One of the key features associated with the substantial increase in life expectancy for individuals with CF is an elevated predisposition to cancer, firmly established by recent studies involving large cohorts. With the recent advances in CFTR modulator therapies and the increased long-term survival rate of individuals with CF, this is a novel challenge emerging at the forefront of this disease. However, the mechanisms linking dysfunctional CFTR to carcinogenesis have yet to be unravelled.
- Cancer
- Cystic Fibrosis
- EMT
- Epithelial differentiation
1. Introduction
Here, we review such studies implicating CFTR in those fundamental cellular processes such as foetal development (Figure 1).
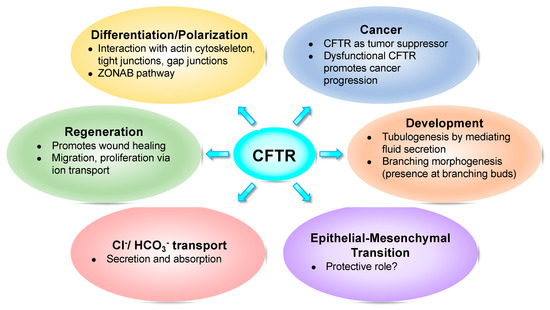
Figure 1. Cystic fibrosis transmembrane conductance regulator (CFTR) impacts several fundamental cellular processes.
2. Development
Another noteworthy question is how CFTR is implicated in epithelial differentiation/ polarization and in the related process of tissue repair. Data collected from wound healing experiments, as an experimental model to study these processes, clearly show that functional CFTR promotes migration while also affecting proliferation. Thus, acting on these two processes could be a novel strategy to promote epithelial repair in CF.
Concrete evidence for the role of CFTR in epithelial differentiation also derives from the observation that its absence leads to an intermediate (partial) state of EMT. Moreover, EMT induction by dysfunctional CFTR also provides a direct (causal) link to its role as tumour suppressor. However, further studies are needed to characterize how functional CFTR prevents progression into EMT.
In this review, we provide state-of-the-art descriptions on the moonlight role(s) of CFTR on these processes, highlighting how the understanding of the underlying mechanisms can contribute to novel therapeutic strategies. However, such roles are still largely unknown, so we need rapid progress in the elucidation of these mechanisms to find the answers and thus tailor the most appropriate therapeutic approaches.
