One of the cutting-edge topics today is the use of magnetic field sensors for applications such as magnetocardiography, magnetotomography, magnetomyography, magnetoneurography, or their application in point-of-care devices. Types of magnetic field sensors include direct current superconducting quantum interference devices, search coil, fluxgate, magnetoelectric, giant magneto-impedance, anisotropic/giant/tunneling magnetoresistance, optically pumped, cavity optomechanical, Hall effect, magnetoelastic, spin wave interferometry, and those based on the behavior of nitrogen-vacancy centers in the atomic lattice of diamond. Current developments of magnetometry in biological diagnostics are revised in review paper DOI: 10.3390/s20061569.
- magnetic field sensors
- biosensors
- biomagnetic fields
- therapeutic application
- noninvasive medical procedures
- diagnosis
1. Introduction
The development of magnetic field sensors for biomedical applications primarily focuses on equivalent magnetic noise reduction or overall design improvement in order to make them smaller and cheaper while keeping the required values of a limit of detection. Contemporary demands of biological systems diagnostics are for low-cost fabrication methods, flexibility of usage, and the quick obtaining of test results. At the same time, new diagnostic platforms have to be more precise and provide the right clinical management decisions. The development of magnetic field sensors for biomedical applications primarily focuses on equivalent magnetic noise reduction or overall design improvement in order to make them smaller and cheaper while keeping the required values of a limit of detection.
Applications of magnetic sensing technology in biomedical fields can be subdivided into two main categories:
- Measuring a magnetic field produced by human organs
- Detecting magnetically labeled biomolecules.
Magnetic field sensors suitable for biomedical applications are:
- Search Coil Magnetometers [[1][2]]
- Direct current superconducting quantum interference devices (dc SQUIDs) Magnetometers [[3][4][5][6]]
- Fluxgate Magnetometers [[7][8][9][10]]
- Magnetometers based on the Anisotropic/Giant/Tunneling Magnetoresistance (AMR/GMR/TMR) Effects [[11][12][13][14][15]]
- Magnetoelectric Magnetometers [[16][17][18]]
- Giant Magneto-Impedance (GMI) Magnetometers [[19][20][21][22]]
- Optically Pumped Atomic Magnetometers [[23][24][25][26][27][28]]
- Cavity Optomechanical Magnetometers [[29][30][31]]
- Magnetometry utilizing Nitrogen-Vacancy Centers in Diamond [[32][33][34][35][36][37]]
- Hall Effect Magnetometers [[38][39][40][41][42]]
- Magnetoelastic Magnetometers [[43][44][45][46]]
- Spin Wave Interferometry Based Magnetometers [[47][48]]
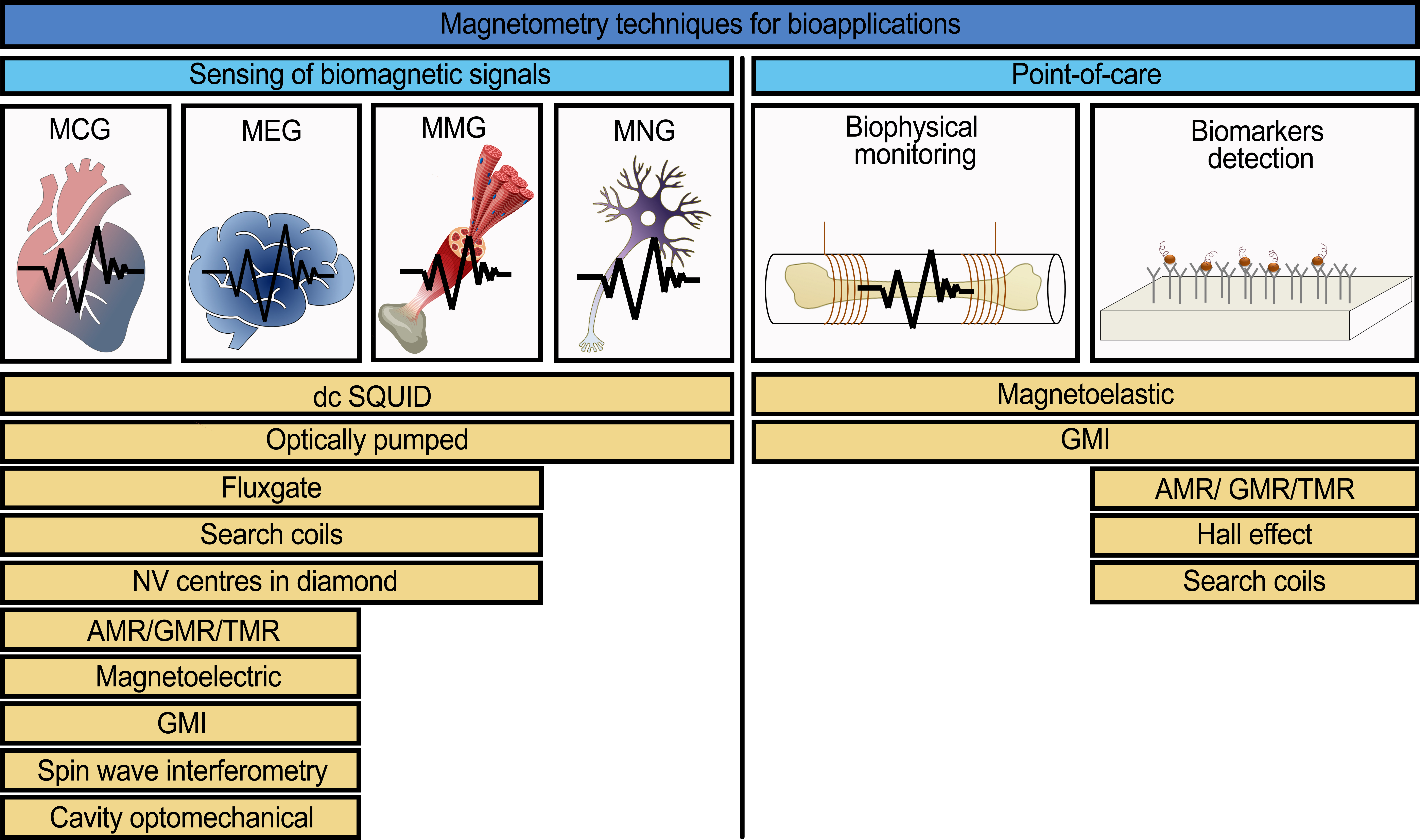
A list of magnetic sensors depending on the type of application (for biomagnetic signals detection or for point-of-care devices) is presented in Figure 1. The list of magnetic sensors is constituted from their potential use in systems for sensing many kinds of biomagnetic signals including point-of-care devices. The working principles and detailed explanation of the potential use of these sensors are presented in the full version of this review [[49]].
Figure 1. A list of the common and modern magnetic field sensors with the potential to be used for the detection of biomagnetic signals (magnetocardiography (MCG), magnetoencephalography (MEG), magnetomyography (MMG), magnetoneurography (MNG)) and for point-of-care devices [[2][16][22][31][48][50][51][52][53][54][55][56][57][58][59][60][61][62]].
2. Magnetic Field Sensors for Detection of Biomagnetic Signals
Biomagnetic signals from a human body provide a lot of useful information about the heart, nervous system, brain or muscle activity. However, magnetic fields generated by most biosystems have a low amplitude in comparison with noise sources [[63]]. The adult heart signal is the largest of the biological magnetic signals with a peak magnitude of about 25 pT [[64]]. For each biomagnetic signal source, one can determine the required limits of detectable magnetic field strength and frequency in terms of an equivalent magnetic noise spectral density. To study magnetocardiography [[65]], magnetoneurography [[54]], magnetoencephalography [[66]] and magnetomyography [[67]], the magnetic field sensor must fulfill those conditions. A chart showing sensors capable of detecting corresponding biomagnetic signals is shown in Figure 2.
