Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Aida Meto.
Monkeypox virus (MPXV) belongs to the Poxviridae species. In the Poxviridae family, the Orthopoxvirus gene contains two enclosed strands of virus DNA (replicating in the cytoplasm and not the nucleus) and is called the monkeypox virus (MPXV).
- monkeypox
- pandemic
- zoonosis
- public health
- infectious disease
1. Introduction
Infectious diseases are currently on the rise, including monkeypox, which is similar to smallpox/Variola virus. Monkeypox (MPX) has emerged as a global threat and has been declared a public health emergency [1]. Although less clinically severe than smallpox, monkeypox is a zoonotic disease with symptoms resembling the latter [2].
In 1958, colonies of “monkeypox” were isolated; the virus was referred to as MPX, and in 1970, the first human case was detected in the Democratic Republic of Congo [3]. Most cases of this virus are found in Central and West Africa. The first wide outbreak of monkeypox outside of Africa was documented in the United States of America in 2003 [4,5][4][5]. Monkeypox might pose a threat to developing countries, such as India.
2. Epidemiology
2.1. Agent
Monkeypox virus (MPXV) belongs to the Poxviridae species [6]. In the Poxviridae family, the Orthopoxvirus gene contains two enclosed strands of virus DNA (replicating in the cytoplasm and not the nucleus) and is called the monkeypox virus (MPXV). It is an enveloped virus that is 200–250 nm length and brick-shaped (Figure 1a) [3,7][3][7].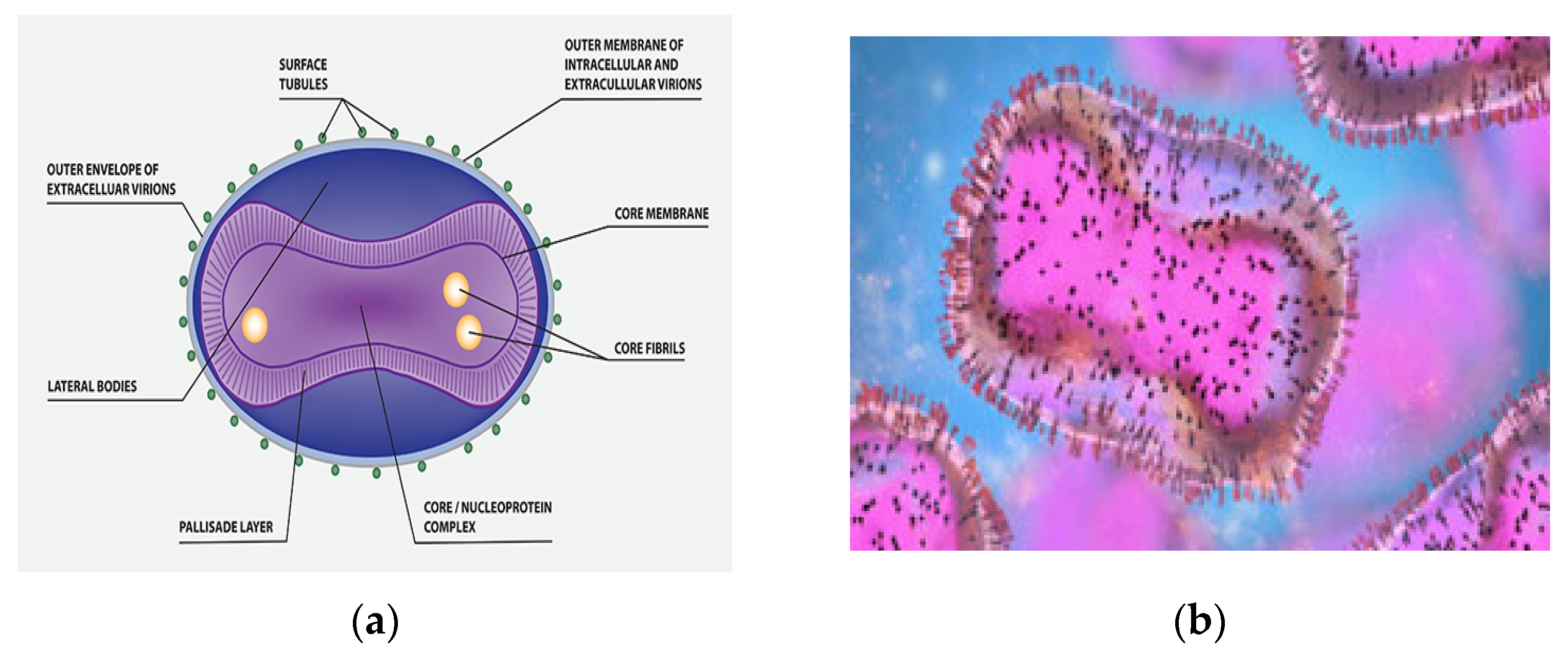
Figure 1. (a) MPXV molecular structure. (b) An electron micrograph displaying oval-shaped mature virus particles (pink) and immature particles (blue), taken from a sample from a patient with monkeypox.
