You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Adonis Sfera.
Similar to previous pandemics, COVID-19 has been succeeded by well-documented post-infectious sequelae, including chronic fatigue, cough, shortness of breath, myalgia, and concentration difficulties. Dysfunctional efferocytosis has been associated with biological barrier disruption, inflammatory bowel disease (IBD), and a constellation of symptoms reminiscent of long COVID and other fatiguing illnesses.
- cortisol
- HMGB1
- microbial translocation
- SARS-CoV-2
1. Introduction
In the post-pandemic era, residual or long COVID-19 sequelae have been gradually emerging as many patients experience prolonged fatigue, cough, shortness of breath, myalgia, and problems with concentration long after the acute illness phase [1]. From a biological pathway perspective, as both SARS-CoV-2 infection and the associated psychological stress upregulate cortisol, the function of macrophages and natural killer (NK) cells may be impaired, disrupting the clearance of senescent, damaged, or virus-infected cells. This may lead to biological barrier dysfunction and chronic fatigue, phenomena well-documented in cancer survivors treated with cellular senescence-inducing chemotherapy or radiation [2,3,4,5][2][3][4][5].
At the molecular level, upregulated cortisol lowers the expression of claudin-1 (CLDN1), an intestinal tight junction protein, facilitating microbial translocation outside of the gastrointestinal (GI) tract [6]. In the central nervous system (CNS), P-glycoprotein (P-gp), a cortisol substrate, functions as a gatekeeper of the blood–brain barrier (BBB), likely accounting for hypercortisolemia-increased barrier permeability [7,8,9][7][8][9]. Interestingly, in the GI tract, gut microbiota and lipopolysaccharide (LPS), a cell wall component of Gram-negative bacteria, regulate P-gp, connecting this protein to microbial translocation [10,11][10][11].
Recent studies have found a direct relationship between circulating levels of cortisol and premature cellular senescence, a phenotype characterized by permanent cell cycle arrest, active metabolism, and a detrimental secretome [12]. The accumulation of senescent cells was found to be associated not only with organismal aging but also with chronic fatigue, pain, and depression, documented in cancer survivors [2]. On the other hand, enhanced elimination of senescent cells via senotherapeutics can correct barrier dysfunction, lowering fatigue [13,14,15][13][14][15].
NK cells and macrophages can execute the phagocytic engulfment (efferocytosis) of damaged or dead cells, including malignant, virus-infected, and senescent cells. Dysfunctional efferocytosis has been associated with biological barrier disruption, inflammatory bowel disease (IBD), and a constellation of symptoms reminiscent of long COVID and other fatiguing illnesses [16,17,18,19,20,21][16][17][18][19][20][21]. Indeed, NK cell dysfunction is one of the most consistent findings in long COVID-19, myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), Gulf War illnesses (GWI), and fibromyalgia (FM), linking these pathologies to dysfunctional efferocytosis [22,23,24,25,26,27][22][23][24][25][26][27]. Moreover, NK cells had been previously linked to fatigue-associated thyroid, adrenal, hypothalamic, and pituitary disorders, thus connecting these neuroendocrine -related pathologies with dysfunctional immunity [28,29,30,31,32][28][29][30][31][32]. Furthermore, NK cells express estrogen, prolactin, and cortisol receptors as well as a functional renin-angiotensin-system (RAS), rendering them sensitive to hormonal fluctuations [33,34,35][33][34][35]. NK cells are capable of paracrine signaling and they secrete biomolecules, including perforin, granzyme B, and the high mobility group box 1 protein (HMGB1) that can facilitate the elimination of damaged, senescent, and/or malignant cells [36,37,38][36][37][38].
Microbial translocation markers, such as LPS, lipopolysaccharide binding protein (LPB), soluble CD14 (sCD14), and HMGB1, were found to be elevated in long COVID, highlighting the role of dysfunctional gut barrier in this condition [30,31,32,33,34,35,36,37,38,39,40,41][30][31][32][33][34][35][36][37][38][39][40][41]. Indeed, accumulation of senescent cells has been associated with HMGB1 spillover into the extracellular space where it can act as an inflammagen and barrier disruptor [42,43,44,45,46][42][43][44][45][46]. Therefore, upregulated HMGB1, documented in ME/CFS, FM, and GWI, and COVID-19, directly links dysfunctional efferocytosis to chronic fatigue [47,48,49,50][47][48][49][50]. For example, gut HMGB1 disrupts the barrier tight junctions and is considered a biomarker of inflammatory bowel disease (IBD), a condition associated with both fatigue and increased GI tract permeability. This may explain certain SARS-CoV-2 bacteriophage-like properties as this virus can likely penetrate the microbial cell walls [51,52,53,54,55,56,57][51][52][53][54][55][56][57].