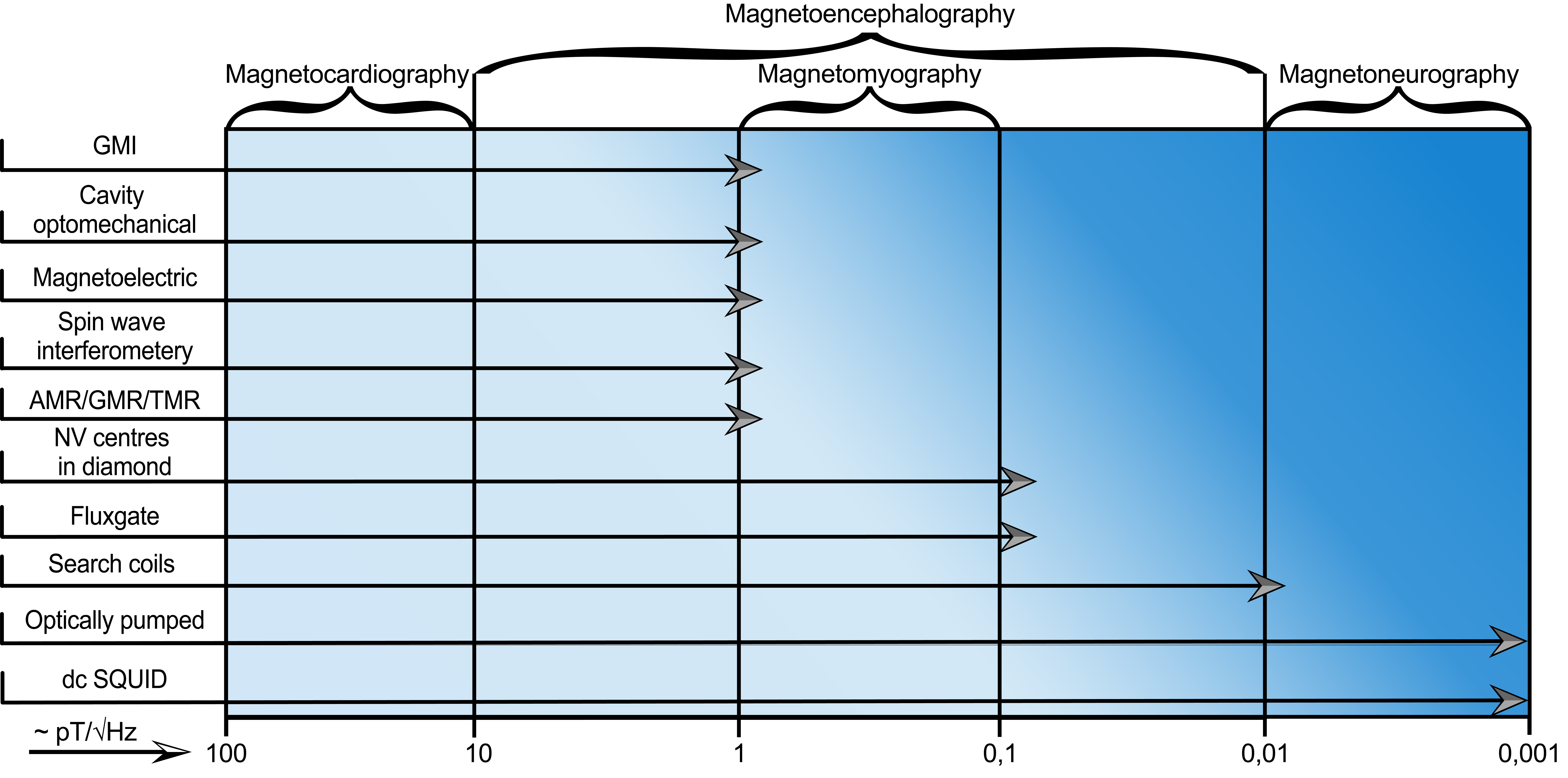
In the field of biomagnetic signal detection, there are some well-established as well as new state-of-the-art magnetometry techniques worth mentioning. Nowadays, the most used technique is SQUID magnetometry [[68]]. However, this method is complicated to achieve in practice, because it generally relies on the use of a magnetically shielded room and cooling of the sensing element with liquid nitrogen or helium [[69][70]]. The resolution problems may be overcome by the implementation of specific hardware and software shielding technologies. Also, new developments in the high-Tc SQUIDs (not requiring liquid helium) provide the possibility of placing sensing elements close to the system to be measured, unfortunately, also followed by a higher noise level [[71]].
Optically pumped magnetometers achieving measurements less than 1 fT/√Hz [[72]] have great potential to replace SQUID magnetometers in some areas, but measuring methods can still be improved in the design, setup, and signal-to-noise ratio [[73]]. Also, these devices measure the magnitude of the field giving no information on its direction. Moreover, there could be zones of zero sensitivity in certain directions. Latest examples of detection limits in the low-frequency regime (<10 Hz) for different volumes of vapor cells include: 7 fT/√Hz by Krzyzewski et al. [[74]], 15 fT/√Hz by Knappe et al. [[75]], 10 fT/√Hz by Boto et al. [[76]]. The versions based on a nonlinear magneto-optical rotation regime are not commonly considered when measuring biomagnetic signals but there is some research devoted to the improvement of their limit of detection [[77][78]].
Both SQUID and optically pumped magnetometers achieve a limit of detection of the order of fT/√Hz only while having milli- or micrometer spatial resolution. The achievement of nanometer spatial resolution with these types of sensors is cost expensive and is followed with the huge drop of detection limit. In turn, nanometer spatial resolution can be provided by one of the newest and most promising types of magnetometers based on the behavior of nitrogen-vacancy (NV) centers in the atomic structure of diamond. Though for now these magnetometers commonly have a nT/√Hz [[79][80]] or pT/√Hz [[81]] limit of detection, they have a number of noteworthy applications due to their special features. In addition, such sensors have potential in fields of magnetocardiography, magnetomyography and magnetoencephalography, due to the possibility of achieving the required limit of detection. Nevertheless, the physics of detection based on NV centers in diamond is complicated and differs from sample to sample. Thus, theoretical studies describing the physics of the processes in the structures considered are still ongoing.
The sensors discussed previously have restrictions on their operating temperature or on the optical excitation power, and there is a demand on the magnetic field sensor operating at room temperature whilst maintaining optimum performance. One of the most promising candidates for this purpose is the cavity optomechanical magnetometer. This currently has low energy consumption, high spatial resolution, and requires no cooling systems [[82]].
An additional type of magnetometer having low energy consumption, wide temperature range and competitive sensitivity is the spin wave interferometry-based magnetometer. One of the latest works devoted to the advantages of this new technique predicts the value of the detection limit of 1 pT/√Hz [[48]].
Highly sensitive and localized magnetic field sensors already discussed are ether technically sophisticated or cost expensive. The topic of relatively simple and cheap magnetic field sensors having sensitivities at a level suitable for magnetocardiography or magnetomyography is expanding. One relatively cheap and available magnetic field sensor able to measure biomagnetic signals is the fluxgate magnetometer. Recently, a new type of flux-gate sensor which utilizes epitaxial iron garnet films with a specially designed edge profile as a core was developed, claiming a reduction of noise level at room temperature down to 0.1 pT/√Hz. This magnetometer was successfully applied in magnetocardiography experiments on animals [[83]].