2.2. Host
Multiple investigations have indicated that this disease is also linked to animal contact. Nonhuman primates (such as squirrels, Gambian pouched rats, and dormice) are known to be naturally prone to contracting the monkeypox virus [6]. Such species include wild squirrels, pet prairie dogs, and imported rodents from Ghana [14]. Additionally, in Africa, various kinds of monkeys have been documented as additional potential animal contacts for MPXV transmission [6,15][6][15]. Small mammals, such as elephant shrews and mice, are considered to play a part in viral transmission, but the reservoir host remains unknown [16].2.3. Mode of Transmission
The main way that diseases spread from one person to another is through their large airways. Droplets typically need a long period of intimate contact. It is transmissible by both direct and indirect interaction with the lesion, and interaction with body fluids, such as through sharing an infected person’s contaminated clothing or bed sheets. Viral transmission through feces may provide an additional exposure source [17]. Additionally, transmission from an infected mother to her fetus is possible [4,6,18][4][6][18]. Known risk factors for MPX transfer from person to person include sharing a bed, as well as practices that transfer the virus directly to oral mucosa, such as using the same fork or cup for eating or drinking. Living in an area that has recently lost its forest cover, not being vaccinated against smallpox, sleeping on the floor, and touching or eating dead bush meat or monkeys are risk factors for viral transmission in endemic areas [3,19][3][19]. Despite the fact that global reporting of MPX cases in this outbreak has asserted sexual associations, MPX is not a sexually transmitted pathogen. Recent cases have indicated a higher infection rate among males [20]. Infection is often caused by dangerous interactions with wild animals. The epidemiological and clinical standards for monkeypox are still being examined because it is unclear how widespread the disease is [21,22][21][22]. Thus, the main goals of research surrounding the virus are to understand its clinical features and complications, to provide symptom relief, and to avoid secondary infection.2.4. Current Worldwide Monkeypox Trends
The Central African clade and the West African clade are two separate genetic clades of MPXV. Historically, infection with the Central African (Congo Basin) clade is linked to high rates of transmission and cases with mortality rates of 10%, whereas infection with the West African clade has been connected to a more self-limiting condition [23]. Numerous other nations in Central and Western Africa have reported an endemic monkeypox outbreak. Additionally, some nonendemic nations such as the USA and UK have reported cases of this [7,24][7][24]. Monkeypox cases were found worldwide in 2103 instances between 1 January 2022 and 15 July 2022. Monkeypox remains a problem in 42 member states throughout five WHO regions, including America, Africa, Europe, the Eastern Mediterranean, and the Western Pacific. Since January 2022, the USA (7525), UK (2980), Spain (5162), France (2424), Germany (2980), and Brazil (2131) have all reported high numbers of confirmed cases. Additionally, eleven deaths have been attributed to monkeypox [25]. In 2022, the first death from monkeypox was reported in Nigeria. Of the reported instances, 99% involved men over 40 years of age (range: 0–65 years) [18,26][18][26]. Around 30,000 cases of monkeypox have been documented in 89 countries as of 2022; India, with a population of 1.38 billion people, is not an exemption.2.5. The Route of Monkeypox to India
India recently verified two instances of monkeypox in the district of Kerala [18,27][18][27]. The first incidence of monkeypox in Southeast Asia was reported in India on July 14; the patient was a 35-year-old male who had previously visited Kerala from the United Arab Emirates (UAE). In recent months, the number of cases in India has continued to rise. India announced nine confirmed cases of monkeypox on 8 August 2022. Five of these occurred in Kerala in the southwest and four occurred in Delhi in the north; these locations are more than 2600 km apart. The Indian Council of Medical Research (ICMR) isolated a viral strain, A.2, from first two reported cases. This strain differs from the one that has affected humans in Europe [6]. Despite the increasing number of cases, the following question remains unanswered: Will MPXV cause a pandemic [28]?2.6. Will It Be Referred to as the “Next COVID”?
