Cancer is a predominant cause of mortality all over the world. Lung, prostate, and colorectal cancer are the more frequent in men while breast and colorectal have a high incidence in women. Major progress aside, some cancers are still frequent and one major issue is improvements in detection methods. Imaging techniques have a major role, but inflammatory, tumoral markers and calculated scores may contribute to the assessment of prognosis. The erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and carcinoembryonic antigen cell adhesion molecule (CEACAM) have been used for decades and do not have a clear use for diagnosis or prognosis yet. The CEACAM family includes 12 human members, and some of them have a cluster differentiation (CD). CD66 may be an interesting indicator of disease severity. Beside interleukin-6 (IL-6), the high level of which is observed in patients with a high mortality rate, other cytokines IL-17A, IL-22, and transforming growth factor -β (TGF-β) are expressed at the tumor level. The detection of circulating tumor cells has been improved but is still of undetermined value. Circulating tumor DNA (ctDNA) was recently studied in CRC stage II patients and may be helpful for chemotherapy management.
- colorectal cancer (CRC)
- C-reactive protein (CRP)
- carcinoembryonic antigen (CEA)
- CEA cell adhesion molecule (CEACAM)
1. Inflammatory Markers in CRC
1.1. Erythrocyte Sedimentation Rate (ESR) and Hemogram
1.2. C-Reactive Protein (CRP)
1.3. Systemic Inflammatory Score or Index
1.4. Cytokines
2. Tumor Markers
2.1. Carcinoembryonic Antigen (CEA)
2.2. CEA Structure
2.3. CEACAM Family
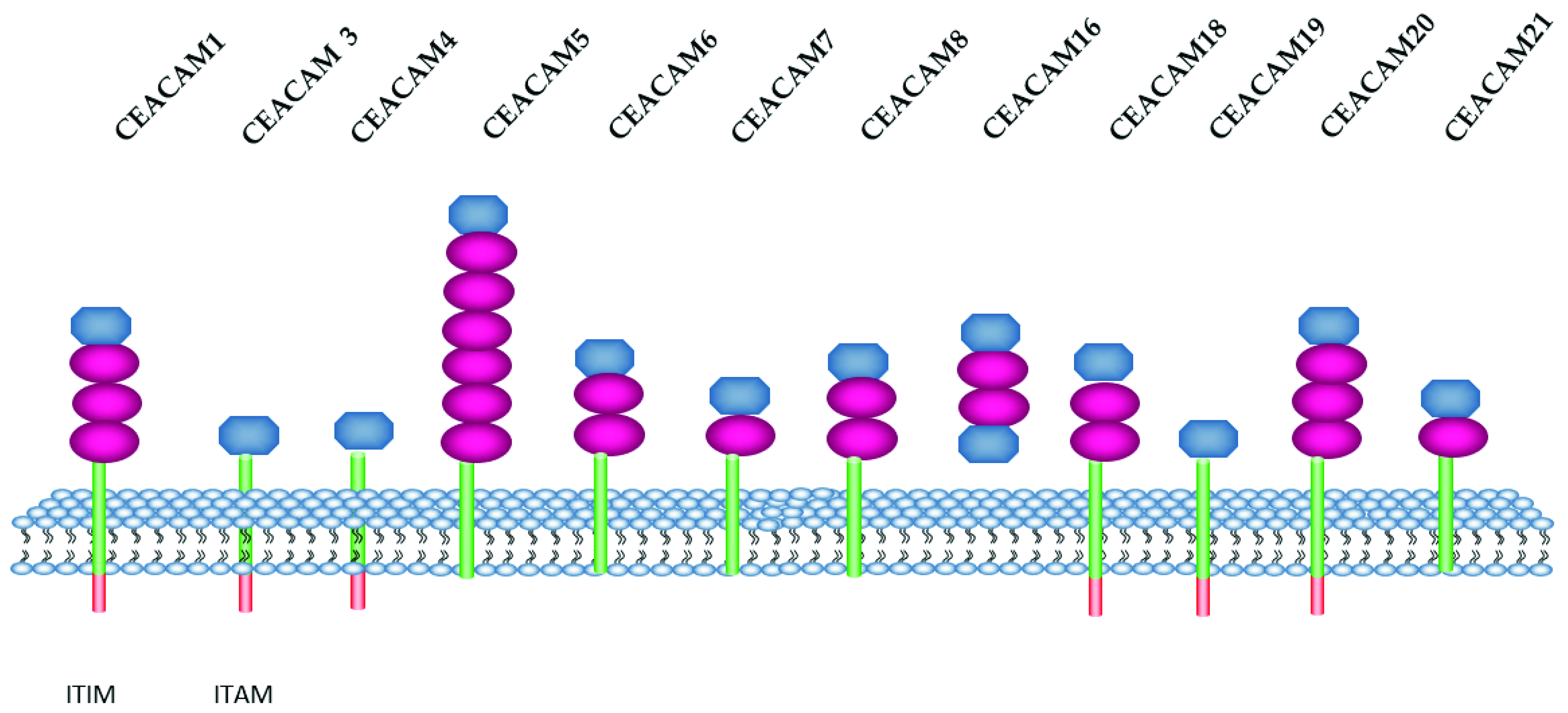
| Name | Amino Acid | Molecular Weight (kDa) |
|---|---|---|
| CEACAM1, CD66a | 526 | 57.6 |
| CEACAM3, CD66d | 252 | 27.1 |
| CEACAM4, CGM7 | 244 | 28.9 |
| CEACAM5, CD66e | 702 | 76.8 |
| CEACAM6, CD66c | 344 | 37.2 |
| CEACAM7, CGM2 | 265 | 29.3 |
| CEACAM8, CD66b | 349 | 38.1 |
| CEACAM16 | 425 | 45.9 |
| CEACAM18 | 384 | 43.3 |
| CEACAM19 | 300 | 32.6 |
| CEACAM20 | 596 | 65.8 |
| CEACAM21 | 293 | 32.3 |
2.4. CEACAM and Leukocytes
3. Micro-RNA and Circulating Tumor DNA (ctDNA) in CRC Patients