2. Efferocytosis and Biological Barriers
Each day billions of cells throughout the body undergo apoptosis and are removed by professional phagocytes, macrophages, monocytes, and neutrophils as well as non-professional phagocytes, including the intestinal epithelial cells (IECs) and the M2 microglia in the BBB [58,59,60][58][59][60]. Professional and non-professional phagocytes are assisted by NK cells that can eliminate defective and pathogen-infected cells without prior sensitization [61,62,63][61][62][63]. NK cells maintain the integrity of BBB and the intestinal barrier as they can promptly clear damaged cells, preventing inflammation and barrier disruption [64,65][64][65]. To accomplish this, NK cells perforate the membrane of targeted cells by releasing HMGB1, perforin, and granzyme, triggering apoptosis by Ca2+ influx [66,67][66][67]. Upregulated cytosolic Ca2+ is known to activate TMEM16F, an enzyme that flips phosphatidylserine (PS) to the outer leaflet of the cell membrane, providing a distress signal, that attracts immune cells, promoting phagocytosis [68]. Exposed PS (ePS) comprises an “eat me” or “fuse with me” signal that can lead to either cell death or syncytia formation, depending on the degree of cell membrane damage [69]. For example, less damaged cells can fuse with each other for protection, a phenomenon documented in in many tissues, including the CNS [70] (Figure 1). Several studies have demonstrated that senescent and cancer cells can avoid elimination by expressing CD47, a “don’t eat me” signal, that inhibits phagocytosis [71,72,73][71][72][73].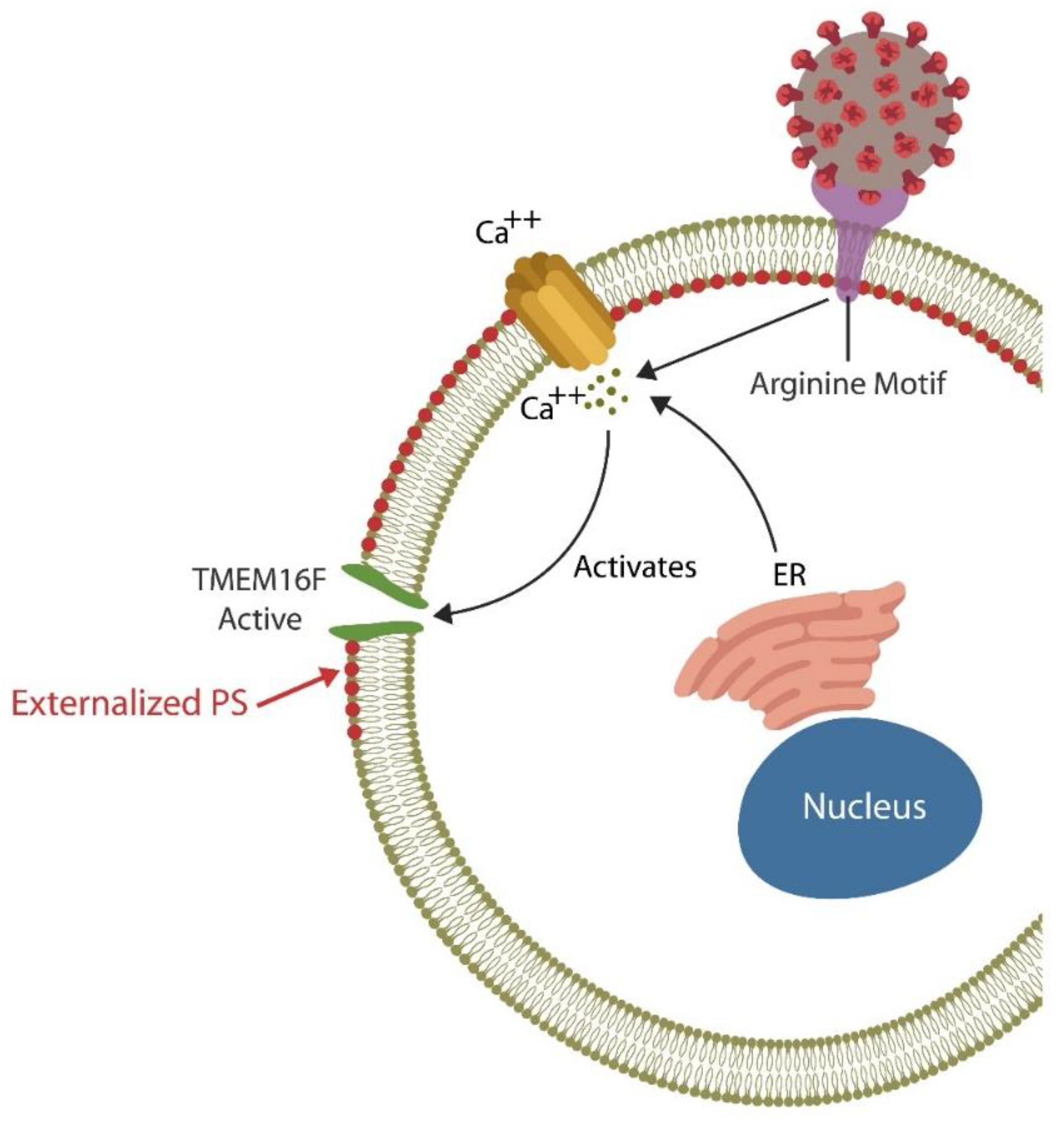
Figure 1. The SARS-CoV-2 receptor binding site (RBS) contains a double arginine insert (PRRA) or arginine motif, that perforates the cell membrane, triggering Ca2+ influx from both the endoplasmic reticulum (ER) and the extracellular compartment. Upregulated cytosolic Ca2+ activates TMEN16F, externalizing phosphatidylserine (ePS), an “eat me” or “fuse me” signal that leads to cell death (if the damage is irreparable) or cell–cell fusion (if the cell can be repaired). Cell–cell fusion or syncytia formation induces premature cellular senescence, disrupting biological barriers. The virus benefits from ePS as this comprises a global immunosuppressive signal, allowing its undetected entry into host cells.