Multiple factors mean that it is unlikely that MPXV will become a pandemic. Firstly, MPXV has existed worldwide for many years, and wthere haveis a reasonable comprehension of the virus’s composition, spread, and pathogenicity. Secondly, generally speaking, MPXV leads to moderate symptoms, as demonstrated by the low number of fatalities occurring since the pandemic began. In contrast, COVID-19 transmits via the respiratory system and has produced a large number of asymptomatic cases; thus, MPXV is less communicable because it requires close personal contact. In MPXV, an individual can transmit disease if the symptoms manifest; consequently, the likelihood of transmission occurring unnoticed is minimal [29]. Fourth, some smallpox vaccines are widely available; their “off-label” use should be advised, and global production can be scaled up as needed. Fifth, the virus is relatively stable, with a modest mutation rate. In this context, the majority of infectious disease specialists anticipate that the epidemic of monkeypox will not develop into a pandemic. Thus, weit can be assumed that an outbreak of monkeypox can be efficiently contained by isolating confirmed cases, quarantining contacts, and using licensed smallpox vaccines “off-label” for “ring vaccination.” Currently, it is not advised to immunize large communities [30]. Hence, with the urgent increase of surveillance programs in various nonendemic nations, the support of rigorous monkeypox case findings, rapid diagnoses, and the provision of appropriate supportive care, weit can be easily prevented the wide transmission of this virus. More consideration must be given to populations at high risk of infection and the potential risks of monkeypox nosocomial transmission. Understanding the dynamic epidemiology of the current outbreak of monkeypox requires international cooperation, which will enable improved surveillance and contact tracing [31,32][31][32].References
- Bhattacharya, M.; Dhama, K.; Chakraborty, C. Recently spreading human monkeypox virus infection and its transmission during COVID-19 pandemic period: A travelers’ prospective. Travel. Med. Infect. Dis. 2022, 49, 102398.
- Petersen, E.; Kantele, A.; Koopmans, M.; Asogun, D.; Yinka-Ogunleye, A.; Ihekweazu, C.; Zumla, A. Human monkeypox: Epidemiologic and clinical characteristics, diagnosis, and prevention. Infect. Dis. Clin. 2019, 33, 1027–1043.
- Brown, K.; Leggat, P.A. Human monkeypox: Current state of knowledge and implications for the future. Trop. Med. Int. 2016, 1, 8.
- Guidelines for Management of Monkeypox Disease. Available online: https://main.mohfw.gov.in/sites/default/files/Guidelines%20for%20Management%20of%20Monkeypox%20Disease.pdf (accessed on 1 August 2022).
- About Monkeypox. Available online: https://www.cdc.gov/poxvirus/monkeypox/about.html (accessed on 1 August 2022).
- WHO—MPXV Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/monkeypox (accessed on 1 August 2022).
- Mukherjee, D.; Roy, S.; Singh, V.; Gopinath, S.; Pokhrel, N.B.; Jaiswal, V. Monkeypox as an emerging global health threat during the COVID-19 time. Ann. Med. Surg. 2022, 79, 104075.
- Moore, M.; Zahra, F. Monkeypox. InStatPearls; StatPearls Publications: Tampa, FL, USA, 2021; Volume 8.
- Isidro, J.; Borges, V.; Pinto, M. First draft genome sequence of Monkeypox virus associated with the suspected multi-country outbreak. Gene Rep. 2022. Available online: https://virological.org/t/first-draft-genome-sequence-of-monkeypox-virus-associated-with-the-suspected-multi-country-outbreak-may-2022-confirmed-case-in-portugal/799 (accessed on 1 August 2022).
- Moss, B. Poxvirus DNA replication. Cold Spring Harb. Perspect. Biol. 2013, 5, a010199.
- Kugelman, J.R.; Johnston, S.C.; Mulembakani, P.M.; Kisalu, N.; Lee, M.S.; Koroleva, G.; McCarthy, S.E.; Gestole, M.C.; Wolfe, N.D.; Fair, J.N.; et al. Genomic variability of MPXV virus among humans, Democratic Republic of the Congo. Emerg. Infect. Dis. 2014, 20, 232–239.
- Hendrickson, R.C.; Wang, C.; Hatcher, E.L.; Lefkowitz, E.J. Orthopoxvirus genome evolution: The role of gene loss. Viruses 2010, 2, 1933–1967.
- Elde, N.C.; Child, S.J.; Eickbush, M.T.; Kitzman, J.O.; Rogers, K.S.; Shendure, J.; Geballe, A.P.; Malik, H.S. Poxviruses deploy genomic accordions to adapt rapidly against host antiviral defenses. Cell 2012, 150, 831–841.
- Khodakevich, L.; Szczeniowski, M.; Jezek, Z.; Marennikova, S.; Nakano, J.; Messinger, D. The role of squirrels in sustaining MPXV virus transmission. Trop. Geogr. Med. 1987, 39, 115–122.
