Different brain disorders display distinctive etiologies and pathogenic mechanisms. However, they also share pathogenic events. One event systematically occurring in different brain disorders, both acute and chronic, is the increase of the extracellular ATP levels. Accordingly, several P2 (ATP/ADP) and P1 (adenosine) receptors, as well as the ectoenzymes involved in the extracellular catabolism of ATP, have been associated to different brain pathologies, either with a neuroprotective or neurodegenerative action. The P2Y1 receptor (P2Y1R) is one of the purinergic receptors associated to different brain diseases. It has a widespread regional, cellular, and subcellular distribution in the brain, it is capable of modulating synaptic function and neuronal activity, and it is particularly important in the control of astrocytic activity and in astrocyte–neuron communication. In diverse brain pathologies, there is growing evidence of a noxious gain-of-function of P2Y1R favoring neurodegeneration by promoting astrocyte hyperactivity, entraining Ca2+-waves, and inducing the release of glutamate by directly or indirectly recruiting microglia and/or by increasing the susceptibility of neurons to damage.
- P2Y1 receptor
- neurodegeneration
- ATP
- ADP
- brain
1. P2Y1 Receptor in Neurodegenerative Disorders
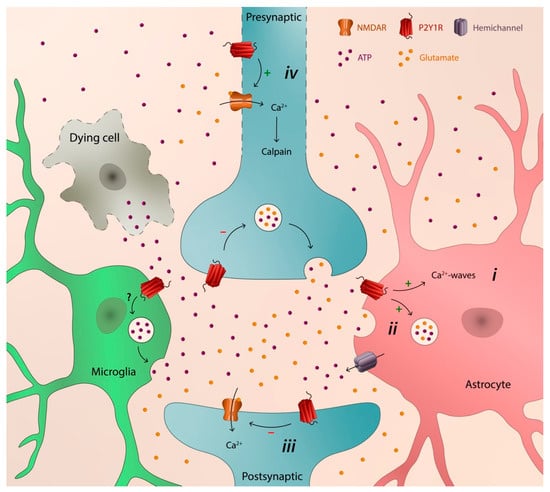
2. P2Y1 Receptor as a Catalyst of Neurodegeneration
References
- Padrão, R.A.; Ariza, C.B.; Canzian, M.; Porcionatto, M.; Araffljo, M.G.L.; Cavalheiro, E.A. The P2 purinergic receptors are increased in the hippocampus of patients with temporal lobe epilepsy: What is the relevance to the epileptogenesis? Purinergic Signal. 2011, 7, 127.
- Alves, M.; Gomez-Villafuertes, R.; Delanty, N.; Farrell, M.A.; O’Brien, D.F.; Miras-Portugal, M.T.; Hernandez, M.D.; Henshall, D.C.; Engel, T. Expression and function of the metabotropic purinergic P2Y receptor family in experimental seizure models and patients with drug-refractory epilepsy. Epilepsia 2017, 58, 1603–1614.
- Alves, M.; Smith, J.; Engel, T. Differential expression of the metabotropic P2Y receptor family in the cortex following status epilepticus and neuroprotection via P2Y1 antagonism in mice. Front. Pharmacol. 2020, 10, 1558.
- Franke, H.; Krugel, U.; Grosche, J.; Heine, C.; Hartig, W.; Allgaier, C.; Illes, P. P2Y receptor expression on astrocytes in the nucleus accumbens of rats. Neuroscience 2004, 127, 431–441.
- Kuboyama, K.; Harada, H.; Tozaki-Saitoh, H.; Tsuda, M.; Ushijima, K.; Inoue, K. Astrocytic P2Y(1) receptor is involved in the regulation of cytokine/chemokine transcription and cerebral damage in a rat model of cerebral ischemia. J. Cereb. Blood Flow Metab. 2011, 31, 1930–1941.
- Delekate, A.; Fuchtemeier, M.; Schumacher, T.; Ulbrich, C.; Foddis, M.; Petzold, G.C. Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an alzheimer’s disease mouse model. Nat. Commun. 2014, 5, 5422.
- Moore, D.; Iritani, S.; Chambers, J.; Emson, P. Immunohistochemical localization of the P2Y1 purinergic receptor in Alzheimer’s disease. Neuroreport 2000, 11, 3799–3803.
