Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 5 by Beatrix Zheng and Version 4 by Beatrix Zheng.
Natural killer (NK) cells are a part of the native immune response to cancer. NK cell-based immunotherapies are an emerging strategy to kill tumor cells.
- nanoparticles
- NK cells
- NK cell-mediated immunotherapy
1. Introduction
Our immune system is a living, breathing medicine that protects the body from various diseases, including cancer [1][2]. Three types of immunity have been identified: innate, adaptive, and passive immunity. Both innate and adaptive immunity systemically fight against the development of neoplastic cells and bacterial and viral infections in the body on a constant basis, which we term “immunosurveillance” [3].
The innate immune system can identify various pathogenic organisms through a molecular immunosurveillance process called pathogen-assisted molecular patterns or “PAMPs” that are not produced by the host (e.g., polysaccharides) [4][5][6]. PAMPs are evolutionarily conserved molecules that are present only in pathogenic organisms. They are recognized by the binding of PAMPs to cell surface and endosomal receptors (e.g., Toll-like receptors) [7]. Immune cells such as macrophages, dendritic cells, Natural NKkiller(NK) cells, and some epithelial cells, which form a network of immunosurveillance processes, recognize PAMPs [8]. The second mechanism by which the immune system is alerted during endogenous immunosurveillance is through danger-assisted molecular patterns or “DAMPs” (e.g., calreticulin or CD91) [4].
Cells that are physically, chemically, and biologically stressed or damaged/dying, which can be due to pathogens or other stressors (e.g., chemo or radiation), emit danger signals called DAMPs that alert the immune system [4]. DAMPs can be highly diverse, and their diversity is responsible for immune stimulation, immune modulation, and producing systemic antitumor immunity [4]. Together, PAMPs and alarmins form a network family of DAMPs that aids the immunosurveillance process [9]. Still, cancer cells evade the immunosurveillance process in several ways: loss of cell-adhesion antigens, impairment of cytotoxic T-lymphocytes and NK cells, generation of ligands that block recognition, and secretion of cytokines (e.g., VEGF, IL-10, and TGF-β) that inhibit maturation of dendritic cells [10][11]. In many cancers, tumor growth is supported through immunosuppression that hampers the effective antitumor response and tumor eradication. The tumor microenvironment is often “cold” or “desert-like” meaning they don’t secrete any molecules that could be identified by immune cells [12]. Additionally, tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MSDC) envelop the tumor mass, which effectively keeps the infiltration of T-cells at bay [13]. TAMs also keep the immune system suppressed through the following additional pathways: regulation of PD-1 and CTLA-4 [14], T-cell suppression and exhaustion [15], recruitment of Tregs through CCL2 [16], and promotion of inflammation of the tumor microenvironment [16]. TAMs also secrete large amounts of cytokines, such as TGF-β and IL10+, that can impair the cytotoxicity of NK cells [17]. Inflammatory cytokines induced by regulatory or suppressive immune cells promote cancer cell proliferation and suppress the antitumor immune response in the tumor microenvironment. Cells expressing indoleamine 2,3-dioxygenase (IDO) can inhibit the T cell response and lead to immunosuppression of the TME [18][19][20]. Arginase is produced by myeloid-derived suppressor cells and is released into circulation in patients with cancer [21][22]. These enzymes deplete the amino acids necessary for the proper functioning of T-cells. Together, the TME, along with immunosuppressive cells and inhibitory molecules, produces a significant barrier preventing immune attack, making it difficult to treat cancer. This explains some of the reasons for the failure of immunotherapy in many patients.
One of the mechanisms of tumor cell escape and subsequent metastasis involves immunoediting of the TME [3][23]. Cancer immunoediting is a dynamic process involving the interplay between tumor cells and immune cells where the tumor cells become less immunogenic over a period of time and develop the capability to escape [24]. Immunoediting can result in protection against cancer through immune-cell-mediated destruction of cancer cells, but it also can result in the escape of cancer cells beyond immune system control, which can lead to proliferation and metastasis. Three phases or “Es” of immunoediting have been identified: elimination, equilibrium, and escape [25] (Figure 1).
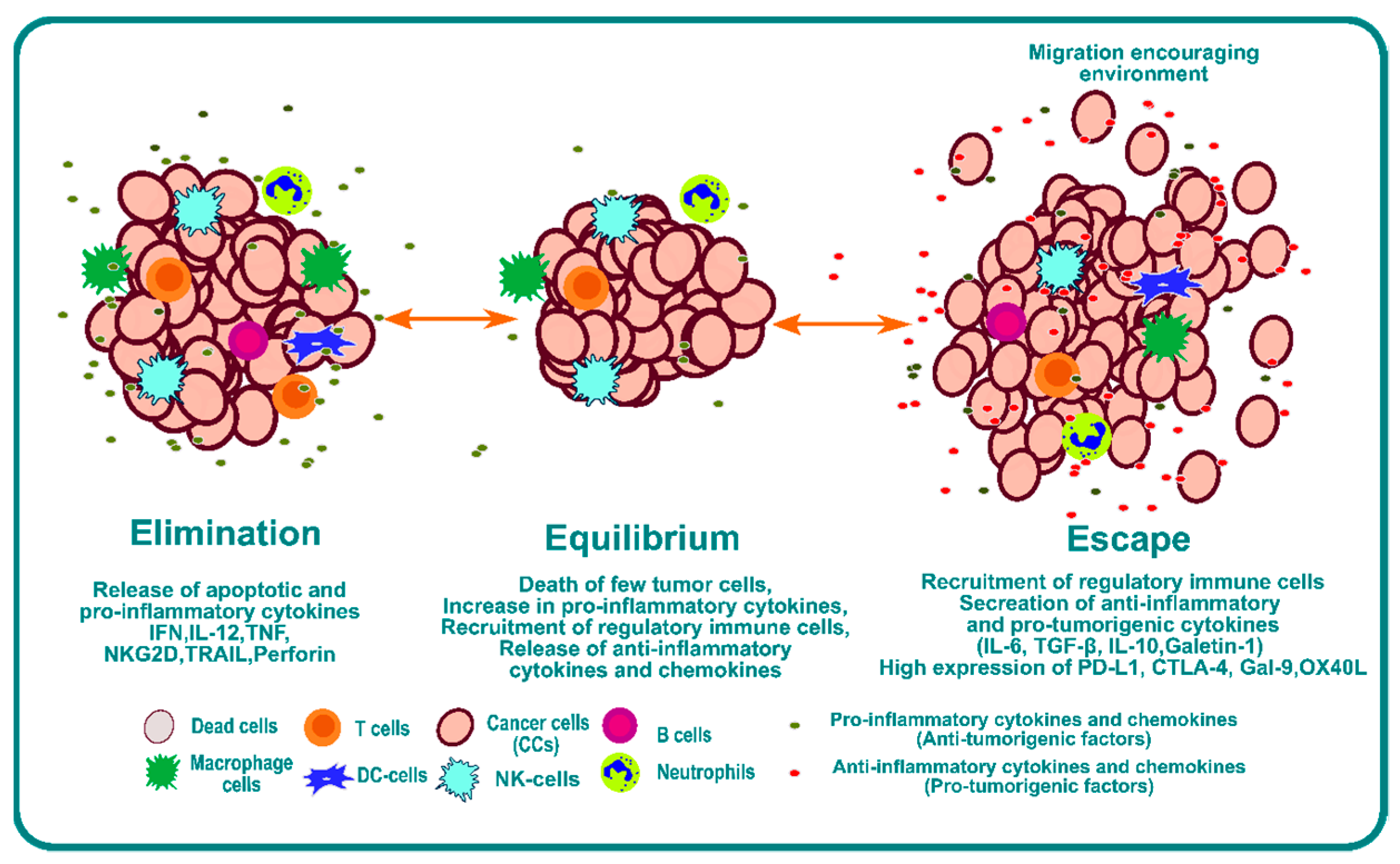
Figure 1. Schematic representation of the phases of immunoediting, namely elimination, equilibrium, and escape.
