Recent research has led to an explosion in our interest and our understanding of the role of vitamin D in regulation of immunity. At the molecular level, the hormonal form of vitamin D signals through the nuclear vitamin D receptor (VDR), a ligand-regulated transcription factor. The VDR and vitamin D metabolic enzymes are expressed throughout the innate and adaptive arms of the immune system. The advent of genome-wide approaches to gene expression profiling led to identification of numerous VDR-regulated genes implicated in regulation of innate and adaptive immunity. The molecular data infer that vitamin D signaling should boost innate immunity against pathogens of bacterial or viral origin.
- vitamin D
- non-classical actions
- innate immunity
- antibacterial activity
- antiviral activity
1. Introduction
The active hormonal form of vitamin D, 1,25-dihydroxyvitamin D (1,25(OH)2D3), signals through the vitamin D receptor (VDR), a member of the nuclear receptor family of ligand-regulated transcription factors[1]. 1,25(OH)2D3 is produced by sequential hydroxylations of vitamin D, first by largely hepatic 25-hydroxylation catalyzed by cytochrome P450 2 R1 (CYP2R1) and other enzymes, followed by tightly regulated 1a-hydroxylation by CYP27B1 in peripheral tissues. The classical view of vitamin D action in calcium homeostasis is based on the conversion of 25-hydroxyvitamin D (25OHD), the major circulating form, into 1,25(OH)2D3 in the kidney. Renal CYP27B1 expression is controlled by key regulators of calcium homeostasis, such as parathyroid hormone and fibroblast growth factor 23 (FGF23)[1]. In the classical model, renal 1,25(OH)2D3 released into the circulation acts in an endocrine manner to maintain circulating calcium concentrations, most notably by enhancing the intestinal uptake of dietary calcium. Indeed, severe neonatal vitamin D deficiency leads to hypocalcemia and rickets, with defects in skeletal growth associated with softening and weakening of the bones. Descriptions of rickets, along with paleontological evidence, date back millennia[2]. Cod liver oil and sun exposure were proposed as treatments for nutritional rickets as early as the beginning of the 19th century[3]. Unfortunately, 200 years later, nutritional rickets remains a clinical problem and is almost certainly under-reported[4][5]. Moreover, rickets diagnosed in the clinic represents the tip of the iceberg both in terms of the extent of pediatric vitamin D deficiency and because it is often associated with an increased prevalence of diseases unrelated to disrupted calcium homeostasis[5]. These include a number of immune-related disorders, such as increased rates of infections and conditions associated with compromised innate immunity; autoimmune diseases, such as type 1 diabetes (T1D) and multiple sclerosis (MS); and allergic conditions, such as atopic dermatitis[1][6][7].
Clinical interest in the links between vitamin D deficiency and these non-classical immune indications has been bolstered by rapidly expanding evidence from laboratory and translational studies that vitamin D signaling is a key regulator of both the innate and adaptive arms of the immune system[8]. The VDR and vitamin D metabolic enzymes are present throughout the immune system, and, importantly, CYP27B1 production in immune cells is induced by pathogen detection and regulated by a complex cytokine network[9][10], and is independent of calcium homeostatic signals. Thus, hormonal 1,25(OH)2D3 can be produced and act locally in the immune system under conditions of pathogen threat. The discovery of induced CYP27B1 expression in myeloid cells in vitro provides a molecular basis for observations of elevated levels of 1,25(OH)2D3 produced by macrophages in granulomatous diseases like sarcoidosis, which in extreme cases can lead to hypercalcemia [11](see Figure 1 for details).
