Baldermann; Dirk Albach; Intraspecific Variation in Carotenoids ofBrassica oleraceavar.sabellica. Journal of Agricultural and Food Chemistry 2016, 64, 3251-3257, 10.1021/acs.jafc.6b00268.
- M.F. Fernández-León; A.M. Fernández-León; M. Lozano; M.C. Ayuso; David González-Gómez; Identification, quantification and comparison of the principal bioactive compounds and external quality parameters of two broccoli cultivars. Journal of Functional Foods 2012, 4, 465-473, 10.1016/j.jff.2012.02.005.
- Anouk Kaulmann; Christelle M. André; Yves-Jacques Schneider; Lucien Hoffmann; Torsten Bohn; Carotenoid and polyphenol bioaccessibility and cellular uptake from plum and cabbage varieties. Food Chemistry 2016, 197, 325-332, 10.1016/j.foodchem.2015.10.049.
- Anna Podsędek; Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT 2007, 40, 1-11, 10.1016/j.lwt.2005.07.023.
- Anne C. Kurilich; Grace J. Tsau; Allan Brown; Lenora Howard; B.P. Klein; Elizabeth H. Jeffery; Mosbah Kushad; M A Wallig; John A. Juvik; Carotene, tocopherol, and ascorbate contents in subspecies of Brassica oleracea.. Journal of Agricultural and Food Chemistry 1999, 47, 1576-1581, 10.1021/jf9810158.
- Dan Lu; Yi Yang; Yongxin Li; Chengjun Sun; Analysis of Tocopherols and Tocotrienols in Pharmaceuticals and Foods: A Critical Review. Current Pharmaceutical Analysis 2014, 11, 66-78, 10.2174/1573412910666140630170055.
- Weiping Yu; Maria Simmons‐Menchaca; Abdul Gapor; Bob G. Sanders; Kimberly Kline; Induction of apoptosis in human breast cancer cells by tocopherols and tocotrienols. Nutrition and Cancer 1999, 33, 26-32, 10.1080/01635589909514744.
- Huanbiao Mo; Charles E Elson; Apoptosis and cell-cycle arrest in human and murine tumor cells are initiated by isoprenoids.. The Journal of Nutrition 1999, 129, 804-813, 10.1093/jn/129.4.804.
- Lampi, A.-M.; Kamal-Eldin, A.; Piironen, V.; Tocopherols and tocotrienols from oil and cereal grains. Funct. Foods Biochem. Process. Asp. 2002, no, 1–38.
- W.H Ling; Peter J. H. Jones; Dietary phytosterols: A review of metabolism, benefits and side effects. Life Sciences 1995, 57, 195-206, 10.1016/0024-3205(95)00263-6.
- Gül, M.; Amar, S.; Sterols and the phytosterol content in oilseed rape (Brassica napus L.). J. Cell & Mol. Biol. 2006, 5, 71–79.
- Anubhuti Sharma; P.K. Rai; Surendra Prasad; GC–MS detection and determination of major volatile compounds in Brassica juncea L. leaves and seeds. Microchemical Journal 2018, 138, 488-493, 10.1016/j.microc.2018.01.015.
- Ishita Ahuja; Ralph Kissen; Atle Bones; Phytoalexins in defense against pathogens. Trends in Plant Science 2012, 17, 73-90, 10.1016/j.tplants.2011.11.002.
- Andrew Klein; Elizabeth S. Sattely; Biosynthesis of cabbage phytoalexins from indole glucosinolate. Proceedings of the National Academy of Sciences 2017, 114, 1910-1915, 10.1073/pnas.1615625114.
- M. Soledade C. Pedras; Qing-An Zheng; Ravi S. Gadagi; S. Roger Rimmer; Phytoalexins and polar metabolites from the oilseeds canola and rapeseed: Differential metabolic responses to the biotroph Albugo candida and to abiotic stress. Phytochemistry 2008, 69, 894-910, 10.1016/j.phytochem.2007.10.019.
- M. Soledade C. Pedras; Adewale M. Adio; Mojmír Suchý; Denis P.O. Okinyo; Qing-An Zheng; Mukund Jha; Mohammed G. Sarwar; Detection, characterization and identification of crucifer phytoalexins using high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry. Journal of Chromatography A 2006, 1133, 172-183, 10.1016/j.chroma.2006.08.015.
- M. Soledade C. Pedras; Pearson W.K. Ahiahonu; Metabolism and detoxification of phytoalexins and analogs by phytopathogenic fungi. Phytochemistry 2005, 66, 391-411, 10.1016/j.phytochem.2004.12.032.
- M. Soledade C. Pedras; S Montaut; I L Zaharia; Y Gai; D E Ward; Transformation of the host-selective toxin destruxin B by wild crucifers: probing a detoxification pathway.. Phytochemistry 2003, 64, 957–963.
- M. Soledade C. Pedras; John L. Sorensen; Francis I. Okanga; Irina L. Zaharia; Wasalexins A and B, new phytoalexins from wasabi: isolation, synthesis, and antifungal activity.. Bioorganic & Medicinal Chemistry Letters 1999, 9, 3015-3020, 10.1016/s0960-894x(99)00523-5.
- Kenji Monde; Mitsuo Takasugi; Akira Shirata; Three sulphur-containing stress metabolites from Japanese radish. Phytochemistry 1995, 39, 581-586, 10.1016/0031-9422(95)00011-u.
- Nee, M. Plant Alkaloids: A Guide to Their Discovery and Distribution; Raffauf, R.F., Ed.; Brittonia: New York, NY, USA, 1998; Volume 50, p.55.
- Guriya, R.; Moon, A.; Talreja, K.; Phytochemical profiling and characterization of bioactive compounds from Brassica oleracea. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 825–831.
- Khalid, A.; Mohammed, A.D.; Al-Maliki, M.; Effect of phenolic and alkaloid compounds extracted from Brassica oleracea var. capitata seed on glucose level in blood of alloxan- induced diabetes rabbits. World J. Exp. Biosci. 2014, 2, 24–29.
- Yannai, S. Dictionary of Food; CRC Press: Boca Raton, FL, USA, 2003.
- Shah, M.A.; Sarker, M.M.R.; Gousuddin, M.; Antidiabetic Potential of Brassica oleracea var. italica in Type 2 Diabetic Sprague Dawley (sd) Rats. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 462–469.
- Chauhan, E.S.T.; Tiwari, A.; Singh, A.; Phytochemical screening of red cabbage (Brassica oleracea) powder and juice—A comparative study. J. Med. Plants Stud. 2016, 4, 196–199.
- Andrea Brock; Tobias Herzfeld; Reinhard Paschke; Marcus A. Koch; Birgit Dräger; Brassicaceae contain nortropane alkaloids. Phytochemistry 2006, 67, 2050-2057, 10.1016/j.phytochem.2006.06.024.