The elimination phase presents a phase where a strong immune response is observed due to a combination of both adaptive and innate immunity. This immunoediting phase is beneficial for systemic protection against cancer. Tumor cells have a high expression of surface calreticulin that acts as “eat me” signals for the dendritic cells [26][27][28][29]. Dendritic cells will process tumor antigens and present them on the surface to educate T and NKT cells, which is one of the most critical steps for the success of any form of immunotherapy [30][31]. The effector cells secrete IFN-γ; anti-angiogenic chemokines such as CXCL9, CXCL10, and CXCL11; and cytokines like IL-12 and IFN-γ that enhance macrophage polarization and NK cell activation [32]. Furthermore, Langerhans cells (LCs) secrete IL-15 and can cause the activation of NK and CD8+ T cells [33][34][35].
The second phase is the equilibrium phase. As the name suggests, it creates a microenvironment that contains a balance of pro-inflammatory cytokines and anti-inflammatory cytokines [36]. This phase of immunoediting results in dormancy of tumor growth. Nothing happens in this phase; the tumor is not eradicated, but it also does not grow. This phase is poorly understood, but it is known that the immune system keeps the tumor in a state of functional dormancy in this phase [36].
The third phase is called escape, and it occurs due to the following reasons: the loss of tumor antigens and subsequent loss of immune recognition of tumor cells [37][38]; an increase in secretion of cytokines such as TGF-β [39][40][41], IL-10 [42][43][44], and VEGF [45][46][47]; an increase in transcription factors such as STAT3 [48][49] and BACH2 [50][51]; and overexpression of molecules such as PDL1 and CTLA4, which act as a bridge to inhibit recognition by immune cells. Together, they provide an overarching barrier that prevents immune attack, and they impair the function of immune cells by inducing checkpoint blockade of the immune system. This environment provides enhanced opportunity for invasion and metastasis.
It is now known that cancers are immunogenic. The discovery of immune checkpoint blockade has revolutionized our understanding and created a new field of immuno-oncology. The initial discovery of PD-1 [52] and CTLA-4 [53] led to several promising therapeutic drugs (e.g., Ipilimumab, the first immunotherapy drug that targets CTLA4) for cancer treatment [54]. Ipilimumab blocks the CTLA-4 pathway, which is a T-cell inhibitor that results in the infiltration of cytotoxic T-cells into the TME [53]. Early investigations with Ipilimumab as a monotherapy in advanced metastatic melanoma were highly promising, showing an objective response rate of 40–60% for two years despite adverse side effects in 10–15% of patients [54][55]. The hefty price tag makes it hard to prescribe this as the first line of treatment for most individuals [55]. However, immunotherapy does not work for everyone, and only a fraction of patients benefits from immune-checkpoint-blockade-based therapies. The percentage of patients estimated to respond to checkpoint inhibitor drugs was 0.14% in 2011 and increased to 12.46% in 2018 [56]. Nanotechnology can assist with increasing the efficacy of immunotherapies in a variety of ways. The small size of nanoparticles, 10–100 nm, makes them ideal for modulating immunotherapies in a variety of ways that may not be possible using traditional antibody delivery approaches. These include delivery of immune checkpoint inhibitors (ICI) or antibodies to the TME using encapsulated nanoparticles, creation of tumor antigens through nanoparticle-mediated local ablation of cancer cells and necroptotic cell death, creation of designer nanoparticles with PAMPs and DAMPs that are customized to the TME, education of T cells and NK cells in vitro using nanoparticles based on the mutational status of the TME, delivery of pro-inflammatory cytokines using nanoparticles to neutralize the immunosuppressive environment, and many others. Thus, nanotechnology could potentially enhance immunotherapies and overcome the immunosuppressive environment in a variety of solid cancers. In this research, the researchers focus on the roles NK cells and how nanotechnology can be used to modulate NK cell activity in tumor eradication.
2. Application of Nanoparticles in NK Cell-Based Immunotherapy
The small size of nanoparticles compared to a cell is conducive for delivering drug molecules or cargo. Nanoparticles are not restricted to a delivery vehicle, and they play a pivotal role in the activation of different immune cells, including NK cells. This section summarizes the potential of nanoparticles to assist NK cell-based immunotherapy. Based on the mechanism of action or the target of the immunotherapy, NK cell-based immunotherapies are further subdivided into the following five categories: nanoparticle-assisted immunomodulation to enhance NK cell activity, nanoparticle enhancing homing of NK cells, nanoparticles delivering RNAi to enhance NK cell function, nanoparticles for genetic modification of NK cells, nanoparticles activating NKG2D receptor (Figure 2). NK cell-based nano-immunotherapies are a nascent and developing field, and these therapies might move toward the clinical phase in the coming decades. Currently, CytoSen Therapeutics and KBI Biopharma have developed nanoparticle-based NK cell therapy, and it has entered phase two of a clinical trial [57]. Phase one clinical trials are ongoing for NK cell-based treatment of esophageal cancer [58]. PRECIOUS-01 is a natural killer T cell (iNKT) activating agent that consists of threitolceramide-6 (ThrCer6, IMM60) and New York Esophageal Squamous Cell Carcinoma-1 (NY-ESO-1) cancer-testis antigen peptides encapsulated in a biodegradable polymer poly (lactic-co-glycolic acid) (PLGA) nanoparticle [58]. NY-ESO-1 is overexpressed in many advanced cancers, including lung cancers, bladder cancers, melanoma, and ovarian cancers.