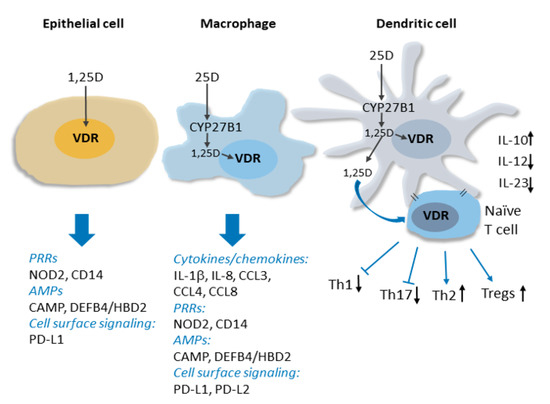
Figure 1. Vitamin D signaling in innate immunity. Intracrine production 1,25D from CYP27B1-catalyzed conversion of circulating 25D in activated macrophages and dendritic cell is shown. The induction of expression by 1,25D signaling through the VDR of genes encoding several types of proteins implicated in innate immune signaling, including cytokines/chemokines, pattern recognition receptors (PRRs) and antimicrobial peptides (AMPs), is indicated. 1,25D signaling within and release from dendritic cells influences dendritic cell maturation and suppresses production of inflammatory Th1 and Th17 cells, favouring Th2 and Tregs. See text for details.
Innate immune responses are initiated by the detection of pathogen-associated antigens (pathogen-associated molecular patterns; PAMPS) by families of so-called pattern recognition receptors (PRRs). PRR activation and signaling leads to the production of antimicrobial peptides and a network of downstream responses, including an array of cytokines and chemokines, which propagate the signal to other components of the immune system. Studies of 1,25(OH)2D3-regulated gene expression in innate immune cells have revealed that vitamin D signaling functions upstream and downstream of PRRs to enhance immune responses. This includes inducing the expression of CD14[12], a cofactor of toll-like receptor 4 PRR signaling, as well as that of NOD2 (nucleotide-binding oligomerization domain protein 2), a PRR defective in a subset of patients with Crohn’s disease[13]. The hormone-bound VDR also directly stimulates the transcription of genes encoding antimicrobial peptides cathelicidin antimicrobial peptide (CAMP/LL37) and human beta/defensin 2 defensin B4 (HBD2/DEFB4)[13][14][15], and readily detectable antibacterial activity is secreted into the media of 1,25(OH)2D3-treated cells in vitro[14][16]. Importantly, antimicrobial activity in pulmonary surface airway fluid was significantly higher in vitamin D-supplemented patients in a placebo-controlled double-blind randomized trial (RCT)[17]. In the presence of 1,25(OH)2D3, the expression of several cytokines, including interleukin 1b (IL-1b), a core component of innate immune responses, and the neutrophil chemokine IL-8/CXCL8 are induced, notably in macrophages infected with Mycobacterium tuberculosis[18]. Vitamin D signaling also regulates the innate-adaptive immune interface by rendering dendritic cells less inflammatory[1][19][20]. This contributes to suppression by 1,25(OH)2D3 of peripheral inflammatory T cell responses and enhanced development of T-regulatory (Treg) cells[19][21][22][23]. In addition to the above, genome-wide analyses of vitamin D signaling have revealed that the VDR regulates the transcription of numerous other genes implicated in immune system function[24]. Thus, we are physiologically wired to produce 1,25(OH)2D3 locally in immune cells in response to pathogens, and vitamin D signaling is a key component of many aspects of immune responses.