A new approach using micro-RNA (miRNA) as a biomarker for CRC was investigated suggesting that miRNA-106a expression was increased and miRNA-30a-3p, miRNA 145, miRNA-125a, and miRNA-133a expression was decreased in CRC tissues. miRNAs may become candidates to develop biomarkers [54]. DNA with tumor specific modification can be found in blood. ctDNA comes from dead tumor cells and are small fragments, including specific alteration in tumor oncogene microsatellites [55][56]. In patients with CRC treated by surgery and chemotherapy, the presence of ctDNA may represent a risk factor, but in several studies the survival time was similar with positive or negative ctDNA [57]. A meta-analysis reveals that detection panels contained genetic or epigenetic alterations. The most frequent mutations were KRAS, but other mutations can be associated. ctDNA appears to be an independent factor of prognosis [58]. Stage II CRC patients with TP53, APC, and KRAS mutations and postoperative ctDNA positivity have a reduced disease-free survival. One of the major problems is the absence of a standardized definition of ctDNA. Another difficulty is the low level of ctDNA and the difference in techniques used for the detection. In most of the studies ctDNA-positive patients have a poorer prognosis. Beside the Dukes’ staging and tumor node and metastasis (TNM) staging [59], the carcinoembryonic antigen blood level provided prognostic information (Figure 2).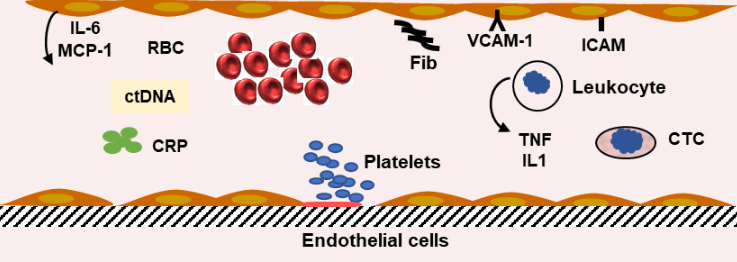
References
- Park, G.; Song, S.Y.; Ahn, J.H.; Kim, W.L.; Lee, J.S.; Jeong, S.Y.; Park, J.W.; Choi, E.K.; Choi, W.; Jung, I.H. The pretreatment erythrocyte sedimentation rate predicts survival outcomes after surgery and adjuvant radiotherapy for extremity soft tissue sarcoma. Radiat. Oncol. 2019, 14, 116.
- Alexandrakis, M.G.; Passam, F.H.; Ganotakis, E.S.; Sfiridaki, K.; Xilouri, I.; Perisinakis, K.; Kyriakou, D.S. The clinical and prognostic significance of erythrocyte sedimentation rate (ESR), serum interleukin-6 (IL-6) and acute phase protein levels in multiple myeloma. Clin. Lab. Haematol. 2003, 25, 41–46.
- Henry-Amar, M.; Friedman, S.; Hayat, M.; Somers, R.; Meerwaldt, J.H.; Carde, P.; Burgers, J.M.; Thomas, J.; Monconduit, M.; Noordijk, E.M.; et al. Erythrocyte sedimentation rate predicts early relapse and survival in early-stage Hodgkin disease. The EORTC Lymphoma Cooperative Group. Ann. Intern. Med. 1991, 114, 361–365.
- Hart, P.C.; Rajab, I.M.; Alebraheem, M.; Potempa, L.A. C-Reactive Protein and Cancer-Diagnostic and Therapeutic Insights. Front. Immunol. 2020, 11, 595835.
- Cesana, C.; Klersy, C.; Barbarano, L.; Nosari, A.M.; Crugnola, M.; Pungolino, E.; Gargantini, L.; Granata, S.; Valentini, M.; Morra, E. Prognostic factors for malignant transformation in monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. J. Clin. Oncol. 2002, 20, 1625–1634.