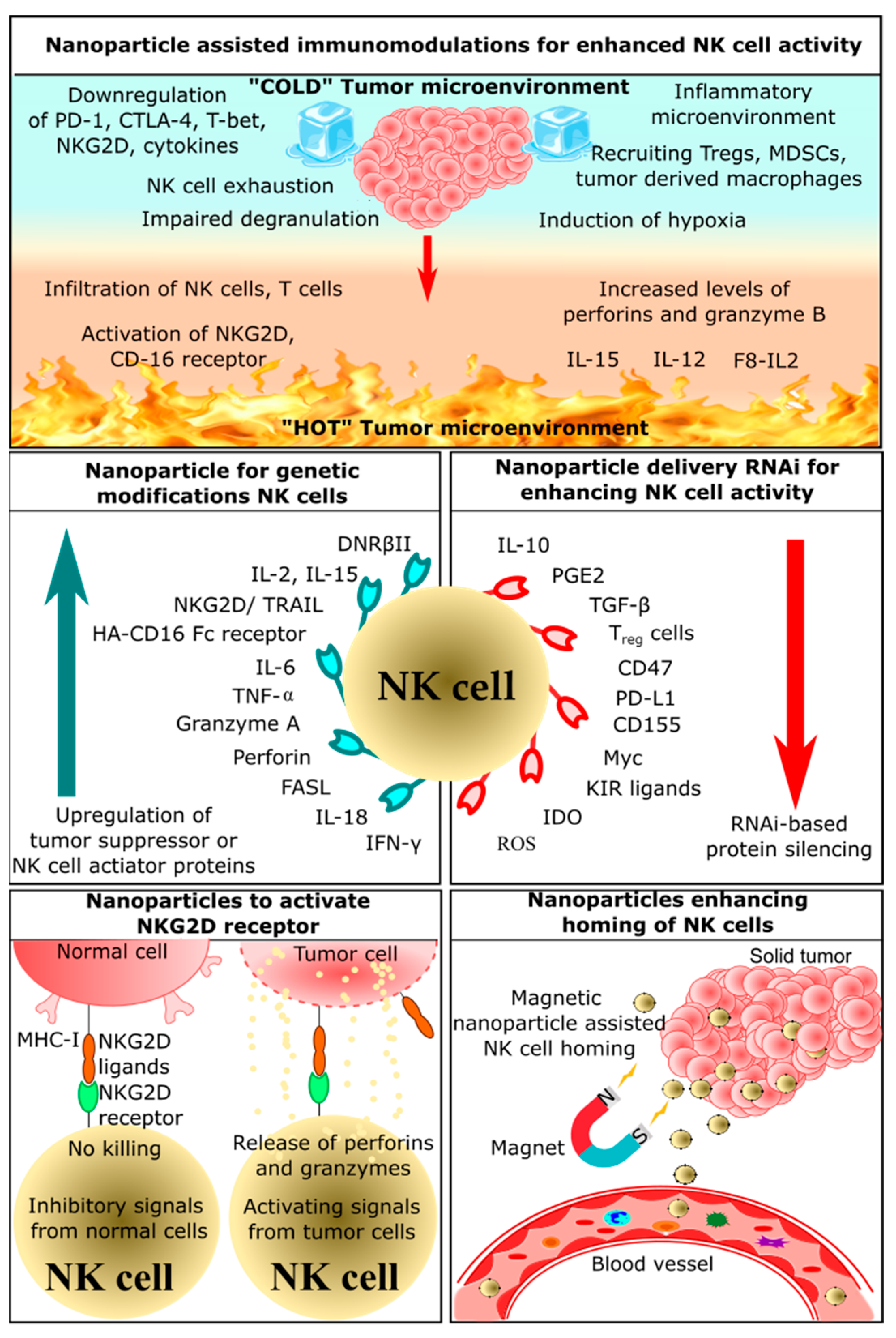
2.1. Nanoparticle-Assisted Immunomodulation for Enhanced NK Cell Activity
Immunomodulation-based cancer therapeutics have recently emerged, as this approach employs the host’s own defense mechanisms to recognize and eliminate cancerous cells. Nanoparticles acting as immunomodulators can improve therapeutic effects and overcome limitations of conventional methods of cancer treatment [62]. There is a broad scope of nanoparticles for drug delivery and an increase in immunotherapeutic efficiency. Some nanoparticles can be used to deliver anticancer drugs, chemokines, and cytokines [63][64][65]. Lipid-based nanoparticles could encapsulate these molecules and effectively deliver them to the tumor site [66][67][68]. Another strategy involves the use of surface-engineered nanoparticles; polymeric or metal-based nanoparticles can be modified such that tumor antigens or antibodies can be docked onto the surface [63][69][70].
2.2. Nanoparticles Enhancing Homing of NK Cells
Homing is a mode of signaling between homing receptors present on immune cells and homing receptor ligands on the affected tissues or tumor-secreted molecules [71]. These interactions in a tumor microenvironment could recruit more effector immune cells and enhance immune cell infiltration [71][72]. Cancer cells secrete cytokines and chemokines like TNF-α and ROS, which attracts other effector immune cells. Depending on the function of the cytokines and chemokines, different immunological signals are triggered [73]. These signals could either induce inflammation or activate cytotoxic activity. Chronic inflammation induces tumor cell proliferation, survival, and metastasis [74]. Immune cells involved in inflammation include neutrophils, macrophages, and myeloid-derived suppressor cells [75]. Some of these cytokines induce effector immune cells that could kill cancer cells by activating T cells, B cells, and NK cells [76]. NK cells have a crucial role in the cytotoxicity-mediated killing of tumor cells, and they secrete various cytokines and chemokines that could recruit other immune cells [75]. Once NK cells infiltrate the tumor microenvironment, multiple signaling pathways are triggered by a ligand-receptor interaction, which results in the release of perforin, granzymes, and apoptosis-inducing factors [77]. Hence, it is essential to induce NK cell homing in the tumor microenvironment for effective tumor reduction.
The use of nanoparticles, especially magnetic nanoparticles, for homing immune cells has been explored extensively. For instance, the conjugation of iron oxide nanoparticles on the surface of primary NK cells would significantly enhance the homing of NK cells in the tumor microenvironment. These nanoparticles showed significant antitumor efficacy by increasing the expression of granzymes and perforins in neuroblastoma cells compared with unmodified NK cells [78]. Moreover, the killing of cancer cells by NK cells was associated with the homing of NK cells as well as by external magnetic guidance [78]. These nanoparticles have attracted significant interest for the delivery of immunotherapeutic drugs. Similarly, immunomodulatory magnetic microspheres also increased NK cell infiltration at the tumor site [79]. These microspheres consist of iron oxide nanocubes and recombinant interferon gamma encapsulated in biodegradable poly(lactide-co-glycolide). This architecture allows sustained release of IFN-γ and sensitive MRI T2 contrast agents, which enables both the homing of immune cells and MRI imaging. These microspheres were evaluated on an orthotropic liver tumor VX2 rabbit model, which showed enhanced proliferation and infiltration of NK cells. There are various strategies being adopted for the development of nanoparticle-based NK cell homing. Some of these strategies involve delivering effector molecules such as granzymes, membrane-bound heat shock proteins, and cytokines such as IL-12 using magnetic nanoparticles, which not only enhance homing but also activate NK cells or kill tumor cells [79].
