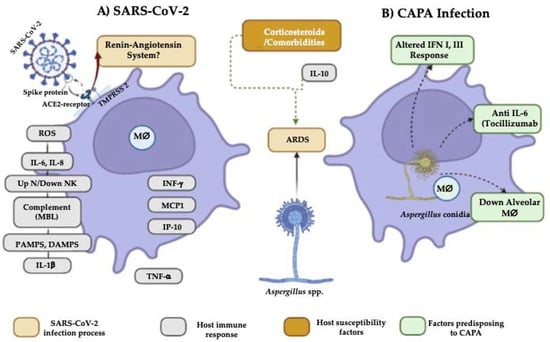
COVID-19-associated pulmonary aspergillosis (CAPA) has had a high incidence. In addition, it has been associated with prolonged hospital stays, as well as several predisposing risk factors, such as fungal factors (nosocomial organism, the size of the conidia, and the ability of the Aspergillus spp. of colonizing the respiratory tract), environmental factors (remodeling in hospitals, use of air conditioning and negative pressure in intensive care units), comorbidities, and immunosuppressive therapies. In addition to these factors, SARS-CoV-2 per se is associated with significant dysfunction of the patient’s immune system, involving both innate and acquired immunity, with reduced CD4+ and CD8+ T cell counts and cytokine storm.
- Aspergillus
- SARS-CoV-2
- CAPA
- interaction
- coinfection
1. Comorbidities
2. Changes in the Immune Response That Predispose to CAPA
3. Influence of Antifungals on the Aspergillus-SARS-CoV-2 Interaction
References
- Pasquier, G.; Bounhiol, A.; Robert Gangneux, F.; Zahar, J.; Gangneux, J.P.; Novara, A.; Bougnoux, M.; Dannaoui, E. A review of significance of Aspergillus detection in airways of ICU COVID-19 patients. Mycoses 2021, 64, 980–988.Arastehfar, A.; Carvalho, A.; van de Veerdonk, F.L.; Jenks, J.D.; Koehler, P.; Krause, R.; Cornely, O.A.; SPerlin, D.; Lass-Flör, C.; Hoenig, M. COVID-19 associated pulmonary aspergillosis (CAPA)-From Immunology to Treatment. J. Fungi Basel Switz. 2020, 6, 91. [CrossRef] [PubMed]
- Feys, S.; Almyroudi, M.P.; Braspenning, R.; Lagrou, K.; Spriet, I.; Dimopoulos, G.; Wauters, J.A. Visual and comprehensive review on COVID-19-Associated Pulmonary Aspergillosis (CAPA). J. Fungi 2021, 7, 1067.Lai, C.C.; Yu, W.L. Covid-19 associated with pulmonary aspergillosis: A literature review. J. Microbiol. Immunol. Infect. 2021, 54, 46–53. [CrossRef]
- Janssen, N.A.F.; Nyga, R.; Vanderbeke, L.; Jacobs, C.; Ergün, M.; Buil, J.B.; van Dijk, K.; Altenburg, J.; Bouman, C.S.C.; van der Spoel, H.I.; et al. Multinational observational cohort study of COVID-19-associated pulmonary aspergillosis. Emerg. Infect. Dis. 2021, 27, 2892–2898.Bartoletti, M.; Pascale, R.; Cricca, M.; Rinaldi, M.; Maccaro, A.; Bussini, L.; Fornaro, G.; Tonetti, T.; Pizzilli, G.; Francalanci, E.; et al. Epidemiology of invasive pulmonary Aspergillosis among intubated patients with COVID-19: A prospective study. Clin. Infect. Dis. 2021, 73, e3606–e3614. [CrossRef]
- Pasquier, G.; Bounhiol, A.; Robert Gangneux, F.; Zahar, J.; Gangneux, J.P.; Novara, A.; Bougnoux, M.; Dannaoui, E. A review of significance of Aspergillus detection in airways of ICU COVID-19 patients. Mycoses 2021, 64, 980–988. Machado, M.; Valerio, M.; Álvarez-Uría, A.; Olmedo, M.; Veintimilla, C.; Padilla, B.; De la Villa, S.; Guinea, J.; Escribano, P.; Ruiz-Serrano, M.J.; et al. Invasive pulmonary aspergillosis in the COVID-19 era: An expected new entity. Mycoses 2020, 64, 132–143. [CrossRef] [PubMed]