Magnetic field sensors competing with the fluxgate for magnetocardiography are those based (separately) on the magnetoimpedance, magnetoelectric and magnetoresistive effects. With the use of GMI gradiometers in a magnetically unshielded environment, the magnetic heart signal from a human subject was detected at nine spatially separated points demonstrating the possibility of obtaining more information in comparison with electrocardiography [[20][22]]. For these measurements, the noise level was approximately 2 pT/√Hz. The possibility of detection with GMI gradiometers of other biomagnetic signals as a magnetic field around a muscle tissue sample was also shown [[19][84]]. Amongst magnetoresistive sensors, TMR-based sensors are good candidates for measuring fields from the heart and, possibly, the brain [[58]]. A future challenge for the low cost, portable magnetic MR sensors is to non-invasively monitor the heart of an unborn baby in the womb, being an area presently covered by SQUID magnetometers. The signals from the electrical activity of the brain, however, are much smaller, so to monitor these a sensitivity in the femto-Tesla range is required - which is currently beyond the range of MR sensors.
3. Magnetic Field Sensors for Point-of-Care Devices
Some recent studies have been dedicated to the development of point-of-care devices for biomedical diagnostics [[85]]. Although biological objects are generally non-ferromagnetic, the latest developments in magnetic nanoparticles’ systems have produced specific magnetic markers which have affinities to conjugating ligands for cells, proteins, nucleic acids, etc. Magnetically labeled targets can be detected by magnetic field sensors or concentrated by a magnetic field on the surface of sensors thus allowing an improvement in the detection efficiency. Point-of-care technologies are generally associated with lab-on-a-chip systems where magnetic nanoparticles can employ different functions [[86][87]]. For example:
- Capture, preconcentration, and separation of analytes bonded with the specifically functionalized surface of the particles;
- Mixing of lateral flows;
- Creation of contrast in magnetic susceptibility of the biological medium for future sensing.
This set of features opens doors for completely new applications as well as for the significant improvement of existing methods. For instance, the lateral flow immunoassay is a well-established method of biodetection which is very prominent because of the immunochromatographic strip test for ascertaining pregnancy [[88]]. This can be improved with the use of magnetic nanoparticles. Recent developments show that the approach of focusing magnetically labeled rare protein biomarkers for early diagnosis of cervical cancer can significantly improve sensitivity [[89]]. Quantitative analysis can be achieved through an optical signal detected by the camera of a smartphone or by the naked eye [[90]]. In some cases, however, the traditional optical methods are not acceptable due to the high background noise or the low sensitivity of detecting devices. Because of these reasons, magnetic sensors have been employed [[91][92][93]].
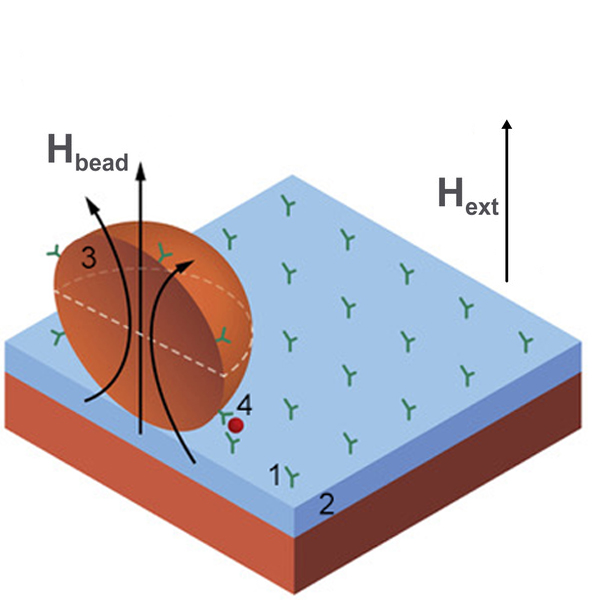
Magnetic field sensors in such devices are used for detection of drugs, cellular proteins or other biomarkers which are usually labeled with magnetic particles (or beads) as shown in Figure 3. There are also tests for blood coagulation, measurement of forces or stresses in artificial bones and the mass evaluation of cell cultures. Typical sensors being used in lab-on-a-chip systems are GMR/TMR [[94][95][96]], search coils [[52][97][98]], GMI [[50][99]], and Hall effect [[100]] magnetometers while for bioengineering purposes the most commonly used magnetic sensors are based on magnetoelasticity [[101]]. A significant advantage of magnetoresistive sensors in these applications is that they are fabricated with the same overall technology used to produce silicon chips, so it is relatively easy to manufacture them as part of an integrated lab-on-a-chip system.
Figure 3. Example of a biochip based on magnetic label detection using a magnetic thin-film sensor. The chip consists of an array of probe biomolecules (1) of known identity immobilized onto the surface of the sensor (2), magnetic labels (3) functionalized with target biomolecules (4) that bind to the sensor surface through biomolecular recognition. The magnetic particle stray field Hbead resulting from the magnetic moment of the label induced by the applied magnetic field Hext is measured by the sensor.
Magnetoelastic magnetic field sensors are used for the monitoring of glucose concentration, growth of bacteria, pH, biliary stent monitoring and other noteworthy in vitro applications [[46][102]]. This is useful in the mechanical detection of loads on orthopedic implants or in vivo monitoring of the force information in bones and joints.
In the field of microfluidics and lateral flow bioanalysis, there is a need for high sensitivity and fast response. A lot of proposed biosensing platforms meeting these needs are based on the magnetoresistive effect. One of the noteworthy articles is dedicated to tuberculosis point-of-care diagnostics with a magnetoresistive biosensor having a limit of detection of 104 cells/mL [[103]]. Recently, AMR-based microstructures [[104][105][106]] were proposed for magnetic beads detection, but, nowadays, because of the low change of resistivity [[106]] (around 2%), magnetoresistive biosensors based on GMR [[107][108]] and TMR [[109]] are more popular. For detailed information about exchange-biased AMR sensors tailored for magnetic bead sensing in lab-on-a-chip systems, we refer to the overview [[110]].
Detection of biomarkers can also be performed with magnetic field sensors based on the magnetoimpedance effect. An example of this is the detection of a-fetoprotein bioconjugates with a GMI magnetometer (with the 100 fg/mL limit of detection) [50]. Also, GMI based magnetic field sensors can be used in microfluidic chips achieving a detection limit of 0.1 ng∙mL−1 and working in the 0.1 ng/mL–20 ng/mL biomarker concentration range [[111]].
4. Conclusions and Future Perspectives
In the field of biomagnetic signal detection, SQUID magnetometry remains the most common tool for magnetocardiography, magnetomyography, magnetoneurography or magnetoencephalography while having the best sensitivity approaching fT/√Hz. In spite of the usefulness of SQUID magnetometers, the requirement of low operating temperature and magnetically shielded room makes it relatively hard to use in practice. The future prospects for SQUID magnetometers may lie in miniaturization and, also, in the reduction of the operational noise values whilst remaining at a low limit of detection.
Among all magnetic field sensors, the most promising candidate for replacing SQUID-based sensors are optically pumped magnetometers (particularly ones based on the spin relaxation free regime). Their potential lies in their ability to achieve a limit of detection of several fT/√Hz while keeping the sensing area (restricted by linear dimensions of vapor cells) to a few tenths of mm2. In comparison with SQUIDs, these magnetometers produce less operational noise and need heating of vapor cells to a temperature up to around 400 K, which is easier to realize. Still, optically pumped magnetometers require a real-time precise magnetic shielding system and some construction issues for the implementation of such sensors in biomedicine are yet to be solved [[112][113][114]].