2.3. Nanoparticles Delivering RNAi for Enhancing NK Cells Activity
RNA effectors such as siRNA, miRNA, and shRNA could silence specific genes, which could alter genomic function and enhance antitumor activity. The use of these RNA effectors to enhance NK cell activity is classified under RNAi-mediated immunotherapy [80]. A manganese dioxide (MnO2) nanoparticle system was used to deliver small interfering RNA (siRNA) targeting transforming growth factor-β receptor-2 (TGFBR2), which is known to inhibit the function of NK cells. These nanoparticles loaded with TGFBR2 siRNA protected NK cells from immunosuppression by inhibiting TGFBR2. Thus, silencing TGFBR2 in NK cells made the tumor microenvironment more immunoresponsive by activating NK cells. This suggests that these nanoparticles can be used to enhance the antitumor effects of NK cells through TGFBR2 knockdown and increased expression of IFN-γ [81]. Thus, RNAi-based adoptive NK cell therapy has great potential to improve the survival of cancer patients.
EpCAM (epithelial cell adhesion molecule)-targeted cationic liposomes containing si-CD47 and si-PD-L1 were used to knock down immunosuppressive CD47 and PD-L1 [82]. These liposomes effectively prevented the growth of tumors and reduced lung metastasis in 4T1 tumor-bearing mice. These siRNAs could slightly increase the percentage of NK cells, promote the NK cell response, and also increase antibody production. It was also reported that this dual blockade of innate and adaptive immune checkpoint increased the expression of IFN-γ and IL-6 in vivo and in vitro. This suggests that this dual blocking system can be employed to stimulate both adaptive and innate immunity to fight breast cancer [82]. Similarly, another study utilized cationic lipid-assisted nanoparticles encapsulated with siCD155. These nanoparticles were efficient in delivering siCD155 to B16-F10 melanoma cells and macrophages in vitro and in vivo [83]. Downregulation of CD155 promoted the activation of NK and T cells while inhibiting the proliferation of melanoma cells. This suggests that nanoparticle-delivered siCD155 can be used to inhibit melanoma cell proliferation and reprogram the tumor microenvironment with proliferated NK cells and T cells [83].
A novel cocktail strategy was developed by combining NK cell-derived exosomes with miRNA-loaded biomimetic nanoparticles for targeting and therapeutic delivery of miRNA to neuroblastoma cells. NK cell-derived exosomes induced miRNA-loaded nanoparticles to leave systemic circulation and concentrate in tumor cells. One of the major advantages of using NK cell-derived exosomes is that these exosomes might have tumor-specific accumulation and may not be cytotoxic to normal tissues. It was demonstrated that when mice bearing CHLA-255-luc-induced tumors were treated with this cocktail, tumor growth inhibition was observed, which involved synergistic activity between exosomes and miRNA-loaded nanoparticles [65]. Another NK cell-derived exosome was utilized by Neviani et al., where they conjugated NK cell-derived exosomes with miRNA-186. These exosomes exhibited significant cytotoxicity against MYCN gene-amplified neuroblastoma cell lines. Moreover, the cytotoxicity was dependent on the expression of miRNA-186. In vitro studies revealed that these exosomes induced downregulation of TGF-β, which is involved in immune escape. These results suggested that NK cell-derived exosomes loaded with miRNA-186 are a promising therapeutic to promote cytotoxicity of NK cells and block immune escape by tumor cells [84].
2.4. Nanoparticles for Genetic Modification of NK Cells
Due to the dynamic role of NK cells in tumor identification and surveillance, adoptive immunotherapy is being developed as a next-generation therapeutic tool. Various strategies have been employed to improve the efficiency and number of NK cells in the tumor microenvironment. Understanding NK cell biology and its interactions with the tumor microenvironment enable the modification of NK cells for better and relevant NK cell immunotherapy [85]. Nanoparticle-based delivery of chimeric antigen receptor genes to patient-derived NK cells could lead to the development of CAR-NK cell-based therapy (Figure 3).
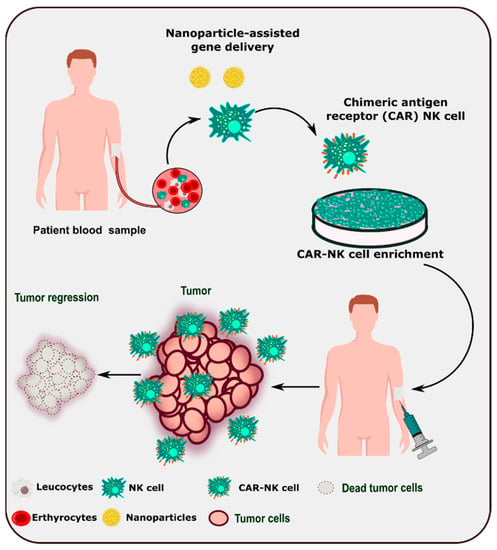
Figure 3. Schematic representation of nanoparticle-assisted development of CAR-NK cell-based therapy.
Chitosan nanoparticles loaded with IL-2 and NKG2D genes activated NK cells and cytotoxic T cells in vitro [86]. They also showed enhanced tumor accumulation due to the enhanced permeability and retention (EPR) effect and the gathering of lymphocytes in the tumor microenvironment [86]. Consistent with the in vitro results, reduced tumor volumes and improved survival time was observed in CT-26 tumor-bearing mice. These results suggested that nanoparticle-mediated delivery of IL-21 stimulated the antitumor effects of NK cells efficiently, which led to enhanced antitumor activity [86]. In another independent study, chitosan nanoparticles successfully delivered IL-15 and NKG2D genes into cancer cells [87]. These nanoparticles, containing genes for the NKG2D-IL-15 fusion protein, bind to the NKG2D receptor of cytotoxic T cells and NK cells and activate them. IL-15 and NKG2D fusion proteins enhanced the antitumor immune response in B16BL6 melanoma cells by activating >5% of cytotoxic T cells and >50% of NK cells. Moreover, they also showed reduced tumor volume and enhanced survival time in B16BL6 tumor-bearing mice. This suggested that these nanoparticles can be used as a fusion gene vaccine for immunomodulation and tumor growth suppression [87].