- Gregoire, E.; Pirotte, B.F.; Moerman, F.; Altdorfer, A.; Gaspard, L.; Firre, E.; Moonen, M.; Fraipont, V.; Ernst, M.; Darcis, G. Incidence and risk factors of COVID-19-associated pulmonary aspergillosis in intensive care unit-A monocentric retrospective observational study. Pathogens 2021, 10, 1370. Pasquier, G.; Bounhiol, A.; Robert Gangneux, F.; Zahar, J.; Gangneux, J.P.; Novara, A.; Bougnoux, M.; Dannaoui, E. A review of significance of Aspergillus detection in airways of ICU COVID-19 patients. Mycoses 2021, 64, 980–988. [CrossRef]
- Xu, J.; Yang, X.; Lv, Z.; Zhou, T.; Liu, H.; Zou, X.; Cao, F.; Zhang, L.; Liu, B.; Chen, W.; et al. Risk factors for invasive aspergillosis in patients admitted to the intensive care unit with coronavirus disease 2019: A multicenter retrospective study. Front. Med. 2021, 8, 753659. Feys, S.; Almyroudi, M.P.; Braspenning, R.; Lagrou, K.; Spriet, I.; Dimopoulos, G.; Wauters, J.A. Visual and comprehensive review on COVID-19-Associated Pulmonary Aspergillosis (CAPA). J. Fungi 2021, 7, 1067. [CrossRef]
- Sagris, D.; Papanikolaou, A.; Kvernland, A.; Korompok Frontera, E.J.A.; Troxe, A.B.; Gavriatopoulou, M.; Milionis, H.; Lip, G.Y.H.; Michel, P.; Yaghi, S.; et al. COVID-19 and ischemic stroke. Eur. J. Neurol. 2021, 28, 3826–3836. Janssen, N.A.F.; Nyga, R.; Vanderbeke, L.; Jacobs, C.; Ergün, M.; Buil, J.B.; van Dijk, K.; Altenburg, J.; Bouman, C.S.C.; van der Spoel, H.I.; et al. Multinational observational cohort study of COVID-19-associated pulmonary aspergillosis. Emerg. Infect. Dis. 2021, 27, 2892–2898. [CrossRef] [PubMed]
- Videla, C.G.; Agustina, M.; de la Iglesia Niveyro, P.X.; Ciarrocchi Nicolas, M. Muerte cerebral debida a aspergilosis cerebral en un paciente de COVID-19. Rev. Esp. Anestesiol. Y Reanim. 2022; 69, 592–596. Gregoire, E.; Pirotte, B.F.; Moerman, F.; Altdorfer, A.; Gaspard, L.; Firre, E.; Moonen, M.; Fraipont, V.; Ernst, M.; Darcis, G. Incidence and risk factors of COVID-19-associated pulmonary aspergillosis in intensive care unit-A monocentric retrospective observational study. Pathogens 2021, 10, 1370. [CrossRef] [PubMed]
- Bhotla, H.K.; Balasubramanian, B.; Meyyazhagan, A.; Pushparaj, K.; Easwaran, M.; Pappusamy, M.; Robert, A.A.; Arumugam, V.A.; Tsibizova, V.; Alfalih, A.M.; et al. Opportunistic mycoses in COVID-19 patients/survivors: Epidemic inside a pandemic. J. Infect. Public Health 2021, 14, 1720–1726. Xu, J.; Yang, X.; Lv, Z.; Zhou, T.; Liu, H.; Zou, X.; Cao, F.; Zhang, L.; Liu, B.; Chen, W.; et al. Risk factors for invasive aspergillosis in patients admitted to the intensive care unit with coronavirus disease 2019: A multicenter retrospective study. Front. Med. 2021, 8, 753659. [CrossRef