2.1. Blood–Brain Barrier
The BBB, a highly regulated interface between the circulatory system and the CNS, consists of cerebral endothelial cells (ECs) that regulate the inward and outward movement of molecules and ions into the CNS [74]. BBB disruption enables viral entry into the brain along with inflammatory cells, and/or deleterious molecules that can trigger infection. Indeed, members of at least 11 viral families, including the human immunodeficiency virus-1 (HIV-1), T-cell leukemia virus, lymphocytic choriomeningitis virus, West Nile virus, and others, can enter the brain, causing encephalitis [75,76,77][75][76][77]. COVID-19-mediated accumulation of senescent ECs compromises the BBB, allowing viral and microbial access to the CNS [78]. In contrast, enhanced elimination of senescent cells via senolytic drugs decreases COVID-19 mortality in rodents, highlighting the role of senescent cells in BBB dysfunction [79,80][79][80]. In addition, the S protein of the SARS-CoV-2 virus was demonstrated to directly bind bacterial LPS, outlining a virus-mediated mechanism of endotoxin entry into the CNS [40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81]. LPS-induced neuroinflammation has been associated with microglial fusion and multinucleation, generating highly phagocytic phenotypes that can cause collateral damage by eliminating viable neurons [82,83][82][83]. Indeed, long COVID has been associated with LPS-activated microglia (M1 phenotype), neuroinflammation and neuronal death [82,83,84,85][82][83][84][85]. On the other hand, the M2 microglial phenotype has been shown to repair the damage, protecting the neurons [58].2.2. Intestinal Barrier
Many viruses, including SARS-CoV-2, enhance infectivity by usurping both the physiological cell–cell fusion and efferocytosis, disrupting biological barriers [75,86][75][86]. For example, the SARS-CoV-2 virus thrives in infected cells and likely inhibits their clearance, causing inflammation and barrier dysfunction [87]. In addition, SARS-CoV-2 promotes pathological cell–cell fusion and syncytia formation by generating cell membrane pores via the PRRA (proline-arginine-arginine-alanine) motif situated at the furin-cleavage site (FCS). This system is reminiscent of microbial twin arginine translocation pathway, a pore-forming mechanism implicated in bacterial virulence [88,89,90][88][89][90]. Moreover, SARS-CoV-2 fusion with Mycoplasma, an arginine dependent microorganism, may explain the high comorbidity of these very different infections [91]. Cell membrane pores lead to ePS, a global immunosuppressive signal, that helps the virus exploit host defenses [92]. The subsequent, syncytia formation can then induce premature cellular senescence and the release of senescence-associated secretory phenotype (SASP), a pathological secretome that disrupts endothelial barriers by promoting premature ECs senescence, a phenotype documented in both ME/CFS and COVID-19 [42,93,94,95][42][93][94][95]. Senescence-induced pathological syncytia can trigger lymphopenia by cell-in-cell phenomena, elimination of viable lymphocytes, including NK cells, a frequent finding in ME/CFS [96,97,98][96][97][98]. In addition, as cellular senescence upregulates HMGB1, it may further predispose to fatiguing disorders [44,47,48,49,99,100,101,102][44][47][48][49][99][100][101][102]. In the GI tract, IECs comprise a single layer of tightly linked columnar cells that are short-lived and need to be replaced every 4 to 5 days to maintain an adequate barrier function [103]. Moreover, IECs acting as non-professional phagocytes, can engulf the translocating microbes and/or antigens, preventing microbial translocation outside the GI tract [104]. However, accumulation of uncleared, defective IECs can trigger inflammation, predisposing to IBD and other illnesses marked by dysfunctional barrier [105]. Macrophages, intestinal NK cells, and Paneth cells contribute to barrier integrity by promptly removing damaged IECs, thus averting necrosis and inflammation-mediated pathology [21,106,107,108][21][106][107][108]. Interestingly, IECs were demonstrated to produce cortisol, a steroid hormone that lowers the expression of CLDN1 that in return increases intestinal permeability [109,110,111][109][110][111]. The role of ANG II: COVID-19-upregulated angiotensin II (ANG II) can disrupt efferocytosis inducing ECs senescence and vascular barrier dysfunction via angiotensin II type 1 receptors (AT-1Rs) which can enhance both cortisol and HMGB1 production, [112,113][112][113] (Figure 2).
Figure 2. SARS-CoV-2 attachment to ACE-2, blocks this enzyme, causing angiotensin II (ANG II) accumulation by inhibiting its hydrolysis. Upregulated ANG II, increases both cortisol and HMGB1, disrupting the efferocytosis of senescent cells. Accumulation of senescent cells triggers inflammation and biological barrier disruption, a common pathology found not only in the disorders marked by chronic fatigue but also in neuropsychiatric and autoimmune diseases.
References
- Raveendran, A.V. Long COVID-19: Challenges in the diagnosis and proposed diagnostic criteria. Diabetes Metab. Syndr. 2021, 15, 145–146.
- Shafqat, S.; Arana Chicas, E.; Shafqat, A.; Hashmi, S.K. The Achilles’ heel of cancer survivors: Fundamentals of accelerated cellular senescence. J. Clin. Investig. 2022, 132, e158452.
- Ekedahl, H.; Isaksson, S.; Ståhl, O.; Bogefors, K.; Romerius, P.; Eberhard, J.; Giwercman, A. Low-grade inflammation in survivors of childhood cancer and testicular cancer and its association with hypogonadism and metabolic risk factors. BMC Cancer 2022, 22, 157.
- Reinertsen, K.V.; Loge, J.H.; Brekke, M.; Kiserud, C.E. Chronic fatigue in adult cancer survivors. Tidsskr. Nor. Laegeforen. 2017, 137.
- Bøhn, S.H.; Thorsen, L.; Kiserud, C.E.; Fosså, S.D.; Lie, H.C.; Loge, J.H.; Wisløff, T.; Haugnes, H.S.; Reinertsen, K.V. Chronic fatigue and associated factors among long-term survivors of cancers in young adulthood. Acta Oncol. 2019, 58, 753–762.
- Zheng, G.; Victor Fon, G.; Meixner, W.; Creekmore, A.; Zong, Y.; Dame, M.K.; Colacino, J.; Dedhia, P.H.; Hong, S.; Wiley, J.W. Chronic stress and intestinal barrier dysfunction: Glucocorticoid receptor and transcription repressor HES1 regulate tight junction protein Claudin-1 promoter. Sci. Rep. 2017, 7, 4502.
- Schinkel, A.H. P-Glycoprotein, a gatekeeper in the blood-brain barrier. Adv. Drug Deliv. Rev. 1999, 36, 179–194.
- Mason, B.L.; Pariante, C.M.; Thomas, S.A. A revised role for P-glycoprotein in the brain distribution of dexamethasone, cortisol, and corticosterone in wild-type and ABCB1A/B-deficient mice. Endocrinology 2008, 149, 5244–5253.
- Ueda, K.; Okamura, N.; Hirai, M.; Tanigawara, Y.; Saeki, T.; Kioka, N.; Komano, T.; Hori, R. Human P-glycoprotein transports cortisol, aldosterone, and dexamethasone, but not progesterone. J. Biol. Chem. 1992, 267, 24248–24252.
- Foley, S.E.; Tuohy, C.; Dunford, M.; Grey, M.J.; De Luca, H.; Cawley, C.; Szabady, R.L.; Maldonado-Contreras, A.; Houghton, J.M.; Ward, D.V.; et al. Gut microbiota regulation of P-glycoprotein in the intestinal epithelium in maintenance of homeostasis. Microbiome 2021, 9, 183.
- Moriguchi, J.; Kato, R.; Nakagawa, M.; Hirotani, Y.; Ijiri, Y.; Tanaka, K. Effects of lipopolysaccharide on intestinal P-glycoprotein expression and activity. Eur. J. Pharmacol. 2007, 565, 220–224.
- Yiallouris, A.; Tsioutis, C.; Agapidaki, E.; Zafeiri, M.; Agouridis, A.P.; Ntourakis, D.; Johnson, E.O. Adrenal Aging and Its Implications on Stress Responsiveness in Humans. Front Endocrinol (Lausanne). Front. Endocrinol. 2019, 10, 54.