In addition, great progress has been achieved in magnetometry using a detection system based on nitrogen-vacancy centers in diamond material. Though the sensitivity of such devices commonly does not extend beyond tenths of pT/√Hz, they operate at room temperature while allowing nanoscale sensing and providing a way to develop novel magnetic field sensors. Due to these prospects, further and current research is mostly aimed at extending the sensitivity and resolution of magnetometers of this type.
Due to recent developments in nano- and microfabrication techniques, new approaches to miniaturize magnetic field sensors with competitive sensitivities may be realized in spin wave interferometry or cavity optomechanical magnetometers. However, these magnetometers are still technically complicated, so magnetometers based on well-established and more simply fabricated AMR/GMR/TMR, GMI, fluxgate, and magnetoelectric technologies are enough for many biomagnetic field measurements.
Concerning point-of-care technologies, sensor integration into lab-on-a-chip, and microfluidic technologies could lead to the replacement of many diagnostic systems currently used in laboratories and clinics. As the sensitivity requirements are determined by a particular device, the path to the miniaturization of diagnostic systems lies in overall design improvements. New advances in the fields of micro- and nano-fabrication will also help to overcome current limitations of the usage of magnetic sensors in this field because of issues concerning high power consumption, single-target detection and system complexity. The most promising magnetic sensors to overcome these limitations are GMR/TMR and GMI magnetometers due to the fact of their flexibility and the convenience of the low-cost integration process. This means that not only the sensor but the complete signal processing system can be built on the same chip using closely related technologies.
References
- C. Coillot; J. Moutoussamy; R. LeBourgeois; S. Ruocco; G. Chanteur; Principle and Performance of a Dual-Band Search Coil Magnetometer: A New Instrument to Investigate Fluctuating Magnetic Fields in Space. CHARM-Deep: Continuous Human Activity Recognition Model Based on Deep Neural Network using IMU Sensors of Smartwatch 2009, 10, 255-260, 10.1109/JSEN.2009.2030977.
- V. Korepanov; R. Berkman; L. Rakhlin; Ye. Klymovych; A. Prystai; A Marussenkov; M. Afanassenko; Advanced field magnetometers comparative study. Measurement 2001, 29, 137-146, 10.1016/s0263-2241(00)00034-8.
- M. Buchner; K. Höfler; Bastian Henne; V. Ney; A. Ney; Tutorial: Basic principles, limits of detection, and pitfalls of highly sensitive SQUID magnetometry for nanomagnetism and spintronics. Journal of Applied Physics 2018, 124, 161101, 10.1063/1.5045299.
- Kang Yang; Hua Chen; Li Lu; Xiangyan Kong; Ruihu Yang; Jia Lei Wang; Yang Ruihu; SQUID Array With Optimal Compensating Configuration for Magnetocardiography Measurement in Different Environments. Toward Increasing the Difficulty of Reverse Engineering of RSFQ Circuits 2019, 29, 1-7, 10.1109/tasc.2019.2904483.
- Karsten Sternickel; Alex I Braginski; Biomagnetism using SQUIDs: status and perspectives. Superconductor Science and Technology 2006, 19, , 10.1088/0953-2048/19/3/024.
- Ethan Y. Cho; Hao Li; Jay C. Lefebvre; Yuchao W. Zhou; R. C. Dynes; Shane A. Cybart; Direct-coupled micro-magnetometer with Y-Ba-Cu-O nano-slit SQUID fabricated with a focused helium ion beam. Applied Physics Letters 2018, 113, 162602, 10.1063/1.5048776.
- David Miles; Miroslaw Ciurzynski; David Barona; B. Barry Narod; John R. Bennest; Andy Kale; Marc Lessard; David K. Milling; Joshua Larson; I. R. Mann; Low-noise permalloy ring cores for fluxgate magnetometers. Geoscientific Instrumentation, Methods and Data Systems 2019, 8, 227-240, 10.5194/gi-8-227-2019.
- P. M. Vetoshko; N. A. Gusev; D. A. Chepurnova; E. V. Samoilova; A. K. Zvezdin; A. A. Korotaeva; V. I. Belotelov; I. I. Syvorotka; I. M. Syvorotka; Flux-gate magnetic field sensor based on yttrium iron garnet films for magnetocardiography investigations. Technical Physics Letters 2016, 42, 860-864, 10.1134/S1063785016080289.
- Jen-Tzong Jeng; Chih-Cheng Lu; Hsiang-Wei Ku; Bo-Rei Huang; Meng-Huan Chia; Xuan Thang Trinh; Three-Axis Micofluxgate With a Fluxguide. Deep Neural Network a Posteriori Probability Detector for Two-dimensional Magnetic Recording 2019, 55, 1-4, 10.1109/tmag.2019.2903107.
- I. Sasada; S. Harada; Ahmed L. Elrefai; Gradiometer and magnetometer integration by using a pair of fundamental mode orthogonal fluxgate sensor heads. 2015 IEEE Magnetics Conference (INTERMAG) 2015, null, 1-1, 10.1109/intmag.2015.7157129.
- Mitra Djamal; Ramli Ramli; Giant Magnetoresistance Sensors Based on Ferrite Material and Its Applications. Magnetic Sensors - Development Trends and Applications 2017, null, , 10.5772/intechopen.70548.
- Maria-Dolores Cubells-Beltran; Càndid Reig; Jordi Madrenas; Andrea De Marcellis; Joana Santos; Susana Cardoso; Susana Cardoso; Integration of GMR Sensors with Different Technologies. Sensors 2016, 16, 939, 10.3390/s16060939.
- Tomas Dias; F. A. Cardoso; Sofia Martins; V. C. Martins; S. Cardoso; J. F. Gaspar; Gabriel A. Monteiro; Susana Cardoso; Implementing a strategy for on-chip detection of cell-free DNA fragments using GMR sensors: A translational application in cancer diagnostics using ALU elements. Analytical Methods 2016, 8, 119-128, 10.1039/c5ay01587a.
- Hui-Min Shen; Liang Hu; Xin Fu; Integrated Giant Magnetoresistance Technology for Approachable Weak Biomagnetic Signal Detections. Sensors 2018, 18, 148, 10.3390/s18010148.
- Meiling Wang; Yang Wang; Lei Peng; Chaofeng Ye; Measurement of Triaxial Magnetocardiography Using High Sensitivity Tunnel Magnetoresistance Sensor. CHARM-Deep: Continuous Human Activity Recognition Model Based on Deep Neural Network using IMU Sensors of Smartwatch 2019, 19, 9610-9615, 10.1109/jsen.2019.2927086.
- E. Yarar; S. Salzer; V. Hrkac; A. Piorra; M. Höft; R. Knöchel; Lorenz Kienle; Eckhard Quandt; Inverse bilayer magnetoelectric thin film sensor. Applied Physics Letters 2016, 109, 022901, 10.1063/1.4958728.
- Yaojin Wang; J. Q. Gao; M. H. Li; Y. Shen; D. Hasanyan; J. F. Li; D. Viehland; A review on equivalent magnetic noise of magnetoelectric laminate sensors. Philosophical Transactions of the Royal Society of London. Series A: Mathematical, Physical and Engineering Sciences 2014, 372, 20120455-20120455, 10.1098/rsta.2012.0455.
- Yaojin Wang; Junqi Gao; Jiefang Li; David Gray; David Berry; Menghui Li; Dwight Viehland; An Extremely Low Equivalent Magnetic Noise Magnetoelectric Sensor. Advanced Materials 2011, 23, 4111-4114, 10.1002/adma.201100773.