- Feys, S.; Almyroudi, M.P.; Braspenning, R.; Lagrou, K.; Spriet, I.; Dimopoulos, G.; Wauters, J.A. Visual and comprehensive review on COVID-19-Associated Pulmonary Aspergillosis (CAPA). J. Fungi 2021, 7, 1067. Sagris, D.; Papanikolaou, A.; Kvernland, A.; Korompok Frontera, E.J.A.; Troxe, A.B.; Gavriatopoulou, M.; Milionis, H.; Lip, G.Y.H.; Michel, P.; Yaghi, S.; et al. COVID-19 and ischemic stroke. Eur. J. Neurol. 2021, 28, 3826–3836. [CrossRef] [PubMed]
- Salazar, F.; Bignell, E.; Brown, G.D.; Cook, P.C.; Warris, A. Pathogenesis of respiratory viral and fungal coinfections. Clin. Microbiol. Rev. 2022, 35, e00094-21. Videla, C.G.; Agustina, M.; de la Iglesia Niveyro, P.X.; Ciarrocchi Nicolas, M. Muerte cerebral debida a aspergilosis cerebral en un paciente de COVID-19. Rev. Esp. Anestesiol. Y Reanim. 2022, 6, 592–596. [CrossRef]
- Aiello, A.; Farzaneh, F.; Candore, G.; Caruso, C.; Davinelli, S.; Gambino, C.M.; Ligotti, M.E.; Zareian, N.; Accardi, G. Immunosenescence and its hallmarks: How to oppose aging strategically? A review of potential options for therapeutic intervention. Front. Immunol. 2019, 10, 2247. Bhotla, H.K.; Balasubramanian, B.; Meyyazhagan, A.; Pushparaj, K.; Easwaran, M.; Pappusamy, M.; Robert, A.A.; Arumugam, V.A.; Tsibizova, V.; Alfalih, A.M.; et al. Opportunistic mycoses in COVID-19 patients/survivors: Epidemic inside a pandemic. J. Infect. Public Health 2021, 14, 1720–1726. [CrossRef]
- Kadambari, S.; Klenerman, P.; Pollard, A.J. Why the elderly appear to be more severely affected by COVID-19: The potential role of immunosenescence and CMV. Rev. Med. Virol. 2020, 30, e2144. Verweij, P.E.; Brüggemann, R.J.M.; Azoulay, E.; Bassetti, M.; Blot, S.; Buil, J.B.; Calandra, T.; Chiller, T.; Clancy, C.J.; Cornely, O.A.; et al. Taskforce report on the diagnosis and clinical management of COVID-19 associated pulmonary aspergillosis. Intensiv. Care Med. 2021, 47, 819–834. [CrossRef] [PubMed]
- Wang, Y.; Pang, S.C.; Yang, Y. A potential association between immunosenescence and high COVID-19 related mortality among elderly patients with cardiovascular diseases. Immun. Ageing 2021, 18, 25. Salazar, F.; Bignell, E.; Brown, G.D.; Cook, P.C.; Warris, A. Pathogenesis of respiratory viral and fungal coinfections. Clin. Microbiol. Rev. 2022, 35, e00094-21. [CrossRef] [PubMed]
- Holman, N.; Knighton, P.; Kar, P.; O’Keefe, J.; Curley, M.; Weaver, A.; Barron, E.; Bakhai, C.; Khunti, K.; Wareham, N.J.; et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: A population-based cohort study. Lancet Diabetes Endocrinol. 2020, 8, 823–833. Aiello, A.; Farzaneh, F.; Candore, G.; Caruso, C.; Davinelli, S.; Gambino, C.M.; Ligotti, M.E.; Zareian, N.; Accardi, G. Immunose nescence and its hallmarks: How to oppose aging strategically? A review of potential options for therapeutic intervention. Front. Immunol. 2019, 10, 2247. [CrossRef]