- Lewis-McDougall, F.C.; Ruchaya, P.J.; Domenjo-Vila, E.; Teoh, T.S.; Prata, L.; Cottle, B.J.; Clark, J.E.; Punjabi, P.P.; Awad, W.; Torella, D.; et al. Aged-senescent cells contribute to impaired heart regeneration. Aging Cell 2019, 18, e12931.
- Short, S.; Fielder, E.; Miwa, S.; von Zglinicki, T. Senolytics and senostatics as adjuvant tumour therapy. eBioMedicine 2019, 41, 683–692.
- Saccon, T.D.; Nagpal, R.; Yadav, H.; Cavalcante, M.B.; Nunes, A.D.C.; Schneider, A.; Gesing, A.; Hughes, B.; Yousefzadeh, M.; Tchkonia, T.; et al. Senolytic Combination of Dasatinib and Quercetin Alleviates Intestinal Senescence and Inflammation and Modulates the Gut Microbiome in Aged Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1895–1905.
- Jeyapalan, J.C.; Sedivy, J.M. Cellular senescence and organismal aging. Mech. Ageing Dev. 2008, 129, 467–474.
- LeBrasseur, N.K.; Tchkonia, T.; Kirkland, J.L. Cellular Senescence and the Biology of Aging, Disease, and Frailty. Frailty Pathophysiol. Phenotype Patient Care 2015, 83, 11–18.
- Lee, J.-H.; Lee, Y.-K.; Lim, J.J.; Byun, H.-O.; Park, I.; Kim, G.-H.; Xu, W.G.; Wang, H.-J.; Yoon, G. Mitochondrial Respiratory Dysfunction Induces Claudin-1 Expression via Reactive Oxygen Species-mediated Heat Shock Factor 1 Activation, Leading to Hepatoma Cell Invasiveness. J. Biol. Chem. 2015, 290, 21421–21431.
- Martínez-Cué, C.; Rueda, N. Cellular senescence in neurodegenerative diseases. Front. Cell Neurosci. 2020, 14, 16.
- Nocerino, A.; Nguyen, A.; Agrawal, M.; Mone, A.; Lakhani, K.; Swaminath, A. Fatigue in Inflammatory Bowel Diseases: Etiologies and Management. Adv. Ther. 2020, 37, 97–112.
- Martin-Rodriguez, O.; Gauthier, T.; Bonnefoy, F.; Couturier, M.; Daoui, A.; Chagué, C.; Valmary-Degano, S.; Gay, C.; Saas, P.; Perruche, S. Pro-Resolving Factors Released by Macrophages After Efferocytosis Promote Mucosal Wound Healing in Inflammatory Bowel Disease. Front. Immunol. 2021, 12, 754475.
- Verma, V.; Drury, G.L.; Parisien, M.; Özdağ Acarli, A.N.; Al-Aubodah, T.A.; Nijnik, A.; Wen, X.; Tugarinov, N.; Verner, M.; Klares, R., 3rd; et al. Unbiased immune profiling reveals a natural killer cell-peripheral nerve axis in fibromyalgia. Pain 2022, 163, e821–e836.
- Bi, J. NK cell dysfunction in patients with COVID-19. Cell Mol. Immunol. 2022, 19, 127–129.
- Galán, M.; Vigón, L.; Fuertes, D.; Murciano-Antón, M.A.; Casado-Fernández, G.; Domínguez-Mateos, S.; Mateos, E.; Ramos-Martín, F.; Planelles, V.; Torres, M.; et al. Persistent Overactive Cytotoxic Immune Response in a Spanish Cohort of Individuals With Long-COVID: Identification of Diagnostic Biomarkers. Front. Immunol. 2022, 13, 848886.
- Whistler, T.; Fletcher, M.A.; Lonergan, W.; Zeng, X.R.; Lin, J.M.; Laperriere, A.; Vernon, S.D.; Klimas, N.G. Impaired immune function in Gulf War Illness. BMC Med. Genom. 2009, 2, 12.
- Rivas, J.L.; Palencia, T.; Fernández, G.; García, M. Association of T and NK Cell Phenotype With the Diagnosis of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Front. Immunol. 2018, 9, 1028.
- Sung, A.P.; Tang, J.J.; Guglielmo, M.J.; Smith-Gagen, J.; Bateman, L.; Navarrete-Galvan, L.; Redelman, D.D.; Hudig, D. Antibody-Dependent Cell-mediated Cytotoxicity (ADCC) in Familial Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Fatigue 2020, 8, 226–244.
- Lee, E.K.; Sunwoo, J.B. Natural Killer Cells and Thyroid Diseases. Endocrinol. Metab. Seoul. 2019, 34, 132–137.
- Bancos, I.; Hazeldine, J.; Chortis, V.; Hampson, P.; Taylor, A.E.; Lord, J.M.; Arlt, W. Primary adrenal insufficiency is associated with impaired natural killer cell function: A potential link to increased mortality. Eur. J. Endocrinol. 2017, 176, 471–480.
- Lamers, M.M.; Beumer, J.; van der Vaart, J.; Knoops, K.; Puschhof, J.; Breugem, I.T.; Ravelli, R.B.G.; van Schayck, J.P.; Mykytyn, A.Z.; Duimel, H.Q.; et al. SARS-CoV-2 productively infects human gut enterocytes. Science 2020, 369, 50–54.
- Sanno, N.; Itoh, J.; Teramoto, A.; Itoh, Y.; Hori, S.; Osamura, R.Y. Immunohistochemical detection of human natural killer cell like immunoreactivity in human pituitary adenomas, using monoclonal antibody NK-1. J. Neurooncol. 1997, 35, 29–38.
- Belluardo, N.; Mudó, G.; Cella, S.; Santoni, A.; Forni, G.; Bindoni, M. Hypothalamic control of certain aspects of natural immunity in the mouse. Immunology 1987, 62, 321–327.
- Godoy-Pacheco, A.; García-Chagollán, M.; Ramírez-De-Arellano, A.; Hernández-Silva, C.D.; Villegas-Pineda, J.C.; Ramírez-López, I.G.; Zepeda-Nuño, J.S.; Aguilar-Lemarroy, A.; Pereira-Suárez, A.L. Differential modulation of natural killer cell cytotoxicity by 17β-estradiol and prolactin through the NKG2D/NKG2DL axis in cervical cancer cells. Oncol. Lett. 2022, 24, 288.