- Tsuyoshi Uchiyama; Shinsuke Nakayama; Kaneo Mohri; Kenichi Bushida; Biomagnetic field detection using very high sensitivity magnetoimpedance sensors for medical applications. physica status solidi (a) 2009, 206, 639-643, 10.1002/pssa.200881251.
- Tsuyoshi Uchiyama; Takashi Takiya; Development of precise off-diagonal magnetoimpedance gradiometer for magnetocardiography. AIP Advances 2017, 7, 056644, 10.1063/1.4975128.
- Uchiyama, T.; Mohri, K.; Honkura, Y.; Panina, L. V Recent Advances of Pico-Tesla Resolution Magneto-Impedance Sensor Based on Amorphous Wire CMOS IC MI Sensor. IEEE Trans. Magn. 2012, 48, 3833–3839.
- Tsuyoshi Uchiyama; J. Ma; Design and Demonstration of Novel Magnetoencephalogram Detectors. Deep Neural Network a Posteriori Probability Detector for Two-dimensional Magnetic Recording 2019, null, 1-8, 10.1109/tmag.2019.2895399.
- Hyun Joon Lee; Seong-Joo Lee; Jeong Hyun Shim; Han Seb Moon; Kiwoong Kim; In-situ Overhauser-enhanced nuclear magnetic resonance at less than 1 μT using an atomic magnetometer.. Journal of Magnetic Resonance 2019, 300, 149-152, 10.1016/j.jmr.2019.02.001.
- Robert J. Cooper; David W. Prescott; Garrett J. Lee; Karen L. Sauer; RF atomic magnetometer array with over 40 dB interference suppression using electron spin resonance.. Journal of Magnetic Resonance 2018, 296, 36-46, 10.1016/j.jmr.2018.08.007.
- Peng-Cheng Du; Jian-Jun Li; Si-Jia Yang; Xu-Tong Wang; Yan Zhuo; Fan Wang; Ruquan Wang; Observing the steady-state visual evoked potentials with a compact quad-channel spin exchange relaxation-free magnetometer. Chinese Physics B 2019, 28, 040702, 10.1088/1674-1056/28/4/040702.
- Amir Borna; Tony R. Carter; Paul Derego; Conrad D. James; Peter D. D. Schwindt; Magnetic Source Imaging Using a Pulsed Optically Pumped Magnetometer Array. Guidance Mechanism for Flexible Wing Aircraft Using Measurement-Interfaced Machine Learning Platform 2018, 68, 493-501, 10.1109/tim.2018.2851458.
- Wenhao Li; Xiang Peng; Songjian Li; Chuanfei Liu; Hong Guo; Pingwei Lin; Wei Zhang; Unshielded scalar magnetometer based on nonlinear magneto-optical rotation with amplitude modulated light. 2016 IEEE International Frequency Control Symposium (IFCS) 2016, null, 1-4, 10.1109/fcs.2016.7546761.
- Wei Fang; Shangzhong Jin; Yufeng Wu; Yongqiang Yan; ZeNan Li; Yiyi Zhang; A new method to simultaneously improve the sensitivity and absolute accuracy for CPT magnetometer. The European Physical Journal D 2020, 74, 1-4, 10.1140/epjd/e2020-100374-5.
- Li, B.-B.; Bulla, D.; Prakash, V.; Forstne, S.; Dehghan-Manshadi, A.; Dunlop, H.R.-; Foster, S.; Bowen, W.P.; Citation: Invited Article: Scalable high-sensitivity optomechanical magnetometers on a chip. APL Photonics 2018, 3, 120806.
- Yimin Yu; Stefan Forstner; Halina Rubinsztein-Dunlop; Warwick P. Bowen; Modelling of Cavity Optomechanical Magnetometers. Sensors 2018, 18, 1558, 10.3390/s18051558.
- Stefan Forstner; Eoin Sheridan; Joachim Knittel; Christopher L. Humphreys; George A. Brawley; Halina Rubinsztein-Dunlop; Warwick P. Bowen; Micrometry: Ultrasensitive Optomechanical Magnetometry (Adv. Mater. 36/2014). Advanced Materials 2014, 26, 6355-6355, 10.1002/adma.201470249.
- Georgios Chatzidrosos; Arne Wickenbrock; Lykourgos Bougas; Nathan Leefer; Teng Wu; Kasper Jensen; Yannick Dumeige; Dmitry Budker; Miniature Cavity-Enhanced Diamond Magnetometer. Physical Review Applied 2017, 8, , 10.1103/PhysRevApplied.8.044019.
- David R Glenn; Kyungheon Lee; Hongkun Park; Ralph Weissleder; Amir Yacoby; Mikhail D Lukin; Hakho Lee; R. L. Walsworth; Colin B Connolly; Single-cell magnetic imaging using a quantum diamond microscope. Nature Chemical Biology 2015, 12, 736-738, 10.1038/nmeth.3449.
- Thomas Wolf; Philipp Neumann; Kazuo Nakamura; Hitoshi Sumiya; Takeshi Ohshima; Junichi Isoya; Jörg Wrachtrup; Subpicotesla Diamond Magnetometry. Physical Review X 2015, 5, 041001, 10.1103/physrevx.5.041001.
- Philipp Fuchs; Michel Challier; Elke Neu; Optimized Single-Crystal Diamond Scanning Probes for High Sensitivity Magnetometry. null 2018, null, , null.
- David Le Sage; K. Arai; D. R. Glenn; Stephen DeVience; L. M. Pham; L. Rahn-Lee; M. D. Lukin; A. Yacoby; A. Komeili; R. L. Walsworth; Optical magnetic imaging of living cells. Nature 2013, 496, 486-489, 10.1038/nature12072.
- Jennifer M. Schloss; John F. Barry; Matthew J. Turner; R. L. Walsworth; Simultaneous Broadband Vector Magnetometry Using Solid-State Spins. Physical Review Applied 2018, 10, 034044, 10.1103/physrevapplied.10.034044.
- Anders Dahl Henriksen; Giovanni Rizzi; Mikkel Fougt Hansen; Experimental comparison of ring and diamond shaped planar Hall effect bridge magnetic field sensors. Journal of Applied Physics 2015, 118, 103901, 10.1063/1.4930068.
- H. Pişkin; Numan Akdoğan; Interface-induced enhancement of sensitivity in NiFe/Pt/IrMn-based planar hall sensors with nanoTesla resolution. Sensors and Actuators A: Physical 2019, 292, 24-29, 10.1016/j.sna.2019.04.003.
- Hyuntai Kim; Venu Reddy; Kun Woo Kim; Ilgyo Jeong; Xing Hao Hu; CheolGi Kim; Single Magnetic Bead Detection in a Microfluidic Chip Using Planar Hall Effect Sensor. Journal of Magnetics 2014, 19, 10-14, 10.4283/jmag.2014.19.1.010.
- Davut Izci; Carl Dale; Neil Keegan; John Hedley; The Construction of a Graphene Hall Effect Magnetometer. CHARM-Deep: Continuous Human Activity Recognition Model Based on Deep Neural Network using IMU Sensors of Smartwatch 2018, 18, 9534-9541, 10.1109/jsen.2018.2872604.
- Pablo Nicolás Granell; Guoliang Wang; Gilbert Santiago Cañón Bermúdez; Tobias Kosub; Federico Golmar; Laura Steren; Jürgen Fassbender; Denys Makarov; Highly compliant planar Hall effect sensor with sub 200 nT sensitivity. npj Flexible Electronics 2019, 3, 3, 10.1038/s41528-018-0046-9.
- Keat Ghee Ong; Ee Lim Tan; Brandon Pereles; Brock Horton; Wireless, magnetic-based sensors for biomedical applications. 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society 2009, 2009, 5436-5439, 10.1109/IEMBS.2009.5332475.