An effective method to destroy tumor cells is to use engineered NK cells that consist of dendrimer-entrapped gold nanoparticles containing human ferritin heavy chain (hFTH1) gene-transfected NK cells [88]. These PEG-modified dendrimer-entrapped gold nanoparticles efficiently provided high-quality imaging of transfected NK cells. This system has hFTH1 transfected effectively at a ratio of 5:1 to allow magnetic resonance imaging (MRI) of NK-92 cells and breast cancer cells. Furthermore, these nanoparticles guided NK cells toward the tumor environment for efficient gene therapy in 4T1 tumor-bearing mice. It was suggested that this system could be an efficient vector for genes and also for real-time monitoring with MRI [88]. Similarly, a multi-kinase inhibitor TUS2 (Tumor suppressor candidate 2) gene was delivered using nanovesicles [89]. TUS2 contributes to significant tumor growth reduction in a Kras-mutant syngeneic mouse lung cancer model. Furthermore, it was experimentally found that it increases the levels of circulating and splenic NK cells and CD8+ T cells, decreases the action of Treg cells and MDSCs, and reduces some of the checkpoint receptors such as PD-1, CTLA-4, and TIM-3 [89]. Later in the same research, the anti-PD-1 antibody and the TUS2 plasmid were loaded in the nanovesicle, which then revealed synergistic action against tumor cells via enhanced cytokine-based NK cell activation. A multifunctional magnetic nanoparticle system was synthesized, and it has been shown to be capable of enhancing NK cell function and tracking nanoparticles using magnetic resonance and fluorescence imaging. These multifunctional nanoparticles were designed by applying cationic polydopamine (PDA) coating and plasmid DNA to the surface of magnetic nanoparticles. The magnetic core enabled MRI of NK cells, and the cationic layer enabled them to serve as plasmid DNA carriers. This system showed better cytocompatibility and induced the expression of EGFR targeting chimeric antigen receptors. In vivo results showed that tumor volumes were reduced when treated with these multifunctional nanoparticles in the MDA-MB-231 xenograft mice model. This shows the excellent ability of these cytocompatible multifunctional nanoparticles to potentiate NK cell-mediated antitumor activity and to enable in vivo monitoring [90].
2.5. Nanoparticles Activating NKG2D Receptor
NKG2D is a C-type lectin-like activating receptor expressed on NK cells, NKT cells, and cytotoxic T cells. The ligands of the NKG2D receptor-retinoic acid early induced transcript-1 (RAE-1), H60, UL-16 binding protein like transcript-1, MHC-I chain-related protein A, and ULBPs. These ligands could initiate the NKG2D signaling pathway, which results in the activation, proliferation, and expansion of immune cells [86]. Many researchers have explored these ligands and other novel methods to activate NK cells.
NKG2D receptors initiate ITAM (tyrosine-based activation motif) signaling. Once the signal is triggered, co-stimulatory molecules CD28 and ICOS are triggered and lead to a cascade of reactions that culminate in the activation of PI3K, including reactions involving transcription factors [91]. Activation of these pathways requires targeted delivery of NKG2D ligands or certain cytokines, and nanoparticles mediate the delivery of these ligands. For instance, nanoemulsion of a TGF-β inhibitor and selenocysteine increases the lytic capability of NK cells by sensitizing NKG2D ligands [92]. The TGF-β inhibitor effectively restricted the TGF-β/TGF-β RI/Smad2/3 signaling pathway, which increased the concentration of NKG2D ligands on the tumor cell surface. Selenocysteine supports the expression of NKG2D receptors and suppresses PD-1 expression in γδ T cells [92]. Thus, these combinations enhance the activity of NK cells and act as an appropriate example of adoptive immunotherapy. Some of the other strategies involved are the use of zinc-doped vascular endothelial growth factor (VEGF) receptor-targeted super magnetic nanoparticles, which not only activate NK cells but also trigger magnetic hyperthermia [93].
References
- Parkin, J.; Cohen, B. An overview of the immune system. Lancet 2001, 357, 1777–1789.
- Delves, P.J.; Roitt, I.M. The Immune System. N. Engl. J. Med. 2000, 343, 37–49.
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The Immunobiology of Cancer Immunosurveillance and Immunoediting. Immunity 2004, 21, 137–148.
- Garg, A.D.; Dudek, A.M.; Agostinis, P. Cancer immunogenicity, danger signals, and DAMPs: What, when, and how? BioFactors 2013, 39, 355–367.
- Hobohm, U.; Stanford, J.L.; Grange, J.M. Pathogen-Associated Molecular Pattern in Cancer Immunotherapy. Crit. Rev. Immunol. 2008, 28, 95–107.
- Locy, H.; DE Mey, S.L.; De Mey, W.; De Ridder, M.; Thielemans, K.; Maenhout, S.K. Immunomodulation of the Tumor Microenvironment: Turn Foe into Friend. Front. Immunol. 2018, 9, 2909.
- Kawasaki, T.; Kawai, T. Toll-Like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461.
- Chalifour, A.; Jeannin, P.; Gauchat, J.-F.; Blaecke, A.; Malissard, M.; N’Guyen, T.; Thieblemont, N.; Delneste, Y. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers α-defensin production. Blood 2004, 104, 1778–1783.
- Bianchi, M.E. DAMPs, PAMPs and alarmins: All we need to know about danger. J. Leukoc. Biol. 2006, 81, 1–5.
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.M.C.S.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015, 35, S185–S198.
- Li, Y.; Sun, R. Tumor immunotherapy: New aspects of natural killer cells. Chin. J. Cancer Res. 2018, 30, 173–196.
- Harjunpää, H.; Asens, M.L.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078.
- Petty, A.J.; Yang, Y. Tumor-associated macrophages: Implications in cancer immunotherapy. Immunotherapy 2017, 9, 289–302.
- Pu, Y.; Ji, Q. Tumor-Associated Macrophages Regulate PD-1/PD-L1 Immunosuppression. Front. Immunol. 2022, 13, 874589.
- Zhang, Z.; Liu, S.; Zhang, B.; Qiao, L.; Zhang, Y. T Cell Dysfunction and Exhaustion in Cancer. Front. Cell Dev. Biol. 2020, 8, 17.
- Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leukoc. Biol. 2009, 86, 1065–1073.
- Yang, H.-L.; Zhou, W.-J.; Chang, K.-K.; Mei, J.; Huang, L.-Q.; Wang, M.-Y.; Meng, Y.; Ha, S.-Y.; Li, D.-J.; Li, M.-Q. The crosstalk between endometrial stromal cells and macrophages impairs cytotoxicity of NK cells in endometriosis by secreting IL-10 and TGF-β. Reproduction 2017, 154, 815–825.