- Mavoungou, E.; Bouyou-Akotet, M.K.; Kremsner, P.G. Effects of prolactin and cortisol on natural killer (NK) cell surface expression and function of human natural cytotoxicity receptors (NKp46, NKp44 and NKp30). Clin. Exp. Immunol. 2005, 139, 287–296.
- Jurewicz, M.; McDermott, D.H.; Sechler, J.M.; Tinckam, K.; Takakura, A.; Carpenter, C.B.; Milford, E.; Abdi, R. Human T and natural killer cells possess a functional renin-angiotensin system: Further mechanisms of angiotensin II-induced inflammation. J. Am. Soc. Nephrol. 2007, 18, 1093–1102.
- Ambrose, A.R.; Hazime, K.S.; Worboys, J.D.; Niembro-Vivanco, O.; Davis, D.M. Synaptic secretion from human natural killer cells is diverse and includes supramolecular attack particles. Proc. Natl. Acad. Sci. USA 2020, 117, 23717–23720.
- Gdynia, G.; Sauer, S.; Kopitz, J.; Fuchs, D.; Duglova, K.; Ruppert, T.; Miller, M.; Pahl, J.; Cerwenka, A.; Enders, M.; et al. The HMGB1 protein induces a metabolic type of tumour cell death by blocking aerobic respiration. Nat. Commun. 2016, 7, 10764.
- Cerwenka, A.; Kopitz, J.; Schirmacher, P.; Roth, W.; Gdynia, G. HMGB1: The metabolic weapon in the arsenal of NK cells. Mol. Cell Oncol. 2016, 3, e1175538.
- Peluso, M.J.; Deitchman, A.N.; Torres, L.; Iyer, N.S.; Munter, S.E.; Nixon, C.C.; Donatelli, J.; Thanh, C.; Takahashi, S.; Hakim, J.; et al. Long-term SARS-CoV-2-specific immune and inflammatory responses in individuals recovering from COVID-19 with and without post-acute symptoms. Cell Rep. 2021, 36, 109518.
- Petruk, G.; Puthia, M.; Petrlova, J.; Samsudin, F.; Strömdahl, A.C.; Cerps, S.; Uller, L.; Kjellström, S.; Bond, P.J.; Schmidtchen, A.A.; et al. SARS-CoV-2 spike protein binds to bacterial lipopolysaccharide and boosts proinflammatory activity. J. Mol. Cell Biol. 2020, 12, 916–932.
- Štros, M.; Polanská, E.V.; Hlaváčová, T.; Skládal, P. Progress in Assays of HMGB1 Levels in Human Plasma-The Potential Prognostic Value in COVID-19. Biomolecules 2022, 12, 544.
- Tripathi, U.; Nchioua, R.; Prata, L.G.P.L.; Zhu, Y.; Gerdes, E.O.W.; Giorgadze, N.; Pirtskhalava, T.; Parker, E.; Xue, A.; Espindola-Netto, J.M.; et al. SARS-CoV-2 causes senescence in human cells and exacerbates the senescence-associated secretory phenotype through TLR-3. Aging 2021, 13, 21838–21854.
- Davalos, A.R.; Kawahara, M.; Malhotra, G.K.; Schaum, N.; Huang, J.; Ved, U.; Beausejour, C.M.; Coppe, J.P.; Rodier, F.; Campisi, J. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J. Cell Biol. 2013, 201, 613–629.
- Sofiadis, K.; Josipovic, N.; Nikolic, M.; Kargapolova, Y.; Übelmesser, N.; Varamogianni-Mamatsi, V.; Zirkel, A.; Papadionysiou, I.; Loughran, G.; Keane, J.; et al. HMGB1 coordinates SASP-related chromatin folding and RNA homeostasis on the path to senescence. Mol. Syst. Biol. 2021, 17, e9760.
- Banerjee, S.; Friggeri, A.; Liu, G.; Abraham, E. The C-terminal acidic tail is responsible for the inhibitory effects of HMGB1 on efferocytosis. J. Leukoc. Biol. 2010, 88, 973–979.
- Friggeri, A.; Yang, Y.; Banerjee, S.; Park, Y.J.; Liu, G.; Abraham, E. HMGB1 inhibits macrophage activity in efferocytosis through binding to the alphavbeta3-integrin. Am. J. Physiol. Cell Physiol. 2010, 299, C1267–C1276.
- Nguyen, T.; Johnston, S.; Chacko, A.; Gibson, D.; Cepon, J.; Smith, P.; Staines, D.; Marshall-Gradisnik, S. Novel characterisation of mast cell phenotypes from peripheral blood mononuclear cells in chronic fatigue syndrome/myalgic encephalomyelitis patients. Asian Pac. J. Allergy Immunol. 2017, 35, 75–81.
- Oktayoglu, P.; Tahtasiz, M.; Bozkurt, M.; Em, S.; Ucar, D.; Yazmalar, L.; Mete, N.; Nas, K.; Gezer, O. Serum levels of high mobility group box 1 protein and its association with quality of life and psychological and functional status in patients with fibromyalgia. Int. J. Rheum. Dis. 2013, 16, 403–407.
- Garza-Lombó, C.; Thang, M.; Greve, H.J.; Mumaw, C.L.; Messenger, E.J.; Ahmed, C.; Quinn, E.; Sullivan, K.; Block, M.L. Circulating HMGB1 is elevated in veterans with Gulf War Illness and triggers the persistent pro-inflammatory microglia phenotype in male C57Bl/6J mice. Transl. Psychiatry 2021, 11, 390.
- Hsiao, I.H.; Lin, Y.W. Electroacupuncture Reduces Fibromyalgia Pain by Attenuating the HMGB1, S100B, and TRPV1 Signalling Pathways in the Mouse Brain. Evid. Based Complement. Altern. Med. 2022, 2022, 2242074.
- Palone, F.; Vitali, R.; Cucchiara, S.; Pierdomenico, M.; Negroni, A.; Aloi, M.; Nuti, F.; Felice, C.; Armuzzi, A.; Stronati, L. Role of HMGB1 as a suitable biomarker of subclinical intestinal inflammation and mucosal healing in patients with inflammatory bowel disease. Inflamm. Bowel. Dis. 2014, 20, 1448–1457.