- S. Atalay; V.S. Kolat; F.E. Atalay; N. Bayri; H. Kaya; T. Izgi; Magnetoelastic sensor for magnetic nanoparticle detection. Journal of Magnetism and Magnetic Materials 2018, 465, 151-155, 10.1016/j.jmmm.2018.05.108.
- Limin Ren; Kun Yu; Yisong Tan; Monitoring and Assessing the Degradation Rate of Magnesium-Based Artificial Bone In Vitro Using a Wireless Magnetoelastic Sensor. Sensors 2018, 18, 3066, 10.3390/s18093066.
- Yisong Tan; Jianhua Zhu; Limin Ren; A Two-Dimensional Wireless and Passive Sensor for Stress Monitoring. Sensors 2019, 19, 135, 10.3390/s19010135.
- Peter M. Vetoshko; Michael V. Valeiko; Petr I. Nikitin; Epitaxial yttrium iron garnet film as an active medium of an even-harmonic magnetic field transducer. Sensors and Actuators A: Physical 2003, 106, 270-273, 10.1016/s0924-4247(03)00182-1.
- M. Balynsky; D. Gutierrez; H. Chiang; A. Kozhevnikov; G. Dudko; Y. Filimonov; A. A. Balandin; A. Khitun; A Magnetometer Based on a Spin Wave Interferometer. Scientific Reports 2017, 7, 11539, 10.1038/s41598-017-11881-y.
- Dmitry Murzin; Desmond Mapps; Kateryna Levada; Victor Belyaev; Alexander Omelyanchik; Larissa Panina; Valeria Rodionova; Ultrasensitive Magnetic Field Sensors for Biomedical Applications. Sensors 2020, 20, 1569, 10.3390/s20061569.
- Ying Zhu; Qing Zhang; Xin Li; Hailin Pan; Jiangtao Wang; Zhenjie Zhao; Detection of AFP with an ultra-sensitive giant magnetoimpedance biosensor. Sensors and Actuators B: Chemical 2019, 293, 53-58, 10.1016/j.snb.2019.05.004.
- Zheng, C.; Zhu, K.; De Freitas, S.C.; Chang, J.Y.; Davies, J.E.; Eames, P.; Freitas, P.P.; Kazakova, O.; Kim, C.G.; Leung, C.W.; et al. Magnetoresistive Sensor Development Roadmap (Non-Recording Applications). IEEE Trans. Magn. 2019, 55, 1–30.
- Petr I. Nikitin; Petr M. Vetoshko; Tatiana Ksenevich; New type of biosensor based on magnetic nanoparticle detection. Journal of Magnetism and Magnetic Materials 2007, 311, 445-449, 10.1016/j.jmmm.2006.10.1180.
- Rasmus Zetter; Joonas Iivanainen; Lauri Parkkonen; Optical Co-registration of MRI and On-scalp MEG. Scientific Reports 2019, 9, 5490, 10.1038/s41598-019-41763-4.
- Bruno-Marcel Mackert; Magnetoneurography: theory and application to peripheral nerve disorders. Clinical Neurophysiology 2004, 115, 2667-2676, 10.1016/j.clinph.2004.07.028.
- Alessandra Manzin; Vahid Nabaei; Riccardo Ferrero; Quantification of Magnetic Nanobeads With Micrometer Hall Sensors. CHARM-Deep: Continuous Human Activity Recognition Model Based on Deep Neural Network using IMU Sensors of Smartwatch 2018, 18, 10058-10065, 10.1109/jsen.2018.2874520.
- Kasper Jensen; Rima Budvytyte; Rodrigo A. Thomas; Tian Wang; Annette Fuchs; Mikhail Balabas; Georgios Vasilakis; Lars Mosgaard; Thomas Heimburg; Søren-Peter Olesen; E. S. Polzik; Non-invasive detection of animal nerve impulses with an atomic magnetometer operating near quantum limited sensitivity. null 2016, null, , null.
- Dale, M.W.; Morley, G.W. Medical applications of diamond magnetometry: commercial viability. arXiv 2017, arXiv:1705.01994.
- Kosuke Fujiwara; Mikihiko Oogane; Akitake Kanno; Masahiro Imada; Junichi Jono; Takashi Terauchi; Tetsuo Okuno; Yuuji Aritomi; Masahiro Morikawa; Masaaki Tsuchida; Nobukazu Nakasato; Yukio Ando; Magnetocardiography and magnetoencephalography measurements at room temperature using tunnel magneto-resistance sensors. Applied Physics Express 2018, 11, 23001, 10.7567/apex.11.023001.
- H. T. Huang; P. Garu; C. H. Li; W. C. Chang; B. W. Chen; S. Y. Sung; C. M. Lee; J. Y. Chen; T. F. Hsieh; W. J. Sheu; H. Ouyang; W. C. Wang; C. R. Chang; C. L. Wang; M. S. Hsu; Z. H. Wei; Magnetoresistive Biosensors for Direct Detection of Magnetic Nanoparticle Conjugated Biomarkers on a Chip. SPIN 2019, 9, , 10.1142/s2010324719400022.
- Wang, Y.; Li, J.; Heidari, H.; Qin, J. Wearable Fluxgate Sensors for the Magnetoencephalography (MEG) for Monitoring the Brain Activities. In Abstract Book of Magnetic Frontiers Magnetic Sensors 2019; Magnetic Frontiers: Lisbon, Portugal, 2019; pp. P2-7.
- Limin Ren; Kun Yu; Yisong Tan; Applications and Advances of Magnetoelastic Sensors in Biomedical Engineering: A Review.. Materials 2019, 12, 1135, 10.3390/ma12071135.
- Venkatramana D. Krishna; Kai Wu; Andres Perez; Jian-Ping Wang; Giant Magnetoresistance-based Biosensor for Detection of Influenza A Virus. Frontiers in Microbiology 2016, 7, 156, 10.3389/fmicb.2016.00400.
- Jens Reermann; Eric Elzenheimer; Gerhard Schmidt; Real-Time Biomagnetic Signal Processing for Uncooled Magnetometers in Cardiology. CHARM-Deep: Continuous Human Activity Recognition Model Based on Deep Neural Network using IMU Sensors of Smartwatch 2019, 19, 4237-4249, 10.1109/jsen.2019.2893236.
- D.J. Mapps; Remote magnetic sensing of people. Sensors and Actuators A: Physical 2003, 106, 321-325, 10.1016/s0924-4247(03)00193-6.
- Stephan Lau; B. Petkovic; Jens Haueisen; Optimal Magnetic Sensor Vests for Cardiac Source Imaging. Sensors 2016, 16, 754, 10.3390/s16060754.
- Elena Boto; Richard Bowtell; Peter Krüger; T. Mark Fromhold; Peter G. Morris; Sofie S. Meyer; Gareth R. Barnes; Matthew J. Brookes; On the Potential of a New Generation of Magnetometers for MEG: A Beamformer Simulation Study. PLOS ONE 2016, 11, e0157655, 10.1371/journal.pone.0157655.
- Marco Antonio Cavalcanti Garcia; Oswaldo Baffa; Magnetic fields from skeletal muscles: a valuable physiological measurement?. Frontiers in Physiology 2015, 6, 1-4, 10.3389/fphys.2015.00228.
- Riitta Hari; Lauri Parkkonen; Cathy Nangini; The brain in time: insights from neuromagnetic recordings. Annals of the New York Academy of Sciences 2010, 1191, 89-109, 10.1111/j.1749-6632.2010.05438.x.