- Falendysz, E.A.; Lopera, J.G.; Lorenzsonn, F.; Salzer, J.S.; Hutson, C.L.; Doty, J.; Gallardo-Romero, N.; Carroll, D.S.; Osorio, J.E.; Rocke, T.E. Further assessment of monkeypox virus infection in Gambian pouched rats (Cricetomys gambianus) using in vivo bioluminescent imaging. PLoS Negl. Trop. Dis. 2015, 9, e0004130.
- Doty, J.B.; Malekani, J.M.; Kalemba, L.S.; Stanley, W.T.; Monroe, B.P.; Nakazawa, Y.U.; Mauldin, M.R.; Bakambana, T.L.; Liyandja, T.L.D.; Braden, Z.H.; et al. Assessing monkeypox virus prevalence in small mammals at the human–animal interface in the Democratic Republic of the Congo. Viruses 2017, 9, 283.
- Kaler, J.; Hussain, A.; Flores, G.; Kheiri, S.; Desrosiers, D. Monkeypox: A Comprehensive Review of Transmission, Pathogenesis, and Manifestation. Cureus 2022, 14, 26531.
- Choudhary, G.; Prabha, P.K.; Gupta, S.; Prakash, A.; Medhi, B. Monkeypox infection: A quick glance. Indian J. Pharmacol. 2022, 54, 161–164.
- Alakunle, E.F.; Okeke, M.I. Monkeypox virus: A neglected zoonotic pathogen spreads globally. Nat. Rev. Microbiol. 2022, 20, 507–508.
- Thakur, V.; Thakur, P.; Srivastava, S.; Kumar, P. Monkeypox virus (MPX) in humans a concern: Trespassing the global boundaries–Correspondence. Int. J. Surg. 2022, 104, 106703.
- Samaranayake, L.; Sukumaran, A. The Monkeypox Outbreak and Implications for Dental Practice. Int. Dent. J. 2022, 72, 589–596.
- Sejvar, J.J.; Chowdary, Y.; Schomogyi, M.; Stevens, J.; Patel, J.; Karem, K.; Fischer, M.; Kuehnert, M.J.; Zaki, S.R.; Paddock, C.D.; et al. Human MPXV infection: A family cluster in the midwestern United States. J. Infect. Dis. 2004, 190, 1833–1840.
- Titanji, B.K.; Tegomoh, B.; Nematollahi, S.; Konomos, M.; Kulkarni, P.A. Monkeypox: A Contemporary Review for Healthcare Professionals. Open. Forum. Infect. 2022, 9, ofac310.
- Petersen, W.; Kabamba, J.; McCollum, A.M.; Lushima, R.S.; Wemakoy, E.O.; Muyembe Tamfum, J.J.; Nguete, B.; Hughes, C.M.; Monroe, B.P.; Reynolds, M.G. Vaccinating against monkeypox in the Democratic Republic of the Congo. Antivir. Res. 2019, 162, 171–177.
- Monkeypox in India—Facing the World’s Latest Health Threat. Available online: https://www.thinkglobalhealth.org/article/monkeypox-india-facing-worlds-latest-health-threat (accessed on 7 August 2022).
- Lahariya, C.; Thakur, A.; Dudeja, N. Monkeypox Disease Outbreak: Epidemiology, Challenges, and the Way Forward. Indian Pediatr. 2022, 59, 636–642.
- Multi-Country Outbreak of Monkeypox. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON396 (accessed on 7 August 2022).
- India Confirms First Case of Monkeypox in WHO South-East Asia Region. Available online: https://www.who.int/southeastasia/news/detail/15-07-2022-india-confirms-first-case-of-monkeypox-in-who-south-east-asia-region (accessed on 7 August 2022).
- Beer, E.M.; Rao, V.B. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl. Trop. Dis. 2019, 13, e0007791.
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl. Trop. Dis. 2022, 16, e0010141.
- Mohapatra, R.K.; Tuli, H.S.; Sarangi, A.K.; Chakraborty, S.; Chandran, D.; Chakraborty, C.; Dhama, K. Unexpected sudden rise of human monkeypox cases in multiple non-endemic countries amid COVID-19 pandemic and salient counteracting strategies: Another potential global threat? Int. J. Surg. 2022, 103, 106705.
- Simpson, K.; Heymann, D.; Brown, C.S.; Edmunds, W.J.; Elsgaard, J.; Fine, P.; Hochrein, H.; Hoff, N.A.; Green, A.; Ihekweazu, C.; et al. Human monkeypox–After 40 years, an unintended consequence of smallpox eradication. Vaccine 2020, 38, 5077–5081.
More