- Huang, L.; Zhang, D.; Han, W.; Guo, C. High-mobility group box-1 inhibition stabilizes intestinal permeability through tight junctions in experimental acute necrotizing pancreatitis. Inflamm. Res. 2019, 68, 677–689.
- Zaiatz Bittencourt, V.; Jones, F.; Tosetto, M.; Doherty, G.A.; Ryan, E.J. Dysregulation of Metabolic Pathways in Circulating Natural Killer Cells Isolated from Inflammatory Bowel Disease Patients. J. Crohns. Colitis. 2021, 15, 1316–1325.
- Brogna, C.; Cristoni, S.; Petrillo, M.; Querci, M.; Piazza, O.; Van den Eede, G. Toxin-like peptides in plasma, urine and faecal samples from COVID-19 patients. F1000Research 2021, 10, 550.
- Groff, A.; Kavanaugh, M.; Ramgobin, D.; McClafferty, B.; Aggarwal, C.S.; Golamari, R.; Jain, R. Gastrointestinal Manifestations of COVID-19: A Review of What We Know. Ochsner J. 2021, 21, 177–180.
- Brogna, C.; Brogna, B.; Bisaccia, D.R.; Lauritano, F.; Marino, G.; Montano, L.; Cristoni, S.; Prisco, M.; Piscopo, M. Could SARS-CoV-2 Have Bacteriophage Behavior or Induce the Activity of Other Bacteriophages? Vaccines 2022, 10, 708.
- Aktaş, E.; Özdemir Özgentürk, N. Revealing In Silico that Bacteria’s Outer Membrane Proteins may Help our Bodies Replicate and Carry Severe Acute Respiratory Syndrome Coronavirus 2. Bioinform. Biol. Insights 2022, 16, 11779322221116320.
- Ronaldson, P.T.; Davis, T.P. Regulation of blood-brain barrier integrity by microglia in health and disease: A therapeutic opportunity. J. Cereb. Blood Flow Metab. 2020, 40, S6–S24.
- Neal, M.D.; Leaphart, C.; Levy, R.; Prince, J.; Billiar, T.R.; Watkins, S.; Li, J.; Cetin, S.; Ford, H.; Schreiber, A.; et al. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J. Immunol. 2006, 176, 3070–3079.
- Lim, J.J.; Grinstein, S.; Roth, Z. Diversity and Versatility of Phagocytosis: Roles in Innate Immunity, Tissue Remodeling, and Homeostasis. Front. Cell Infect. Microbiol. 2017, 7, 191.
- Belizário, J.E.; Neyra, J.M.; Setúbal Destro Rodrigues, M.F. When and how NK cell-induced programmed cell death benefits immunological protection against intracellular pathogen infection. Innate Immun. 2018, 24, 452–465.
- Vann, J.M.; Proctor, R.A. Phagocytosis of bacteria by endothelial cells. In Pathogenesis of Wound and Biomaterial-Associated Infections; Wadström, T., Eliasson, I., Holder, I., Ljungh, A., Eds.; Springer: Berlin/Heidelberg, Germany, 1990.
- Seeberg, J.C.; Loibl, M.; Moser, F.; Schwegler, M.; Büttner-Herold, M.; Daniel, C.; Engel, F.B.; Hartmann, A.; Schlötzer-Schrehardt, U.; Goppelt-Struebe, M.; et al. Non-professional phagocytosis: A general feature of normal tissue cells. Sci. Rep. 2019, 9, 11875.
- Spits, H.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.; Mebius, R.E.; et al. Innate lymphoid cells-a proposal for uniform nomenclature. Nat. Rev. Immunol. 2013, 13, 145–149.
- Sedgwick, A.J.; Ghazanfari, N.; Constantinescu, P.; Mantamadiotis, T.; Barrow, A.D. The Role of NK Cells and Innate Lymphoid Cells in Brain Cancer. Front. Immunol. 2020, 11, 1549.
- Ermak, G.; Davies, K.J. Calcium and oxidative stress: From cell signaling to cell death. Mol. Immunol. 2002, 38, 713–721.
- Kale, A.; Sharma, A.; Stolzing, A.; Desprez, P.Y.; Campisi, J. Role of immune cells in the removal of deleterious senescent cells. Immun. Ageing 2020, 17, 16.
- Bricogne, C.; Fine, M.; Pereira, P.M.; Sung, J.; Tijani, M.; Wang, Y.; Henriques, R.; Collins, M.K.; Hilgemann, D.W. TMEM16F activation by Ca2+ triggers plasma membrane expansion and directs PD-1 trafficking. Sci. Rep. 2019, 9, 619.
- Whitlock, J.M.; Chernomordik, L.V. Flagging fusion: Phosphatidylserine signaling in cell-cell fusion. J. Biol. Chem. 2021, 296, 100411.
- Kemp, K.; Wilkins, A.; Scolding, N. Cell fusion in the brain: Two cells forward, one cell back. Acta Neuropathol. 2014, 128, 629–638.
- Schürch, C.M.; Forster, S.; Brühl, F.; Yang, S.H.; Felley-Bosco, E.; Hewer, E. The “don’t eat me” signal CD47 is a novel diagnostic biomarker and potential therapeutic target for diffuse malignant mesothelioma. Oncoimmunology 2017, 7, e1373235.
- Song, P.; An, J.; Zou, M.H. Immune Clearance of Senescent Cells to Combat Ageing and Chronic Diseases. Cells 2020, 9, 671.
- Barrera, L.; Montes-Servín, E.; Hernandez-Martinez, J.M.; García-Vicente, M.L.Á.; Montes-Servín, E.; Herrera-Martínez, M.; Crispín, J.C.; Borbolla-Escoboza, J.R.; Arrieta, O. CD47 overexpression is associated with decreased neutrophil apoptosis/phagocytosis and poor prognosis in non-small-cell lung cancer patients. Br. J. Cancer 2017, 117, 385–397.
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78.
- Spindler, K.R.; Hsu, T.H. Viral disruption of the blood-brain barrier. Trends Microbiol. 2012, 20, 282–290.