- Mellor, A.L.; Keskin, D.B.; Johnson, T.; Chandler, P.; Munn, D. Cells Expressing Indoleamine 2,3-Dioxygenase Inhibit T Cell Responses. J. Immunol. 2002, 168, 3771–3776.
- Zou, W.; Chen, L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008, 8, 467–477.
- Hou, D.-Y.; Muller, A.J.; Sharma, M.D.; DuHadaway, J.; Banerjee, T.; Johnson, M.; Mellor, A.L.; Prendergast, G.C.; Munn, D.H. Inhibition of Indoleamine 2,3-Dioxygenase in Dendritic Cells by Stereoisomers of 1-Methyl-Tryptophan Correlates with Antitumor Responses. Cancer Res. 2007, 67, 792–801.
- Rodríguez, P.C.; Ochoa, A.C. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: Mechanisms and therapeutic perspectives. Immunol. Rev. 2008, 222, 180–191.
- Rodriguez, P.C.; Ernstoff, M.S.; Hernandez, C.; Atkins, M.; Zabaleta, J.; Sierra, R.; Ochoa, A.C. Arginase I–Producing Myeloid-Derived Suppressor Cells in Renal Cell Carcinoma Are a Subpopulation of Activated Granulocytes. Cancer Res. 2009, 69, 1553–1560.
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The Three Es of Cancer Immunoediting. Annu. Rev. Immunol. 2004, 22, 329–360.
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New insights into cancer immunoediting and its three component phases—Elimination, equilibrium and escape. Curr. Opin. Immunol. 2014, 27, 16–25.
- Gubin, M.M.; Vesely, M.D. Cancer Immunoediting in the Era of Immuno-oncology. Clin. Cancer Res. 2022, 28, 3917–3928.
- Gameiro, S.R.; Jammeh, M.L.; Wattenberg, M.M.; Tsang, K.Y.; Ferrone, S.; Hodge, J.W. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget 2014, 5, 403–416.
- Fucikova, J.; Kline, J.P.; Galluzzi, L.; Spisek, R. Calreticulin arms NK cells against leukemia. OncoImmunology 2019, 9, 1671763.
- Porcellini, S.; Traggiai, E.; Schenk, U.; Ferrera, D.; Matteoli, M.; Lanzavecchia, A.; Michalak, M.; Grassi, F. Regulation of peripheral T cell activation by calreticulin. J. Exp. Med. 2006, 203, 461–471.
- Zitvogel, L.; Kepp, O.; Senovilla, L.; Menger, L.; Chaput, N.; Kroemer, G. Immunogenic Tumor Cell Death for Optimal Anticancer Therapy: The Calreticulin Exposure Pathway. Clin. Cancer Res. 2010, 16, 3100–3104.
- Palucka, K.; Banchereau, J. Cancer immunotherapy via dendritic cells. Nat. Cancer 2012, 12, 265–277.
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167.
- Romagnani, P.; Lasagni, L.; Annunziato, F.; Serio, M.; Romagnani, S. CXC chemokines: The regulatory link between inflammation and angiogenesis. Trends Immunol. 2004, 25, 201–209.
- Yanofsky, V.R.; Mitsui, H.; Felsen, D.; Carucci, J.A. Understanding Dendritic Cells and Their Role in Cutaneous Carcinoma and Cancer Immunotherapy. Clin. Dev. Immunol. 2013, 2013, 624123.
- Conlon, K.C.; Lugli, E.; Welles, H.C.; Rosenberg, S.A.; Fojo, A.T.; Morris, J.C.; Fleisher, T.A.; Dubois, S.P.; Perera, L.P.; Stewart, D.M.; et al. Redistribution, Hyperproliferation, Activation of Natural Killer Cells and CD8 T Cells, and Cytokine Production During First-in-Human Clinical Trial of Recombinant Human Interleukin-15 in Patients with Cancer. J. Clin. Oncol. 2015, 33, 74–82.
- Romano, E.; Rossi, M.; Ratzinger, G.; De Cos, M.-A.; Chung, D.J.; Panageas, K.S.; Wolchock, J.D.; Houghton, A.N.; Chapman, P.B.; Heller, G.; et al. Peptide-Loaded Langerhans Cells, Despite Increased IL15 Secretion and T-Cell Activation In Vitro, Elicit Antitumor T-Cell Responses Comparable to Peptide-Loaded Monocyte-Derived Dendritic Cells In Vivo. Clin. Cancer Res. 2011, 17, 1984–1997.
- Padoan, A.; Plebani, M.; Basso, D. Inflammation and Pancreatic Cancer: Focus on Metabolism, Cytokines, and Immunity. Int. J. Mol. Sci. 2019, 20, 676.
- Olson, B.M.; McNeel, D. Antigen loss and tumor-mediated immunosuppression facilitate tumor recurrence. Expert Rev. Vaccines 2012, 11, 1315–1317.
- Saleh, R.; Elkord, E. Acquired resistance to cancer immunotherapy: Role of tumor-mediated immunosuppression. Semin. Cancer Biol. 2019, 65, 13–27.
- Pardali, K.; Moustakas, A. Actions of TGF-β as tumor suppressor and pro-metastatic factor in human cancer. Biochim. Biophys. Acta Rev. Cancer. 2007, 1775, 21–62.
- Wojtowicz-Praga, S. Reversal of Tumor-Induced Immunosuppression: A New Approach to Cancer Therapy. J. Immunother. 1997, 20, 165–177.
- Gold, L.I. The role for transforming growth factor-beta (TGF-beta) in human cancer. Crit. Rev. Oncog. 1999, 10, 303–360.
- Mittal, S.K.; Cho, K.-J.; Ishido, S.; Roche, P.A. Interleukin 10 (IL-10)-mediated Immunosuppression. J. Biol. Chem. 2015, 290, 27158–27167.
- Lamichhane, P.; Karyampudi, L.; Shreeder, B.; Krempski, J.; Bahr, D.; Daum, J.; Kalli, K.R.; Goode, E.L.; Block, M.S.; Cannon, M.J.; et al. IL10 Release upon PD-1 Blockade Sustains Immunosuppression in Ovarian Cancer. Cancer Res. 2017, 77, 6667–6678.
- Frumento, G.; Piazza, T.; Di Carlo, E.; Ferrini, S. Targeting Tumor-Related Immunosuppression for Cancer Immunotherapy. Endocr. Metab. Immune Disord. Drug Targets 2006, 6, 223–237.
- Lapeyre-Prost, A.; Terme, M.; Pernot, S.; Pointet, A.-L.; Voron, T.; Tartour, E.; Taieb, J. Immunomodulatory Activity of VEGF in Cancer. Int. Rev. Cell Mol. Biol. 2017, 330, 295–342.