- Lim, S.; Bae, J.H.; Kwon, H.S.; Nauck, M.A. COVID-19 and diabetes mellitus: From pathophysiology to clinical management. Nat. Rev. Endocrinol. 2021, 17, 11–30. Kadambari, S.; Klenerman, P.; Pollard, A.J. Why the elderly appear to be more severely affected by COVID-19: The potential role of immunosenescence and CMV. Rev. Med. Virol. 2020, 30, e2144. [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. Wang, Y.; Pang, S.C.; Yang, Y. A potential association between immunosenescence and high COVID-19 related mortality among elderly patients with cardiovascular diseases. Immun. Ageing 2021, 18, 25. [CrossRef]
- Sudhakar, M.; Winfred, S.B.; Meiyazhagan, G.; Venkatachalam, D.P. Mechanisms contributing to adverse outcomes of COVID-19 in obesity. Mol. Cell Biochem. 2022, 477, 1155–1193. Holman, N.; Knighton, P.; Kar, P.; O’Keefe, J.; Curley, M.; Weaver, A.; Barron, E.; Bakhai, C.; Khunti, K.; Wareham, N.J.; et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: A population-based cohort study. Lancet Diabetes Endocrinol. 2020, 8, 823–833. [CrossRef]
- Wang, B.; Li, R.; Lu, Z.; Huang, Y. Does comorbidity increase the risk of patients with COVID-19: Evidence from meta-analysis. Aging 2020, 12, 6049–6057. Lim, S.; Bae, J.H.; Kwon, H.S.; Nauck, M.A. COVID-19 and diabetes mellitus: From pathophysiology to clinical management. Nat. Rev. Endocrinol. 2021, 17, 11–30. [CrossRef]
- Chong, W.H.; Saha, B.K.; Neu, K.P. Comparing the clinical characteristics and outcomes of COVID-19-associate pulmonary aspergillosis (CAPA): A systematic review and meta-analysis. Infection 2022, 50, 43–56. Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [CrossRef]
- Apostolopoulou, A.; Garrigos, Z.E.; Vijayvargiya, P.; Lerner, A.H.; Farmakiotis, D. Invasive pulmonary aspergillosis in patients with SARS-CoV-2 infection: A systematic review of the literature. Diagnostics 2020, 10, 807. Sudhakar, M.; Winfred, S.B.; Meiyazhagan, G.; Venkatachalam, D.P. Mechanisms contributing to adverse outcomes of COVID-19 in obesity. Mol. Cell Biochem. 2022, 477, 1155–1193. [CrossRef] [PubMed]
- Borger, P.; Koeter, G.H.; Timmerman, A.J.; Vellenga, A.; Tomee, J.F.; Kauffman, H.F. Protease from Aspergillus fumigatus induce interleukin (IL)-6 and IL-8 producin in airway epithelial cell lines by transcriptional mechanisms. J. Infect. Dis. 1999, 180, 1267–1274. Wang, B.; Li, R.; Lu, Z.; Huang, Y. Does comorbidity increase the risk of patients with COVID-19: Evidence from meta-analysis. Aging 2020, 12, 6049–6057. [CrossRef] [PubMed]
- Arastehfar, A.; Carvalho, A.; van de Veerdonk, F.L.; Jenks, J.D.; Koehler, P.; Krause, R.; Cornely, O.A.; SPerlin, D.; Lass-Flör, C.; Hoenig, M. COVID-19 associated pulmonary aspergillosis (CAPA)-From Immunology to Treatment. J. Fungi Basel Switz. 2020, 6, 91. Chong, W.H.; Saha, B.K.; Neu, K.P. Comparing the clinical characteristics and outcomes of COVID-19-associate pulmonary aspergillosis (CAPA): A systematic review and meta-analysis. Infection 2022, 50, 43–56. [CrossRef]