- Traini, C.; Pedata, F.; Cipriani, S.; Mello, T.; Galli, A.; Giovannini, M.G.; Cerbai, F.; Volpini, R.; Cristalli, G.; Pugliese, A.M. P2 receptor antagonists prevent synaptic failure and extracellular signal-regulated kinase 1/2 activation induced by oxygen and glucose deprivation in rat CA1 hippocampus in vitro. Eur. J. Neurosci. 2011, 33, 2203–2215.
- Maraula, G.; Lana, D.; Coppi, E.; Gentile, F.; Mello, T.; Melani, A.; Galli, A.; Giovannini, M.G.; Pedata, F.; Pugliese, A.M. The selective antagonism of P2X7 and P2Y1 receptors prevents synaptic failure and affects cell proliferation induced by oxygen and glucose deprivation in rat dentate gyrus. PLoS ONE 2014, 9, e115273.
- Chin, Y.; Kishi, M.; Sekino, M.; Nakajo, F.; Abe, Y.; Terazono, Y.; Hiroyuki, O.; Kato, F.; Koizumi, S.; Gachet, C.; et al. Involvement of glial P2Y1 receptors in cognitive deficit after focal cerebral stroke in a rodent model. J. Neuroinflamm. 2013, 10, 95.
- Sun, J.J.; Liu, Y.; Ye, Z.R. Effects of P2Y1 receptor on glial fibrillary acidic protein and glial cell line-derived neurotrophic factor production of astrocytes under ischemic condition and the related signaling pathways. Neurosci. Bull. 2008, 24, 231–243.
- Carmo, M.R.; Simões, A.P.; Fonteles, A.A.; Souza, C.M.; Cunha, R.A.; Andrade, G.M. ATP P2Y1 receptors control cognitive deficits and neurotoxicity but not glial modifications induced by brain ischemia in mice. Eur. J. Neurosci. 2014, 39, 614–622.
- Fukumoto, Y.; Tanaka, K.F.; Parajuli, B.; Shibata, K.; Yoshioka, H.; Kanemaru, K.; Gachet, C.; Ikenaka, K.; Koizumi, S.; Kinouchi, H. Neuroprotective effects of microglial P2Y1 receptors against ischemic neuronal injury. J. Cereb. Blood Flow Metab. 2019, 39, 2144–2156.
- Zheng, W.; Watts, L.T.; Holstein, D.M.; Wewer, J.; Lechleiter, J.D. P2Y1R-initiated, IP3R-dependent stimulation of astrocyte mitochondrial metabolism reduces and partially reverses ischemic neuronal damage in mouse. J. Cereb. Blood Flow Metab. 2013, 33, 600–611.
- Zheng, W.; Watts, L.T.; Holstein, D.M.; Prajapati, S.I.; Keller, C.; Grass, E.H.; Walter, C.A.; Lechleiter, J.D. Purinergic receptor stimulation reduces cytotoxic edema and brain infarcts in mouse induced by photothrombosis by energizing glial mitochondria. PLoS ONE 2010, 5, e14401.
- Fujita, T.; Tozaki-Saitoh, H.; Inoue, K. P2Y1 receptor signaling enhances neuroprotection by astrocytes against oxidative stress via IL-6 release in hippocampal cultures. Glia 2009, 57, 244–257.
- Simões, A.P.; Silva, C.G.; Marques, J.M.; Pochmann, D.; Porciúncula, L.O.; Ferreira, S.; Oses, J.P.; Beleza, R.O.; Real, J.I.; Köfalvi, A.; et al. Glutamate-induced and NMDA receptor-mediated neurodegeneration entails P2Y1 receptor activation. Cell Death Dis. 2018, 9, 297.
- Maiolino, M.; O’Neill, N.; Lariccia, V.; Amoroso, S.; Sylantyev, S.; Angelova, P.R.; Abramov, A.Y. Inorganic polyphosphate regulates AMPA and NMDA receptors and protects against glutamate excitotoxicity via activation of P2Y receptors. J. Neurosci. 2018, 39, 6038–6048.
- Alves, M.; De Diego Garcia, L.; Conte, G.; Jimenez-Mateos, E.M.; D’Orsi, B.; Sanz-Rodriguez, A.; Prehn, J.H.M.; Henshall, D.C.; Engel, T. Context-specific switch from anti- to pro-epileptogenic function of the P2Y1 receptor in experimental epilepsy. J. Neurosci. 2019, 39, 5377–5392.
- Luthardt, J.; Borvendeg, S.J.; Sperlagh, B.; Poelchen, W.; Wirkner, K.; Illes, P. P2Y1 receptor activation inhibits NMDA receptor-channels in layer V pyramidal neurons of the rat prefrontal and parietal cortex. Neurochem. Int. 2003, 42, 161–172.