- Johnson, B.F.; Clay, T.M.; Hobeika, A.C.; Lyerly, H.; Morse, M.A. Vascular endothelial growth factor and immunosuppression in cancer: Current knowledge and potential for new therapy. Expert Opin. Biol. Ther. 2007, 7, 449–460.
- Li, Y.-L.; Zhao, H.; Ren, X.-B. Relationship of VEGF/VEGFR with immune and cancer cells: Staggering or forward? Cancer Biol. Med. 2016, 13, 206–214.
- Bu, L.; Yu, G.; Wu, L.; Mao, L.; Deng, W.; Liu, J.; Kulkarni, A.; Zhang, W.; Zhang, L.; Sun, Z. STAT3 Induces Immunosuppression by Upregulating PD-1/PD-L1 in HNSCC. J. Dent. Res. 2017, 96, 1027–1034.
- Lee, H.; Pal, S.K.; Reckamp, K.; Figlin, R.A.; Yu, H. STAT3: A Target to Enhance Antitumor Immune Response. Curr. Top Microbiol. Immunol. 2010, 344, 41–59.
- Roychoudhuri, R.; Eil, R.L.; Clever, D.; Klebanoff, C.A.; Sukumar, M.; Grant, F.M.; Yu, Z.; Mehta, G.; Liu, H.; Jin, P.; et al. The transcription factor BACH2 promotes tumor immunosuppression. J. Clin. Investig. 2016, 126, 599–604.
- Grant, F.M.; Yang, J.; Nasrallah, R.; Clarke, J.; Sadiyah, F.; Whiteside, S.K.; Imianowski, C.J.; Kuo, P.; Vardaka, P.; Todorov, T.; et al. BACH2 drives quiescence and maintenance of resting Treg cells to promote homeostasis and cancer immunosuppression. J. Exp. Med. 2020, 217, e20190711.
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895.
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of Antitumor Immunity by CTLA-4 Blockade. Science 1996, 271, 1734–1736.
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723.
- Fellner, C. Ipilimumab (yervoy) prolongs survival in advanced melanoma: Serious side effects and a hefty price tag may limit its use. Pharm. Ther. 2012, 37, 503–530.
- Haslam, A.; Prasad, V. Estimation of the Percentage of US Patients with Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw. Open 2019, 2, e192535.
- Press Release|CytoSen Therapeutics and KBI Biopharma Enter into Strategic Partnership to Manufacture NK Cells and Nanoparticles. Available online: https://www.kbibiopharma.com/news/cytosen-therapeutics-and-kbi-biopharma-enter-into-strategic-partnership-to-manufacture-nk-cells-and-nanoparticles (accessed on 30 October 2022).
- Dolen, Y.; Kreutz, M.; Gileadi, U.; Tel, J.; Vasaturo, A.; Van Dinther, E.A.W.; Van Hout-Kuijer, M.A.; Cerundolo, V.; Figdor, C.G. Co-delivery of PLGA encapsulated invariant NKT cell agonist with antigenic protein induce strong T cell-mediated antitumor immune responses. OncoImmunology 2015, 5, e1068493.
- Burga, R.A.; Khan, D.H.; Agrawal, N.; Bollard, C.M.; Fernandes, R. Designing Magnetically Responsive Biohybrids Composed of Cord Blood-Derived Natural Killer Cells and Iron Oxide Nanoparticles. Bioconjug. Chem. 2019, 30, 552–560.
- Nayyar, G.; Chu, Y.; Cairo, M.S. Overcoming Resistance to Natural Killer Cell Based Immunotherapies for Solid Tumors. Front. Oncol. 2019, 9, 51.
- Jonjic, S. Manipulation of NKG2D ligands by cytomegaloviruses: Impact on innate and adaptive immune response. Front. Immunol. 2011, 2, 85.
- Jindal, A.; Sarkar, S.; Alam, A. Nanomaterials-Mediated Immunomodulation for Cancer Therapeutics. Front. Chem. 2021, 9, 629635.
- Mikelez-Alonso, I.; Magadán, S.; González-Fernández, F.; Borrego, F. Natural killer (NK) cell-based immunotherapies and the many faces of NK cell memory: A look into how nanoparticles enhance NK cell activity. Adv. Drug Deliv. Rev. 2021, 176, 113860.
- Xia, F.; Hou, W.; Liu, Y.; Wang, W.; Han, Y.; Yang, M.; Zhi, X.; Li, C.; Qi, D.; Li, T.; et al. Cytokine induced killer cells-assisted delivery of chlorin e6 mediated self-assembled gold nanoclusters to tumors for imaging and immuno-photodynamic therapy. Biomaterials 2018, 170, 1–11.
- Wang, G.; Hu, W.; Chen, H.; Shou, X.; Ye, T.; Xu, Y. Cocktail Strategy Based on NK Cell-Derived Exosomes and Their Biomimetic Nanoparticles for Dual Tumor Therapy. Cancers 2019, 11, 1560.
- Koshy, S.T.; Cheung, A.S.; Gu, L.; Graveline, A.R.; Mooney, D.J. Liposomal Delivery Enhances Immune Activation by STING Agonists for Cancer Immunotherapy. Adv. Biosyst. 2017, 1, 1600013.
- Huang, S.; Fong, C.I.; Xu, M.; Han, B.-N.; Yuan, Z.; Zhao, Q. Nano-loaded natural killer cells as carriers of indocyanine green for synergetic cancer immunotherapy and phototherapy. J. Innov. Opt. Health Sci. 2019, 12, 19410025.
- Pitchaimani, A.; Nguyen, T.D.T.; Aryal, S. Natural killer cell membrane infused biomimetic liposomes for targeted tumor therapy. Biomaterials 2018, 160, 124–137.
- Liu, Z.; Jiang, W.; Nam, J.; Moon, J.J.; Kim, B.Y.S. Immunomodulating Nanomedicine for Cancer Therapy. Nano Lett. 2018, 18, 6655–6659.
- Christian, D.A.; Hunter, C.A. Particle-mediated delivery of cytokines for immunotherapy. Immunotherapy 2012, 4, 425–441.
- Kwak, M.; Erdag, G.; Leick, K.M.; Bekiranov, S.; Engelhard, V.H.; Slingluff, C.L. Associations of immune cell homing gene signatures and infiltrates of lymphocyte subsets in human melanomas: Discordance with CD163+ myeloid cell infiltrates. J. Transl. Med. 2021, 19, 371.
- Zhang, S.-C.; Hu, Z.-Q.; Long, J.-H.; Zhu, G.-M.; Wang, Y.; Jia, Y.; Zhou, J.; Ouyang, Y.; Zeng, Z. Clinical Implications of Tumor-Infiltrating Immune Cells in Breast Cancer. J. Cancer 2019, 10, 6175–6184.