- Lai, C.C.; Yu, W.L. Covid-19 associated with pulmonary aspergillosis: A literature review. J. Microbiol. Immunol. Infect. 2021, 54, 46–53. Apostolopoulou, A.; Garrigos, Z.E.; Vijayvargiya, P.; Lerner, A.H.; Farmakiotis, D. Invasive pulmonary aspergillosis in patients with SARS-CoV-2 infection: A systematic review of the literature. Diagnostics 2020, 10, 807. [CrossRef]
- Machado, M.; Valerio, M.; Álvarez-Uría, A.; Olmedo, M.; Veintimilla, C.; Padilla, B.; De la Villa, S.; Guinea, J.; Escribano, P.; Ruiz-Serrano, M.J.; et al. Invasive pulmonary aspergillosis in the COVID-19 era: An expected new entity. Mycoses 2020, 64, 132–143. Borger, P.; Koeter, G.H.; Timmerman, A.J.; Vellenga, A.; Tomee, J.F.; Kauffman, H.F. Protease from Aspergillus fumigatus induce interleukin (IL)-6 and IL-8 producin in airway epithelial cell lines by transcriptional mechanisms. J. Infect. Dis. 1999, 180, 1267–1274. [CrossRef]
- Kuri-Cervantes, L.; Pampena, M.B.; Meng, W.; Rosenfeld, A.M.; Ittner, C.A.G.; Weisman, A.R.; Agyekum, R.S.; Mathew, D.; Baxter, A.E.; Vella, L.A.; et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci. Immunol. 2020, 5, eabd7114. Kuri-Cervantes, L.; Pampena, M.B.; Meng, W.; Rosenfeld, A.M.; Ittner, C.A.G.; Weisman, A.R.; Agyekum, R.S.; Mathew, D.; Baxter, A.E.; Vella, L.A.; et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci. Immunol. 2020, 5, eabd7114. [CrossRef] [PubMed]
- Lozada-Requena, I.; Núñez Ponce, C. COVID-19: Respuesta inmune y perspectivas terapéuticas. Rev. Peru Med. Exp. Salud Pública 2020, 37, 312–319. Lozada-Requena, I.; Núñez Ponce, C. COVID-19: Respuesta inmune y perspectivas terapéuticas. Rev. Peru Med. Exp. Salud Pública 2020, 37, 312–319. [CrossRef]
- Giudicessi, J.R.; Noseworthy, P.A.; Friedman, P.A.; Ackerman, M.J. Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmaco-therapies for Coronavirus Disease 19 (COVID-19). Mayo Clin. Proc. 2020, 95, 1213–1221. Giudicessi, J.R.; Noseworthy, P.A.; Friedman, P.A.; Ackerman, M.J. Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmaco-therapies for Coronavirus Disease 19 (COVID-19). Mayo Clin. Proc. 2020, 95, 1213–1221. [CrossRef]
- Varshneya, M.; Irurzun-Arana, I.; Campana, C.; Dariolli, R.; Gutierrez, A.; Pullinger, T.K.; Sobie, E.A. Investigational treatments for COVID-19 may increase ventricular arrhythmia risk through drug interactions. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 100–107. Varshneya, M.; Irurzun-Arana, I.; Campana, C.; Dariolli, R.; Gutierrez, A.; Pullinger, T.K.; Sobie, E.A. Investigational treatments for COVID-19 may increase ventricular arrhythmia risk through drug interactions. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 100–107. [CrossRef]
- Jenks, J.D.; Mehta, S.R.; Hoenigl, M. Broad spectrum triazoles for invasive mould infections in adults: Which drug and when? Med. Mycol. 2019, 57, S168–S178. Jenks, J.D.; Mehta, S.R.; Hoenigl, M. Broad spectrum triazoles for invasive mould infections in adults: Which drug and when? Med. Mycol. 2019, 57, S168–S178. [CrossRef] [PubMed]