- Strazza, M.; Pirrone, V.; Wigdahl, B.; Nonnemacher, M.R. Breaking down the barrier: The effects of HIV-1 on the blood-brain barrier. Brain Res. 2011, 1399, 96–115.
- Diamond, M.S.; Klein, R.S. West Nile virus: Crossing the blood-brain barrier. Nat Med. 2004, 10, 1294–1295.
- Choi, J.Y.; Park, J.H.; Jo, C.; Kim, K.C.; Koh, Y.H. SARS-CoV-2 spike S1 subunit protein-mediated increase of beta-secretase 1 (BACE1) impairs human brain vessel cells. Biochem. Biophys. Res. Commun. 2022, 626, 66–71.
- Camell, C.D.; Yousefzadeh, M.J.; Zhu, Y.; Prata, L.G.P.L.; Huggins, M.A.; Pierson, M.; Zhang, L.; O’Kelly, R.D.; Pirtskhalava, T.; Xun, P.; et al. Senolytics reduce coronavirus-related mortality in old mice. Science 2021, 373, eabe4832.
- Adesse, D.; Gladulich, L.; Alvarez-Rosa, L.; Siqueira, M.; Marcos, A.C.; Heider, M.; Motta, C.S.; Torices, S.; Toborek, M.; Stipursky, J. Role of aging in Blood–Brain Barrier dysfunction and susceptibility to SARS-CoV-2 infection: Impacts on neurological symptoms of COVID-19. Fluids Barriers CNS 2022, 19, 63.
- Teixeira, P.C.; Dorneles, G.P.; Filho, P.C.S.; da Silva, I.M.; Schipper, L.L.; Postiga, I.A.; Neves, C.A.M.; Junior, L.C.R.; Peres, A.; de Souto, J.T.; et al. Increased LPS levels coexist with systemic inflammation and result in monocyte activation in severe COVID-19 patients. Int. Immunopharmacol. 2021, 100, 108125.
- Gomes-Leal, W. Why microglia kill neurons after neural disorders? The friendly fire hypothesis. Neural. Regen. Res. 2019, 14, 1499–1502.
- Hornik, T.C.; Neniskyte, U.; Brown, G.C. Inflammation induces multinucleation of Microglia via PKC inhibition of cytokinesis, generating highly phagocytic multinucleated giant cells. J. Neurochem. 2014, 128, 650–661.
- Lu, Z.; Liu, S.; Lopes-Virella, M.F.; Wang, Z. LPS and palmitic acid Co-upregulate microglia activation and neuroinflammatory response. Compr. Psychoneuroendocrinol. 2021, 6, 100048.
- Tate, W.; Walker, M.; Sweetman, E.; Helliwell, A.; Peppercorn, K.; Edgar, C.; Blair, A.; Chatterjee, A. Molecular Mechanisms of Neuroinflammation in ME/CFS and Long COVID to Sustain Disease and Promote Relapses. Front. Neurol. 2022, 13, 877772.
- Sencio, V.; Gallerand, A.; Gomes Machado, M.; Deruyter, L.; Heumel, S.; Soulard, D.; Barthelemy, J.; Cuinat, C.; Vieira, A.T.; Barthelemy, A.; et al. Influenza Virus Infection Impairs the Gut’s Barrier Properties and Favors Secondary Enteric Bacterial Infection through Reduced Production of Short-Chain Fatty Acids. Infect. Immun. 2021, 89, e0073420.
- Salina, A.C.G.; Dos-Santos, D.; Rodrigues, T.S.; Fortes-Rocha, M.; Freitas-Filho, E.G.; Alzamora-Terrel, D.L.; Castro, I.M.S.; Fraga da Silva, T.F.C.; de Lima, M.H.F.; Nascimento, D.C.; et al. Efferocytosis of SARS-CoV-2-infected dying cells impairs macrophage anti-inflammatory functions and clearance of apoptotic cells. Elife 2022, 11, e74443.
- Liu, S.; Selvaraj, P.; Lien, C.Z.; Nunez, I.A.; Wu, W.W.; Chou, C.K.; Wang, T.T. The PRRA insert at the S1/S2 site modulates cellular tropism of SARS-CoV-2 and ACE2 usage by the closely related Bat RaTG13. J. Virol. 2021, 95, e01751-20.
- Lee, P.A.; Tullman-Ercek, D.; Georgiou, G. The bacterial twin-arginine translocation pathway. Annu. Rev. Microbiol. 2006, 60, 373–395.
- Yan, X.; Hu, S.; Yang, Y.; Xu, D.; Li, H.; Liu, W.; He, X.; Li, G.; Cai, W.; Bu, Z. The Twin-Arginine Translocation System Is Important for Stress Resistance and Virulence of Brucella melitensis. Infect. Immun. 2020, 88, e00389-20.
- Pereyre, S.; Sirand-Pugnet, P.; Beven, L.; Charron, A.; Renaudin, H.; Barré, A.; Avenaud, P.; Jacob, D.; Couloux, A.; Barbe, V.; et al. Life on arginine for Mycoplasma hominis: Clues from its minimal genome and comparison with other human urogenital mycoplasmas. PLoS Genet. 2009, 5, e1000677.
- Birge, R.B.; Boeltz, S.; Kumar, S.; Carlson, J.; Wanderley, J.; Calianese, D.; Barcinski, M.; Brekken, R.A.; Huang, X.; Hutchins, J.T.; et al. Phosphatidylserine is a global immunosuppressive signal in efferocytosis, infectious disease, and cancer. Cell Death Differ. 2016, 23, 962–978.
- Gal, H.; Krizhanovsky, V. Cell fusion induced senescence. Aging 2014, 6, 353–354.
- Urata, R.; Ikeda, K.; Yamazaki, E.; Ueno, D.; Katayama, A.; Shin-Ya, M.; Ohgitani, E.; Mazda, O.; Matoba, S. Senescent endothelial cells are predisposed to SARS-CoV-2 infection and subsequent endothelial dysfunction. Sci. Rep. 2022, 12, 1–9.
- Rajeevan, M.S.; Murray, J.; Oakley, L.; Lin, J.S.; Unger, E.R. Association of chronic fatigue syndrome with premature telomere attrition. J. Transl. Med. 2018, 16, 44.