- Tan, Z.; Xue, H.; Sun, Y.; Zhang, C.; Song, Y.; Qi, Y. The Role of Tumor Inflammatory Microenvironment in Lung Cancer. Front. Pharmacol. 2021, 12, 1168.
- Zhang, S.; Yang, X.; Wang, L.; Zhang, C. Interplay between inflammatory tumor microenvironment and cancer stem cells (Review). Oncol. Lett. 2018, 16, 679–686.
- Gun, S.Y.; Lee, S.W.L.; Sieow, J.L.; Wong, S.C. Targeting immune cells for cancer therapy. Redox Biol. 2019, 25, 101174.
- Melero, I.; Rouzaut, A.; Motz, G.T.; Coukos, G. T-Cell and NK-Cell Infiltration into Solid Tumors: A Key Limiting Factor for Efficacious Cancer Immunotherapy. Cancer Discov. 2014, 4, 522–526.
- Ran, G.H.; Lin, Y.Q.; Tian, L.; Zhang, T.; Yan, D.M.; Yu, J.H.; Deng, Y.C. Natural killer cell homing and trafficking in tissues and tumors: From biology to application. Signal Transduct. Target. Ther. 2022, 7, 205.
- Cifaldi, L.; Doria, M.; Cotugno, N.; Zicari, S.; Cancrini, C.; Palma, P.; Rossi, P. DNAM-1 Activating Receptor and Its Ligands: How Do Viruses Affect the NK Cell-Mediated Immune Surveillance during the Various Phases of Infection? Int. J. Mol. Sci. 2019, 20, 3715.
- Park, W.; Gordon, A.C.; Cho, S.; Huang, X.; Harris, K.R.; Larson, A.C.; Kim, D.-H. Immunomodulatory Magnetic Microspheres for Augmenting Tumor-Specific Infiltration of Natural Killer (NK) Cells. ACS Appl. Mater. Interfaces 2017, 9, 13819–13824.
- Monty, M.A.; Islam, A.; Nan, X.; Tan, J.; Tuhin, I.J.; Tang, X.; Miao, M.; Wu, D.; Yu, L. Emerging role of RNA interference in immune cells engineering and its therapeutic synergism in immunotherapy. Br. J. Pharmacol. 2021, 178, 1741–1755.
- Adjei, I.M.; Jordan, J.; Tu, N.; Le Trinh, T.; Kandell, W.; Wei, S.; Sharma, B. Functional recovery of natural killer cell activity by nanoparticle-mediated delivery of transforming growth factor beta 2 small interfering RNA. J. Interdiscip. Nanomed. 2019, 4, 98–112.
- Lian, S.; Xie, R.; Ye, Y.; Xie, X.; Li, S.; Lu, Y.; Li, B.; Cheng, Y.; Katanaev, V.; Jia, L. Simultaneous blocking of CD47 and PD-L1 increases innate and adaptive cancer immune responses and cytokine release. eBioMedicine 2019, 42, 281–295.
- Wang, Y.; Luo, Y.-L.; Chen, Y.-F.; Lu, Z.-D.; Wang, Y.; Czarna, A.; Shen, S.; Xu, C.-F.; Wang, J. Dually regulating the proliferation and the immune microenvironment of melanoma via nanoparticle-delivered siRNA targeting onco-immunologic CD155. Biomater. Sci. 2020, 8, 6683–6694.
- Neviani, P.; Wise, P.M.; Murtadha, M.; Liu, C.W.; Wu, C.-H.; Jong, A.Y.; Seeger, R.C.; Fabbri, M. Natural killer–derived exosomal miR-186 inhibits neuroblastoma growth and immune escape mechanisms. Cancer Res. 2019, 79, 1151–1164.
- Islam, R.; Pupovac, A.; Evtimov, V.; Boyd, N.; Shu, R.; Boyd, R.; Trounson, A. Enhancing a Natural Killer: Modification of NK Cells for Cancer Immunotherapy. Cells 2021, 10, 1058.
- Tan, L.; Han, S.; Ding, S.; Xiao, W.; Ding, Y.; Qian, L.; Wang, C.; Gong, W. Chitosan nanoparticle-based delivery of fused NKG2D-IL-21 gene suppresses colon cancer growth in mice. Int. J. Nanomed. 2017, 12, 3095–3107.
- Yan, C.; Jie, L.; Yongqi, W.; Weiming, X.; Juqun, X.; Yanbing, D.; Li, Q.; Xingyuan, P.; Mingchun, J.; Weijuan, G. Delivery of human NKG2D-IL-15 fusion gene by chitosan nanoparticles to enhance antitumor immunity. Biochem. Biophys. Res. Commun. 2015, 463, 336–343.
- Zhuo, Y.; Chen, F.; Kong, L.; Li, T.; Lu, L.; Yang, J.; Yu, T.; Shi, X.; Li, K. Magnetic Resonance Imaging of the Human Ferritin Heavy Chain Reporter Gene Carried by Dendrimer-Entrapped Gold Nanoparticles. J. Biomed. Nanotechnol. 2019, 15, 518–530.
- Meraz, I.M.; Majidi, M.; Cao, X.; Lin, H.; Li, L.; Wang, J.; Baladandayuthapani, V.; Rice, D.; Sepesi, B.; Ji, L.; et al. TUSC2 Immunogene Therapy Synergizes with Anti–PD-1 through Enhanced Proliferation and Infiltration of Natural Killer Cells in Syngeneic Kras-Mutant Mouse Lung Cancer Models. Cancer Immunol. Res. 2018, 6, 163–177.
- Kim, K.-S.; Han, J.-H.; Park, J.-H.; Kim, H.-K.; Choi, S.H.; Kim, G.R.; Song, H.; An, H.J.; Han, D.K.; Park, W.; et al. Multifunctional nanoparticles for genetic engineering and bioimaging of natural killer (NK) cell therapeutics. Biomaterials 2019, 221, 119418.
- Spear, P.; Wu, M.R.; Sentman, M.L.; Sentman, C.L. NKG2D ligands as therapeutic targets. Cancer Immun. 2013, 13, 8. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3700746/ (accessed on 4 October 2022).
- Liu, C.; Lai, H.; Chen, T. Boosting Natural Killer Cell-Based Cancer Immunotherapy with Selenocystine/Transforming Growth Factor-Beta Inhibitor-Encapsulated Nanoemulsion. ACS Nano 2020, 14, 11067–11082.
- Pan, J.; Xu, Y.; Wu, Q.; Hu, P.; Shi, J. Mild Magnetic Hyperthermia-Activated Innate Immunity for Liver Cancer Therapy. J. Am. Chem. Soc. 2021, 143, 8116–8128.
More