- Gonçalves, S.S.; Stchigel, A.; Cano, J.; Guarro, J.; Colombo, A.L. In vitro antifungal susceptibility of clinically relevant species belonging to Aspergillus section Flavi. Antimicrob. Agents Chemother. 2013, 57, 1944–1947. Gonçalves, S.S.; Stchigel, A.; Cano, J.; Guarro, J.; Colombo, A.L. In vitro antifungal susceptibility of clinically relevant species belonging to Aspergillus section Flavi. Antimicrob. Agents Chemother. 2013, 57, 1944–1947. [CrossRef]
- Aruanno, M.; Glampedakis, E.; Lamoth, F. Echinocandins for the treatment of invasive aspergillosis: From laboratory to bedside. Antimicrob. Agents Chemother. 2019, 63, e00399-19. Aruanno, M.; Glampedakis, E.; Lamoth, F. Echinocandins for the treatment of invasive aspergillosis: From laboratory to bedside. Antimicrob. Agents Chemother. 2019, 63, e00399-19. [CrossRef] [PubMed]
- Ergün, M.; Brüggemann, R.J.M.; Alanio, A.; Delliére, S.; van Arkel, A.; Bentvelsen, R.G.; Rijpstra, T.; van der Sar-van der Brugge, S.; Lagrou, K.; Janssen, N.A.F.; et al. Aspergillus test profiles and mortality in critically Ill COVID-19 patients. J. Clin. Microbiol. 2021, 59, e01229-21. Ergün, M.; Brüggemann, R.J.M.; Alanio, A.; Delliére, S.; van Arkel, A.; Bentvelsen, R.G.; Rijpstra, T.; van der Sar-van der Brugge, S.; Lagrou, K.; Janssen, N.A.F.; et al. Aspergillus test profiles and mortality in critically Ill COVID-19 patients. J. Clin. Microbiol. 2021, 59, e01229-21. [CrossRef] [PubMed]
- Itoh, K.; Tsutani, H.; Iwasaki, H. Multifaceted efficacy of caspofungin against fungal infections in COVID-19 patients. Med. Hypotheses 2022, 164, 110876. Itoh, K.; Tsutani, H.; Iwasaki, H. Multifaceted efficacy of caspofungin against fungal infections in COVID-19 patients. Med. Hypotheses 2022, 164, 110876. [CrossRef] [PubMed]
- Mohamed, A.; Rogers, T.R.; Talento, A.F. COVID-19 Associated invasive pulmonary aspergillosis: Diagnostic and therapeutic challenges. J. Fungi. 2020, 6, 115. Mohamed, A.; Rogers, T.R.; Talento, A.F. COVID-19 Associated invasive pulmonary aspergillosis: Diagnostic and therapeutic challenges. J. Fungi. 2020, 6, 115. [CrossRef] [PubMed]
- Hatzl, S.; Reisinger, A.C.; Posch, F.; Prattes, J.; Stradner, M.; Pilz, S.; Eller, P.; Schoerghuber, M.; Toller, W.; Gorkiewicz, G.; et al. Antifungal prophylaxis 815 for prevention of COVID-19-associated pulmonary aspergillosis in critically ill patients: An observational study. Crit. Care 2021, 25, 335. Hatzl, S.; Reisinger, A.C.; Posch, F.; Prattes, J.; Stradner, M.; Pilz, S.; Eller, P.; Schoerghuber, M.; Toller, W.; Gorkiewicz, G.; et al. Antifungal prophylaxis 815 for prevention of COVID-19-associated pulmonary aspergillosis in critically ill patients: An observational study. Crit. Care 2021, 25, 335. [CrossRef]