- Zhang, Z.; Zheng, Y.; Niu, Z.; Zhang, B.; Wang, C.; Yao, X.; Peng, H.; Franca, D.N.; Wang, Y.; Zhu, Y.; et al. SARS-CoV-2 spike protein dictates syncytium-mediated lymphocyte elimination. Cell Death Differ. 2021, 28, 2765–2777.
- Qiang, S.; Wei, C. Cell-in-cell: An Emerging Player in COVID-19 and Immune Disorders. Natl. Sci. Open 2022, 1, 20220001.
- Fluge, Ø.; Rekeland, I.G.; Lien, K.; Thürmer, H.; Borchgrevink, P.C.; Schäfer, C.; Sørland, K.; Aßmus, J.; Ktoridou-Valen, I.; Herder, I.; et al. B-Lymphocyte Depletion in Patients With Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Ann. Tern. Med. 2019, 170, 585–593.
- Sweetman, E.; Kleffmann, T.; Edgar, C.; de Lange, M.; Vallings, R.; Tate, W. A SWATH-MS analysis of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome peripheral blood mononuclear cell proteomes reveals mitochondrial dysfunction. J. Transl. Med. 2020, 18, 365.
- Hassett, A.L.; Clauw, D.J.; Williams, D.A. Premature Aging in Fibromyalgia. Curr. Aging Sci. 2015, 8, 178–185.
- Zundel, C.G.; Krengel, M.H.; Heeren, T.; Yee, M.K.; Grasso, C.M.; Janulewicz Lloyd, P.A.; Coughlin, S.S.; Sullivan, K. Rates of Chronic Medical Conditions in 1991, Gulf War Veterans Compared to the General Population. Int. J. Environ. Res. Public Health 2019, 16, 949.
- Clark, I.A. Chronic cerebral aspects of long COVID, post-stroke syndromes and similar states share their pathogenesis and perispinal etanercept treatment logic. Pharmacol. Res. Perspect. 2022, 10, e00926, Erratum in: Pharmacol Res Perspect. 2022, 10, e00942.
- Subramanian, S.; Geng, H.; Tan, X.D. Cell death of intestinal epithelial cells in intestinal diseases. Sheng Li Xue Bao 2020, 72, 308–324.
- Geng, H.; Bu, H.F.; Liu, F.; Wu, L.; Pfeifer, K.; Chou, P.M.; Wang, X.; Sun, J.; Lu, L.; Pandey, A.; et al. In Inflamed Intestinal Tissues and Epithelial Cells, Interleukin 22 Signaling Increases Expression of H19 Long Noncoding RNA, Which Promotes Mucosal Regeneration. Gastroenterology 2018, 155, 144–155.
- Lechuga, S.; Ivanov, A.I. Disruption of the epithelial barrier during intestinal inflammation: Quest for new molecules and mechanisms. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1183–1194.
- Shankman, L.S.; Fleury, S.T.; Evans, W.B.; Penberthy, K.K.; Arandjelovic, S.; Blumberg, R.S.; Agaisse, H.; Ravichandran, K.S. Efferocytosis by Paneth cells within the intestine. Curr. Biol. 2021, 31, 2469–2476.e5.
- Satoh-Takayama, N.; Vosshenrich, C.A.; Lesjean-Pottier, S.; Sawa, S.; Lochner, M.; Rattis, F.; Mention, J.J.; Thiam, K.; Cerf-Bensussan, N.; Mandelboim, O.; et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 2008, 29, 958–970.
- Wen, S.; Ling, Y.; Yang, W.; Shen, J.; Li, C.; Deng, W.; Liu, W.; Liu, K. Necroptosis is a key mediator of enterocytes loss in intestinal ischaemia/reperfusion injury. J. Cell Mol. Med. 2017, 21, 432–443.
- Mueller, M.; Cima, I.; Noti, M.; Fuhrer, A.; Jakob, S.; Dubuquoy, L.; Schoonjans, K.; Brunner, T. The nuclear receptor LRH-1 critically regulates extra-adrenal glucocorticoid synthesis in the intestine. J. Exp. Med. 2006, 203, 2057–2062.
- Zheng, G.; Wu, S.P.; Hu, Y.; Smith, D.E.; Wiley, J.W.; Hong, S. Corticosterone mediates stress-related increased intestinal permeability in a region-specific manner. Neurogastroenterol. Motil. 2013, 25, e127–e139.
- Cima, I.; Corazza, N.; Dick, B.; Fuhrer, A.; Herren, S.; Jakob, S.; Ayuni, E.; Mueller, C.; Brunner, T. Intestinal epithelial cells synthesize glucocorticoids and regulate T cell activation. J. Exp. Med. 2004, 200, 1635–1646.
- Zhang, Y.; Wang, Y.; Zhou, D.; Zhang, L.S.; Deng, F.X.; Shu, S.; Wang, L.J.; Wu, Y.; Guo, N.; Zhou, J.; et al. Angiotensin II deteriorates advanced atherosclerosis by promoting MerTK cleavage impairing efferocytosis through the AT1R/ROS/p38 MAPK/ADAM17 pathway. Am. J. Physiol. Cell Physiol. 2019, 317, C776–C787.
- Schulte-Schrepping, J.; Reusch, N.; Paclik, D.; Baßler, K.; Schlickeiser, S.; Zhang, B.; Krämer, B.; Krammer, T.; Brumhard, S.; Bonaguro, L.; et al. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell 2020, 182, 1419–1440.e23.
- Miesbach, W. Pathological Role of Angiotensin II in Severe COVID-19. TH Open 2020, 4, e138–e144.
- Kossmann, S.; Schwenk, M.; Hausding, M.; Karbach, S.H.; Schmidgen, M.I.; Brandt, M.; Knorr, M.; Hu, H.; Kröller-Schön, S.; Schönfelder, T.; et al. Angiotensin II-induced vascular dysfunction depends on interferon-γ-driven immune cell recruitment and mutual activation of monocytes and NK-cells. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1313–1319.
- Biancardi, V.C.; Son, S.J.; Ahmadi, S.; Filosa, J.A.; Stern, J.E. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier. Hypertension 2014, 63, 572–579.
- Liu, T.J.; Shi, Y.Y.; Wang, E.B.; Zhu, T.; Zhao, Q. AT1R blocker losartan attenuates intestinal epithelial cell apoptosis in a mouse model of Crohn’s disease. Mol. Med. Rep. 2016, 13, 1156–1162.
More