2. Antiviral Activity of Vitamin D Signaling: Specific Reference to COVID-19.
At this writing, the world is in the grips of the COVID-19 pandemic, which is caused by the SARS‐CoV‐2 (severe acute respiratory syndrome-Covonavirus-2) virus. As such, along with SARS and MERS (Middle East respiratory syndrome), it represents the third and most severe coronavirus outbreak of this century. Notably, a recent British Medical Journal editorial on COVID-19 led to an extended discussion of vitamin D deficiency as a potential risk factor[25]. While COVID-19 is particularly severe in elderly populations, all age groups, including pediatric populations, are susceptible. One study provided evidence that pediatric COVID-19 was associated with coinfections[26], and fears of the spread of SARS-COV-2 in children will grow in many countries with a return to school. Clinical trials have yet to be registered to test the effects of vitamin D supplementation in the prevention/treatment of COVID-19 in children, although they are sure to come. However, clinical evidence is presented below that vitamin D supplementation reduces the rates of respiratory tract infections many of which are viral in nature. There is molecular evidence to support such antiviral activity. The antimicrobial peptide CAMP/LL37, whose expression is strongly inducible by 1,25(OH)2D3, has antiviral activity against enveloped viruses in vitro and influenza A in vivo[27]. 1,25(OH)2D3 also enhances the antiviral activity of bronchial epithelial cells in vitro and diminishes rhinovirus replication[28]. While these findings support the notion that hormonal vitamin D induces antiviral activity, it should also be noted that vitamin D signaling acts as a negative regulator of the renin-angiotensin system[29], which includes ACE2 (angiotensin converting enzyme 2), the receptor for SARS-COV-2 ACE2[30]. ACE2 itself functions as a negative regulator of the renin-angiotensin cascade, and in an animal model, a 1,25(OH)2D3 analogue enhanced ACE2 expression in vitro[31]. This may not be beneficial in the context of a SARS-COV-2 infection; it has been hypothesized that patients being treated with ACE inhibitors for hypertension, which enhance ACE2 expression, may be at an increased risk for the development of severe COVID-19[32].
References
- R. Bouillon; Claudio Marcocci; Geert Carmeliet; Daniel Bikle; John H. White; Bess Dawson-Hughes; Paul Lips; Craig F Munns; Marise Lazaretti-Castro; Andrea Giustina; et al.John Bilezikian Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions.. Endocrine Reviews 2019, 40, 1109-1151, 10.1210/er.2018-00126.
- Pettifor, J.M.; Thandrayen, K.; Thacher, T.D. Chapter 67—Vitamin D Deficiency and Nutritional Rickets in Children. In Vitamin D, 4th ed.; Feldman, D., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 179–201.
- Ruth A. Guy; THE HISTORY OF COD LIVER OIL AS A REMEDY. Archives of Pediatrics & Adolescent Medicine 1923, 26, 112, 10.1001/archpedi.1923.04120140011002.
- Andrea J. Aul; Philip R. Fischer; Jason S. O'grady; Kristin C. Mara; Julie A. Maxson; Alicia M. Meek; Tanya M. Petterson; Tom D. Thacher; Population-Based Incidence of Potentially Life-Threatening Complications of Hypocalcemia and the Role of Vitamin D Deficiency. The Journal of Pediatrics 2019, 211, 98-104.e4, 10.1016/j.jpeds.2019.02.018.
- Carol L. Wagner; Seeing Beyond Our Expectations: The Case of Pediatric Hypocalcemia.. The Journal of Pediatrics 2019, 211, 9-12, 10.1016/j.jpeds.2019.03.037.
- Michelle Denburg; Andrew N. Hoofnagle; Samir Sayed; Jayanta Gupta; Ian H. De Boer; Lawrence J. Appel; Ramon Durazo-Arvizu; Krista Whitehead; Harold I. Feldman; Mary B. Leonard; Chronic Renal Insufficiency Cohort Study Investigators; on behalf of the Chronic Renal Insufficiency Cohort study investigators; Comparison of Two ELISA Methods and Mass Spectrometry for Measurement of Vitamin D-Binding Protein: Implications for the Assessment of Bioavailable Vitamin D Concentrations Across Genotypes.. Journal of Bone and Mineral Research 2016, 31, 1128-36, 10.1002/jbmr.2829.
- Arturo Borzutzky; Francisca Grob; Carlos A. Camargo; Alejandro Martinez-Aguayo; Vitamin D Deficiency Rickets in an Adolescent With Severe Atopic Dermatitis. Pediatrics 2014, 133, e451-e454, 10.1542/peds.2013-1114.
- R. Bouillon; Leen Antonio; Nutritional rickets: Historic overview and plan for worldwide eradication. The Journal of Steroid Biochemistry and Molecular Biology 2020, 198, 105563, 10.1016/j.jsbmb.2019.105563.
- Martin Hewison; Fiona Burke; Katie N. Evans; David A. Lammas; David Sansom; Philip Liu; Robert L. Modlin; John Adams; Extra-renal 25-hydroxyvitamin D3-1α-hydroxylase in human health and disease. The Journal of Steroid Biochemistry and Molecular Biology 2007, 103, 316-321, 10.1016/j.jsbmb.2006.12.078.