- Jia Lei Wang; Kang Yang; Ruihu Yang; Xiangyan Kong; Wei Chen; Yang Ruihu; SQUID Gradiometer Module for Fetal Magnetocardiography Measurements Inside a Thin Magnetically Shielded Room. Toward Increasing the Difficulty of Reverse Engineering of RSFQ Circuits 2018, 29, 1-4, 10.1109/tasc.2018.2889865.
- Y P Pan; S Y Wang; X Y Liu; Yishi Lin; L X Ma; Yang Feng; Zhen Wang; Lei Chen; Y H Wang; 3D nano-bridge-based SQUID susceptometers for scanning magnetic imaging of quantum materials. Nanotechnology 2019, 30, 305303, 10.1088/1361-6528/ab1792.
- Faley, M.I.; Dammers, J.; Maslennikov, Y.V.; Schneiderman, J.F.; Winkler, D.; Koshelets, V.P.; Shah, N.J.; Dunin-Borkowski, R.E.; High T c SQUID Precautions : Supercond. Sci. Technol. 2017, 30, 1-5.
- H. B. Dang; A. C. Maloof; M. V. Romalis; Ultrahigh sensitivity magnetic field and magnetization measurements with an atomic magnetometer. Applied Physics Letters 2010, 97, 151110, 10.1063/1.3491215.
- Tim M Tierney; Niall Holmes; Stephanie Mellor; José David López; Gillian Roberts; Ryan M. Hill; Elena Boto; James Leggett; Vishal Shah; Matthew J. Brookes; et al.Richard BowtellGareth R. Barnes Optically pumped magnetometers: From quantum origins to multi-channel magnetoencephalography. NeuroImage 2019, 199, 598-608, 10.1016/j.neuroimage.2019.05.063.
- S. P. Krzyzewski; A. R. Perry; V. Gerginov; S. Knappe; Characterization of noise sources in a microfabricated single-beam zero-field optically-pumped magnetometer. Journal of Applied Physics 2019, 126, 044504, 10.1063/1.5098088.
- Svenja Knappe; Orang Alem; Dong Sheng; John E. Kitching; Microfabricated Optically-Pumped Magnetometers for Biomagnetic Applications. Journal of Physics: Conference Series 2016, 723, 12055, 10.1088/1742-6596/723/1/012055.
- Elena Boto; Sofie S. Meyer; Vishal Shah; Orang Alem; Svenja Knappe; Peter Kruger; T. Mark Fromhold; Mark Lim; Paul Glover; Peter G. Morris; et al.Richard BowtellGareth R. BarnesMatthew J. Brookes A new generation of magnetoencephalography: Room temperature measurements using optically-pumped magnetometers.. NeuroImage 2017, 149, 404-414, 10.1016/j.neuroimage.2017.01.034.
- Feng Zhou; Chengjie J. Zhu; Edward W. Hagley; Lu Deng; Symmetry-breaking inelastic wave-mixing atomic magnetometry. Science Advances 2017, 3, e1700422, 10.1126/sciadv.1700422.
- Vladislav Gerginov; Sean Krzyzewski; Svenja Knappe; Pulsed operation of a miniature scalar optically pumped magnetometer. Journal of the Optical Society of America B 2017, 34, 1429-1434, 10.1364/JOSAB.34.001429.
- Xu, L.; Yuan, H.; Zhang, N.; Zhang, J.; Bian, G.; Fan, P.; Li, M.; Zhang, C.; Zhai, Y.; Fang, J. High-efficiency fluorescence collection for NV - center ensembles in diamond . Opt. Express 2019, 27, 10787.
- Zongmin Ma; Shaowen Zhang; Yueping Fu; Hua Yuan; Yunbo Shi; Jian Gao; Li Qin; Jun Tang; Jun Liu; Yanjun Li; Magnetometry for precision measurement using frequency-modulation microwave combined efficient photon-collection technique on an ensemble of nitrogen-vacancy centers in diamond.. Optics Express 2018, 26, 382-390, 10.1364/OE.26.000382.
- John F. Barry; Matthew J. Turner; Jennifer M. Schloss; David R. Glenn; Yuyu Song; Mikhail D. Lukin; Hongkun Park; R. L. Walsworth; Optical magnetic detection of single-neuron action potentials using quantum defects in diamond.. Proceedings of the National Academy of Sciences 2016, 113, 14133-14138, 10.1073/pnas.1601513113.
- Beibei Li; Jan Bílek; Ulrich Busk Hoff; Lars S. Madsen; Stefan Forstner; Varun Prakash; Clemens Schäfermeier; Tobias Gehring; Warwick P. Bowen; Ulrik L. Andersen; et al. Quantum enhanced optomechanical magnetometry. Optica 2018, 5, 850-856, 10.1364/optica.5.000850.
- P. M. Vetoshko; N. A. Gusev; D. A. Chepurnova; E. V. Samoilova; A. K. Zvezdin; A. A. Korotaeva; V. I. Belotelov; Rat Magnetocardiography Using a Flux-Gate Sensor Based on Iron Garnet Films. Biomedical Engineering 2016, 50, 237-240, 10.1007/s10527-016-9628-9.
- Tsuyoshi Uchiyama; Kaneo Mohri; Shinsuke Nakayama; Measurement of Spontaneous Oscillatory Magnetic Field of Guinea-Pig Smooth Muscle Preparation Using Pico-Tesla Resolution Amorphous Wire Magneto-Impedance Sensor. Deep Neural Network a Posteriori Probability Detector for Two-dimensional Magnetic Recording 2011, 47, 3070-3073, 10.1109/tmag.2011.2148165.
- Hakho Lee; Tae-Hyun Shin; Jinwoo Cheon; Ralph Weissleder; Recent Developments in Magnetic Diagnostic Systems. Chemical Reviews 2015, 115, 10690-10724, 10.1021/cr500698d.
- Alexander Van Reenen; Arthur M. De Jong; Jaap Den Toonder; Menno W. J. Prins; Integrated lab-on-chip biosensing systems based on magnetic particle actuation ? a comprehensive review. Lab on a Chip 2014, 14, 1966-1986, 10.1039/C3LC51454D.
- Lucy Gloag; Milad Mehdipour; Dongfei Chen; Richard D. Tilley; J. Justin Gooding; Advances in the Application of Magnetic Nanoparticles for Sensing. Advanced Materials 2019, 31, e1904385, 10.1002/adma.201904385.
- Laura Anfossi; Fabio Di Nardo; Simone Cavalera; Cristina Giovannoli; Claudio Baggiani; Multiplex Lateral Flow Immunoassay: An Overview of Strategies towards High-throughput Point-of-Need Testing. Biosensors 2018, 9, 2, 10.3390/bios9010002.
- Wen Ren; Sulma I. Mohammed; Steven T. Wereley; Joseph Irudayaraj; Magnetic Focus Lateral Flow Sensor for Detection of Cervical Cancer Biomarkers. Analytical Chemistry 2019, 91, 2876-2884, 10.1021/acs.analchem.8b04848.
- Jing Wu; Mingling Dong; Cheng Zhang; Yu Wang; Mengxia Xie; Yiping Chen; Magnetic Lateral Flow Strip for the Detection of Cocaine in Urine by Naked Eyes and Smart Phone Camera. Sensors 2017, 17, 1286, 10.3390/s17061286.
- M. Rivas; David Lago-Cachón; J.C. Martínez-García; Antonio Calleja; Eddy-current sensing of superparamagnetic nanoparticles with spiral-like copper circuits. Sensors and Actuators A: Physical 2014, 216, 123-127, 10.1016/j.sna.2014.05.023.