- Bowser, D.N.; Khakh, B.S. ATP excites interneurons and astrocytes to increase synaptic inhibition in neuronal networks. J. Neurosci. 2004, 24, 8606–8620.
- Kawamura, M.; Gachet, C.; Inoue, K.; Kato, F. Direct excitation of inhibitory interneurons by extracellular ATP mediated by P2Y1 receptors in the hippocampal slice. J. Neurosci. 2004, 24, 10835–41085.
- Domercq, M.; Brambilla, L.; Pilati, E.; Marchaland, J.; Volterra, A.; Bezzi, P. P2Y1 receptor-evoked glutamate exocytosis from astrocytes: Control by tumor necrosis factor-alpha and prostaglandins. J. Biol. Chem. 2006, 281, 30684–93066.
- Jourdain, P.; Bergersen, L.H.; Bhaukaurally, K.; Bezzi, P.; Santello, M.; Domercq, M.; Matute, C.; Tonello, F.; Gundersen, V.; Volterra, A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat. Neurosci. 2007, 10, 331–339.
- Engel, T.; Smith, J.; Alves, M. Targeting neuroinflammation via purinergic P2 receptors for disease modification in drug-refractory epilepsy. J. Inflamm. Res. 2021, 14, 3367–3392.
- Shen, W.; Nikolic, L.; Meunier, C.; Pfrieger, F.; Audinat, E. An autocrine purinergic signaling controls astrocyte-induced neuronal excitation. Sci. Rep. 2017, 7, 11280.
- Nikolic, L.; Shen, W.; Nobili, P.; Virenque, A.; Ulmann, L.; Audinat, E. Blocking TNFα-driven astrocyte purinergic signaling restores normal synaptic activity during epileptogenesis. Glia 2018, 66, 2673–2683.
- Nobili, P.; Shen, W.; Milicevic, K.; Bogdanovic Pristov, J.; Audinat, E.; Nikolic, L. Therapeutic potential of astrocyte purinergic signalling in epilepsy and multiple sclerosis. Front. Pharmacol. 2022, 13, 900337.
- Su, L.; Bai, X.; Niu, T.; Zhuang, X.; Dong, B.; Wang, G.; Yu, Y. P2Y1 purinergic receptor inhibition attenuated remifentanil-induced postoperative hyperalgesia via decreasing NMDA receptor phosphorylation in dorsal root ganglion. Brain Res. Bull. 2021, 177, 352–362.
- Nedergaard, M.; Dirnagl, U. Role of glial cells in cerebral ischemia. Glia 2005, 50, 281–286.
- Shinozaki, Y.; Shibata, K.; Yoshida, K.; Shigetomi, E.; Gachet, C.; Ikenaka, K.; Tanaka, K.F.; Koizumi, S. Transformation of astrocytes to a neuroprotective phenotype by microglia via P2Y1 receptor downregulation. Cell Rep. 2017, 19, 1151–1164.
- Martorell, A.; Wellmann, M.; Guiffa, F.; Fuenzalida, M.; Bonansco, C. P2Y1 receptor inhibition rescues impaired synaptic plasticity and astroglial Ca2+-dependent activity in the epileptic hippocampus. Neurobiol. Dis. 2020, 146, 105132.
- Choo, A.M.; Miller, W.J.; Chen, Y.C.; Nibley, P.; Patel, T.P.; Goletiani, C.; Morrison, B., 3rd; Kutzing, M.K.; Firestein, B.L.; Sul, J.Y.; et al. Antagonism of purinergic signalling improves recovery from traumatic brain injury. Brain 2013, 136, 65–80.
- Moro, N.; Ghavim, S.S.; Sutton, R.L. Massive efflux of adenosine triphosphate into the extracellular space immediately after experimental traumatic brain injury. Exp. Ther. Med. 2021, 21, 575.
- Kumagawa, T.; Moro, N.; Maeda, T.; Kobayashi, M.; Furukawa, Y.; Shijo, K.; Yoshino, A. Anti-inflammatory effect of P2Y1 receptor blocker MRS2179 in a rat model of traumatic brain injury. Brain Res. Bull. 2022, 181, 46–54.
- Forster, D.; Reiser, J. Supportive or detrimental roles of P2Y receptors in brain pathology?—The two faces of P2Y receptors in stroke and neurodegeneration detected in neural cell and in animal model studies. Purinergic Signal. 2015, 11, 441–454.