- Philip Liu; Steffen Stenger; Huiying Li; Linda Wenzel; Belinda H. Tan; Stephan R Krutzik; Maria Teresa Ochoa; Jürgen Schauber; Kent Wu; Christoph Meinken; Diane Kamen; Manfred Wagner; Robert Bals; Andreas Steinmeyer; Ulrich Zügel; Richard L. Gallo; David S. Eisenberg; Martin Hewison; Bruce W. Hollis; John Adams; Barry R. Bloom; Robert L. Modlin; Toll-Like Receptor Triggering of a Vitamin D-Mediated Human Antimicrobial Response. Science 2006, 311, 1770-1773, 10.1126/science.1123933.
- Michael C. Iannuzzi, M.D., Benjamin A. Rybicki, Ph.D., and Alvin S. Teirstein, M.D.; Sarcoidosis. N Engl J Med 2007, 357, 2153-2165, 10.1056/NEJMra071714.
- M. Hewison; S. Barker; A. Brennan; D.R. Katz; J.L.H. O'riordan; Modulation of myelomonocytic U937 cells by vitamin D metabolites. Bone and Mineral 1989, 5, 323-333, 10.1016/0169-6009(89)90010-x.
- Tian-Tian Wang; Basel Dabbas; David Laperriere; Ari J. Bitton; Hafid Soualhine; Luz E. Tavera-Mendoza; Serge Dionne; Marc J. Servant; Alain Bitton; Ernest G. Seidman; et al.Sylvie MaderMarcel A. BehrJohn H. White Direct and Indirect Induction by 1,25-Dihydroxyvitamin D3 of the NOD2/CARD15-Defensin β2 Innate Immune Pathway Defective in Crohn Disease*. Journal of Biological Chemistry 2009, 285, 2227-2231, 10.1074/jbc.C109.071225.
- Tian-Tian Wang; Frederick P. Nestel; Véronique Bourdeau; Yoshihiko Nagai; Qiuyu Wang; Jie Liao; Luz Tavera-Mendoza; Roberto Lin; John W. Hanrahan; Sylvie Mader; John H. White; Cutting Edge: 1,25-Dihydroxyvitamin D3 Is a Direct Inducer of Antimicrobial Peptide Gene Expression. The Journal of Immunology 2004, 173, 2909-2912, 10.4049/jimmunol.173.5.2909.
- Adrian F. Gombart; Niels Borregaard; H. Phillip Koeffler; Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up‐regulated in myeloid cells by 1,25‐dihydroxyvitamin D 3. The FASEB Journal 2005, 19, 1067-1077, 10.1096/fj.04-3284com.
- Vassil Dimitrov; John H. White; Species-specific regulation of innate immunity by vitamin D signaling. The Journal of Steroid Biochemistry and Molecular Biology 2016, 164, 246-253, 10.1016/j.jsbmb.2015.09.016.
- Luis G Vargas Buonfiglio; Marlene Cano; Alejandro A Pezzulo; Oriana G Vanegas Calderon; Joseph Zabner; Alicia K Gerke; Alejandro P Comellas; Effect of vitamin D3 on the antimicrobial activity of human airway surface liquid: preliminary results of a randomised placebo-controlled double-blind trial. BMJ Open Respiratory Research 2017, 4, e00021, 10.1136/bmjresp-2017-000211.
- Mark Verway; Manuella Bouttier; Tian-Tian Wang; Marilyn Carrier; Mario R Calderon; Beum-Soo An; Emmanuelle Devemy; Fiona McIntosh; Maziar Divangahi; Marcel A. Behr; et al.John H. White Vitamin D Induces Interleukin-1β Expression: Paracrine Macrophage Epithelial Signaling Controls M. tuberculosis Infection. PLOS Pathogens 2013, 9, e1003407, 10.1371/journal.ppat.1003407.