- Amanda Moyano; María Salvador; José C. Martínez-García; Vlad Socoliuc; Ladislau Vékás; Davide Peddis; Miguel A. Alvarez; María Fernández; M. Rivas; Maria C. Blanco-López; Magnetic immunochromatographic test for histamine detection in wine.. Analytical and Bioanalytical Chemistry 2019, 411, 6615-6624, 10.1007/s00216-019-02031-6.
- David Lago-Cachón; M Rivas; J C Martinez-García; J A García; Cu impedance-based detection of superparamagnetic nanoparticles. Nanotechnology 2013, 24, 245501, 10.1088/0957-4484/24/24/245501.
- Yasuhiro Shirai; Kenzo Hirao; Tomohiko Shibuya; Shuichi Okawa; Yuki Hasegawa; Yoshiaki Adachi; Kensuke Sekihara; Shigenori Kawabata; Magnetocardiography Using a Magnetoresistive Sensor Array. International Heart Journal 2019, 60, 50-54, 10.1536/ihj.18-002.
- Susana Cardoso; Diana Leitao; Tomas Dias; João Valadeiro; Marília Silva; Alexandre Chícharo; Vânia Silverio; João Gaspar; Susana Cardoso; Challenges and trends in magnetic sensor integration with microfluidics for biomedical applications. Journal of Physics D: Applied Physics 2017, 50, 213001, 10.1088/1361-6463/aa66ec.
- Chinthaka P. Gooneratne; Rimantas Kodzius; Fuquan Li; Ian G. Foulds; Jurgen Kosel; On-Chip Magnetic Bead Manipulation and Detection Using a Magnetoresistive Sensor-Based Micro-Chip: Design Considerations and Experimental Characterization. Sensors 2016, 16, 1369, 10.3390/s16091369.
- M. P. Nikitin; A. V. Orlov; I. L. Sokolov; A. A. Minakov; P. I. Nikitin; J. Ding; S. D. Bader; E. A. Rozhkova; V. Novosad; Ultrasensitive Detection Enabled by Nonlinear Magnetization of Nanomagnetic Labels. null 2018, null, , null.
- Hua Wang; Alborz Mahdavi; David A. Tirrell; Ali Hajimiri; A magnetic cell-based sensor. Lab on a Chip 2012, 12, 4465, 10.1039/c2lc40392g.
- Tao Wang; Yong Zhou; Chong Lei; Jun Luo; Shaorong Xie; Huayan Pu; Magnetic impedance biosensor: A review. Biosensors and Bioelectronics 2017, 90, 418-435, 10.1016/j.bios.2016.10.031.
- D. Issadore; Y. I. Park; H. Shao; C. Min; K. Lee; M. Liong; Ralph Weissleder; Hakho Lee; Magnetic sensing technology for molecular analyses.. Lab on a Chip 2014, 14, 2385-2397, 10.1039/c4lc00314d.
- Alessandro Dalponte; Eduardo Bastos; Frank Missell; Enhanced response from field-annealed magnetoelastic strain sensor. Journal of Applied Physics 2016, 120, 064502, 10.1063/1.4960687.
- Kun Yu; Limin Ren; Yisong Tan; Junyao Wang; Wireless Magnetoelasticity-Based Sensor for Monitoring the Degradation Behavior of Polylactic Acid Artificial Bone In Vitro. Applied Sciences 2019, 9, 739, 10.3390/app9040739.
- Teresa G. Barroso; Rui Martins; Elisabete Fernandes; Susana Cardoso; Jose Rivas; Susana Cardoso; Detection of BCG bacteria using a magnetoresistive biosensor: A step towards a fully electronic platform for tuberculosis point-of-care detection. Biosensors and Bioelectronics 2018, 100, 259-265, 10.1016/j.bios.2017.09.004.
- L T Hien; L K Quynh; V T Huyen; B D Tu; N T Hien; D M Phuong; P H Nhung; D T H Giang; N H Duc; DNA-magnetic bead detection using disposable cards and the anisotropic magnetoresistive sensor. Advances in Natural Sciences: Nanoscience and Nanotechnology 2016, 7, 45006, 10.1088/2043-6262/7/4/045006.
- L.K. Quynh; B.D. Tu; D.X. Dang; D.Q. Viet; L.T. Hien; D.T. Huong Giang; N.H. Duc; Detection of magnetic nanoparticles using simple AMR sensors in Wheatstone bridge. Journal of Science: Advanced Materials and Devices 2016, 1, 98-102, 10.1016/j.jsamd.2016.04.006.
- D. Louis; T. Hauet; S. Petit-Watelot; D. Lacour; M. Hehn; François Montaigne; Four states magnetic dots: a design selection by micromagnetic modeling. Spintronics IX 2016, 9931, 99311, 10.1117/12.2239071.
- Dieter Suess; Anton Bachleitner-Hofmann; Armin Satz; Herbert Weitensfelder; Christoph Vogler; Florian Bruckner; Claas Abert; Klemens Prügl; Jürgen Zimmer; Christian Huber; Sebastian Luber; Wolfgang Raberg; Thomas Schrefl; Hubert Bruckl; Topologically protected vortex structures for low-noise magnetic sensors with high linear range. Nature Electronics 2018, 1, 362-370, 10.1038/s41928-018-0084-2.
- Todd Klein; Wei Wang; Lina Yu; Kai Wu; Kristin L.M. Boylan; Rachel I. Vogel; Amy P.N. Skubitz; Jian-Ping Wang; Development of a multiplexed giant magnetoresistive biosensor array prototype to quantify ovarian cancer biomarkers. Biosensors and Bioelectronics 2019, 126, 301-307, 10.1016/j.bios.2018.10.046.
- Yuanzhao Wu; Yiwei Liu; J. Ping Liu; Run-Wei Li; Qingfeng Zhan; Rapid detection of Escherichia coli O157:H7 using tunneling magnetoresistance biosensor. AIP Advances 2017, 7, 056658, 10.1063/1.4977017.
- Mikkel Fougt Hansen; Giovanni Rizzi; Exchange-Biased AMR Bridges for Magnetic Field Sensing and Biosensing. Deep Neural Network a Posteriori Probability Detector for Two-dimensional Magnetic Recording 2017, 53, 1-11, 10.1109/tmag.2016.2614012.
- Feng, Z.; Zhi, S.; Guo, L.; Zhou, Y.; Lei, C.; An integrated magnetic microfluidic chip for rapid immunodetection of the prostate specific antigen using immunomagnetic beads. . Microchim. Acta 2019, 186, 252.
- Joonas Iivanainen; Rasmus Zetter; Mikael Grön; Karoliina Hakkarainen; Lauri Parkkonen; On-scalp MEG system utilizing an actively shielded array of optically-pumped magnetometers. NeuroImage 2019, 194, 244-258, 10.1016/j.neuroimage.2019.03.022.
- Robert J. Cooper; David W. Prescott; Peter Matz; Karen L. Sauer; Nezih Dural; Michael V. Romalis; Elizabeth L. Foley; Thomas W. Kornack; Mark Monti; Jeffrey Okamitsu; Atomic Magnetometer Multisensor Array for rf Interference Mitigation and Unshielded Detection of Nuclear Quadrupole Resonance. Physical Review Applied 2016, 6, 64014, 10.1103/physrevapplied.6.064014.
- Niall Holmes; James Leggett; Elena Boto; Gillian Roberts; Ryan M. Hill; Tim M. Tierney; Vishal Shah; Gareth R. Barnes; Matthew J. Brookes; Richard Bowtell; A bi-planar coil system for nulling background magnetic fields in scalp mounted magnetoencephalography. NeuroImage 2018, 181, 760-774, 10.1016/j.neuroimage.2018.07.028.