- Shinozaki, Y.; Koizumi, S.; Ishida, S.; Sawada, J.; Ohno, Y.; Inoue, K. Cytoprotection against oxidative stress-induced damage of astrocytes by extracellular ATP via P2Y1 receptors. Glia 2005, 49, 288–300.
- Shinozaki, Y.; Koizumi, S.; Ohno, Y.; Nagao, T.; Inoue, K. Extracellular ATP counteracts the ERK1/2-mediated death-promoting signaling cascades in astrocytes. Glia 2006, 54, 606–618.
- Guo, H.; Liu, Z.Q.; Zhou, H.; Wang, Z.L.; Tao, Y.H.; Tong, Y. P2Y1 receptor antagonists mitigate oxygen and glucose deprivation-induced astrocyte injury. Mol. Med. Rep. 2018, 17, 1819–1824.
- Locovei, S.; Wang, J.; Dahl, G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006, 580, 239–244.
- Miller, W.J.; Leventhal, I.; Scarsella, D.; Haydon, P.G.; Janmey, P.; Meaney, D.F. Mechanically induced reactive gliosis causes ATP-mediated alterations in astrocyte stiffness. J. Neurotrauma 2009, 26, 789–797.
- Pascual, O.; Achour, S.B.; Rostaing, P.; Triller, A.; Bessis, A. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc. Natl. Acad. Sci. USA 2012, 109, E197–E205.
- Reichenbach, N.; Delekate, A.; Breithausen, B.; Keppler, K.; Poll, S.; Schulte, T.; Peter, J.; Plescher, M.; Hansen, J.N.; Blank, N.; et al. P2Y1 receptor blockade normalizes network dysfunction and cognition in an Alzheimer’s disease model. J. Exp. Med. 2018, 215, 1649–1663.
- Rodrigues, R.J.; Tomé, A.R.; Cunha, R.A. ATP as a multi-target danger signal in the brain. Front. Neurosci. 2015, 9, 148.
- Illes, P. P2X7 receptors amplify CNS damage in neurodegenerative diseases. Int. J. Mol. Sci. 2020, 21, 5996.
- Cunha, R.A. How does adenosine control neuronal dysfunction and neurodegeneration? J. Neurochem. 2016, 139, 1019–1055.
- Miras-Portugal, M.T.; Queipo, M.J.; Gil-Redondo, J.C.; Ortega, F.; Gómez-Villafuertes, R.; Gualix, J.; Delicado, E.G.; Pérez-Sen, R. P2 receptor interaction and signalling cascades in neuroprotection. Brain Res. Bull. 2019, 151, 74–83.
- Guzman, S.J.; Gerevich, Z. P2Y receptors in synaptic transmission and plasticity: Therapeutic potential in cognitive dysfunction. Neural Plast. 2016, 2016, 1207393.
- Woods, L.T.; Ajit, D.; Camden, J.M.; Erb, L.; Weisman, G.A. Purinergic receptors as potential therapeutic targets in Alzheimer’s disease. Neuropharmacology 2016, 104, 169–179.
- Madeira, D.; Dias, L.; Santos, P.; Cunha, R.A.; Canas, P.M.; Agostinho, P. Association between adenosine A2A receptors and connexin 43 regulates hemichannels activity and ATP release in astrocytes exposed to amyloid-β peptides. Mol. Neurobiol. 2021, 58, 6232–6248.
- Duan, S.; Neary, J.T. P2X7 receptors: Properties and relevance to CNS function. Glia 2006, 54, 738–746.
- Cisneros-Mejorado, A.; Pérez-Samartín, A.; Gottlieb, M.; Matute, C. ATP signaling in brain: Release, excitotoxicity and potential therapeutic targets. Cell. Mol. Neurobiol. 2015, 35, 1–6.
- Quintas, C.; Vale, N.; Gonçalves, J.; Queiroz, G. Microglia P2Y13 receptors prevent astrocyte proliferation mediated by P2Y1 receptors. Front. Pharmacol. 2018, 9, 418.
- Dias, L.; Madeira, D.; Dias, R.; Tomé, Â.R.; Cunha, R.A.; Agostinho, P. Aβ1-42 peptides blunt the adenosine A2A receptor-mediated control of the interplay between P2X7 and P2Y1 receptors mediated calcium responses in astrocytes. Cell. Mol. Life Sci. 2022, 79, 457.