- G. Penna; Luciano Adorini; 1α,25-Dihydroxyvitamin D3 Inhibits Differentiation, Maturation, Activation, and Survival of Dendritic Cells Leading to Impaired Alloreactive T Cell Activation. The Journal of Immunology 2000, 164, 2405-2411, 10.4049/jimmunol.164.5.2405.
- Luciano Adorini; Intervention in autoimmunity: The potential of vitamin D receptor agonists. Cellular Immunology 2005, 233, 115-124, 10.1016/j.cellimm.2005.04.013.
- Diane L. Kamen; Vin Tangpricha; Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. Journal of Molecular Medicine 2010, 88, 441-50, 10.1007/s00109-010-0590-9.
- Femke Baeke; T Takiishi; Hannelie Korf; Conny Gysemans; Chantal Mathieu; Vitamin D: modulator of the immune system. Current Opinion in Pharmacology 2010, 10, 482-496, 10.1016/j.coph.2010.04.001.
- Zoë Urry; Emma S. Chambers; Emmanuel Xystrakis; Sarah Dimeloe; David F. Richards; Leona Gabryšová; Jillian Christensen; Atul Gupta; Sejal Saglani; Andrew Bush; Anne O’Garra; Zarin Brown; Catherine M. Hawrylowicz; The role of 1α,25-dihydroxyvitamin D3 and cytokines in the promotion of distinct Foxp3+and IL-10+CD4+T cells. European Journal of Immunology 2012, 42, 2697-708, 10.1002/eji.201242370.
- Rene Chun; Philip T. Liu; Robert L. Modlin; John S. Adams; Martin Hewison; Impact of vitamin D on immune function: lessons learned from genome-wide analysis. Frontiers in Physiology 2014, 5, 151, 10.3389/fphys.2014.00151.
- John Watkins; Preventing a covid-19 pandemic. BMJ 2020, 368, m810, 10.1136/bmj.m810.
- Wei Xia; Jianbo Shao; Yu Guo; Xuehua Peng; Zhen Li; Daoyu Hu; Clinical and CT features in pediatric patients with COVID‐19 infection: Different points from adults. Pediatric Pulmonology 2020, 55, 1169-1174, 10.1002/ppul.24718.
- Peter G. Barlow; Pavel Svoboda; Annie Mackellar; Anthony A. Nash; Ian York; Jan Pohl; Nald J. Davidson; Ruben O. Donis; Antiviral Activity and Increased Host Defense against Influenza Infection Elicited by the Human Cathelicidin LL-37. PLOS ONE 2011, 6, e25333, 10.1371/journal.pone.0025333.
- Aurica G. Telcian; Mihnea Zdrenghea; Michael R. Edwards; Vasile Laza-Stanca; Patrick Mallia; Sebastian L. Johnston; Luminita A. Stanciu; Vitamin D increases the antiviral activity of bronchial epithelial cells in vitro. Antiviral Research 2017, 137, 93-101, 10.1016/j.antiviral.2016.11.004.
- Yan Chun Li; Guilin Qiao; Milan Uskokovic; Wei Xiang; Wei Zheng; Juan Kong; Vitamin D: a negative endocrine regulator of the renin–angiotensin system and blood pressure. The Journal of Steroid Biochemistry and Molecular Biology 2004, 89, 387-392, 10.1016/j.jsbmb.2004.03.004.
- Renhong Yan; Yuanyuan Zhang; Yaning Li; Lu Xia; Yingying Guo; Qiang Zhou; Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444-1448, 10.1126/science.abb2762.
- Marta Riera; Lidia Anguiano; Sergi Clotet; Heleia Roca Ho; Marta Rebull; Julio Pascual; María José Soler; Paricalcitol modulates ACE2 shedding and renal ADAM17 in NOD mice beyond proteinuria. American Journal of Physiology-Renal Physiology 2016, 310, F534-F546, 10.1152/ajprenal.00082.2015.
- Lei Fang; George Karakiulakis; Michael Roth; Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?. The Lancet Respiratory Medicine 2020, 8, e21, 10.1016/S2213-2600(20)30116-8.