- Proctor, M.J.; Horgan, P.G.; Talwar, D.; Fletcher, C.D.; Morrison, D.S.; McMillan, D.C. Optimization of the systemic inflammation-based Glasgow prognostic score: A Glasgow Inflammation Outcome Study. Cancer 2013, 119, 2325–2332.
- McMillan, D.C. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat. Rev. 2013, 39, 534–540.
- Emir, S.; Aydin, M.; Can, G.; Bali, I.; Yildirim, O.; Oznur, M.; Yildiz, Z.D.; Sozen, S.; Gurel, A. Comparison of colorectal neoplastic polyps and adenocarcinoma with regard to NLR and PLR. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3613–3618.
- Haram, A.; Boland, M.R.; Kelly, M.E.; Bolger, J.C.; Waldron, R.M.; Kerin, M.J. The prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer: A systematic review. J. Surg. Oncol. 2017, 115, 470–479.
- Stotz, M.; Pichler, M.; Absenger, G.; Szkandera, J.; Arminger, F.; Schaberl-Moser, R.; Samonigg, H.; Stojakovic, T.; Gerger, A. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br. J. Cancer 2014, 110, 435–440.
- Shibutani, M.; Maeda, K.; Nagahara, H.; Ohtani, H.; Iseki, Y.; Ikeya, T.; Sugano, K.; Hirakawa, K. The prognostic significance of the postoperative prognostic nutritional index in patients with colorectal cancer. BMC Cancer 2015, 15, 521.
- Climent, M.; Ryan, E.J.; Stakelum, A.; Khaw, Y.L.; Creavin, B.; Lloyd, A.; Alhassan, D.; Mohan, H.M.; Kennelly, R.; Sheahan, K.; et al. Systemic inflammatory response predicts oncological outcomes in patients undergoing elective surgery for mismatch repair-deficient colorectal cancer. Int. J. Colorectal. Dis. 2019, 34, 1069–1078.
- Zhong, B.; Lin, Z.Y.; Ma, D.D.; Shang, Z.H.; Shen, Y.B.; Zhang, T.; Zhang, J.X.; Jin, W.D. A preoperative prediction model based on Lymphocyte-C-reactive protein ratio predicts postoperative anastomotic leakage in patients with colorectal carcinoma: A retrospective study. BMC Surg. 2022, 22, 283.
- Tan, D.; Fu, Y.; Su, Q.; Wang, H. Prognostic role of platelet-lymphocyte ratio in colorectal cancer: A systematic review and meta-analysis. Medicine 2016, 95, e3837.
- Tan, D.; Fu, Y.; Tong, W.; Li, F. Prognostic significance of lymphocyte to monocyte ratio in colorectal cancer: A meta-analysis. Int. J. Surg. 2018, 55, 128–138.
- Rossi, S.; Basso, M.; Strippoli, A.; Schinzari, G.; D’Argento, E.; Larocca, M.; Cassano, A.; Barone, C. Are Markers of Systemic Inflammation Good Prognostic Indicators in Colorectal Cancer? Clin. Colorectal. Cancer 2017, 16, 264–274.
- Ullman, T.A.; Itzkowitz, S.H. Intestinal inflammation and cancer. Gastroenterology 2011, 140, 1807–1816.
- Waldner, M.J.; Foersch, S.; Neurath, M.F. Interleukin-6--a key regulator of colorectal cancer development. Int. J. Biol. Sci. 2012, 8, 1248–1253.
- Xu, X.; Fu, X.Y.; Plate, J.; Chong, A.S. IFN-gamma induces cell growth inhibition by Fas-mediated apoptosis: Requirement of STAT1 protein for up-regulation of Fas and FasL expression. Cancer Res. 1998, 58, 2832–2837.
- Tannenbaum, C.S.; Hamilton, T.A. Immune-inflammatory mechanisms in IFNgamma-mediated anti-tumor activity. Semin. Cancer Biol. 2000, 10, 113–123.
- Street, S.E.; Trapani, J.A.; MacGregor, D.; Smyth, M.J. Suppression of lymphoma and epithelial malignancies effected by interferon gamma. J. Exp. Med. 2002, 196, 129–134.
- Fridman, W.H.; Galon, J.; Pages, F.; Tartour, E.; Sautes-Fridman, C.; Kroemer, G. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res. 2011, 71, 5601–5605.
- Tosolini, M.; Kirilovsky, A.; Mlecnik, B.; Fredriksen, T.; Mauger, S.; Bindea, G.; Berger, A.; Bruneval, P.; Fridman, W.H.; Pages, F.; et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011, 71, 1263–1271.
- De Simone, V.; Franze, E.; Ronchetti, G.; Colantoni, A.; Fantini, M.C.; Di Fusco, D.; Sica, G.S.; Sileri, P.; MacDonald, T.T.; Pallone, F.; et al. Th17-type cytokines, IL-6 and TNF-alpha synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene 2015, 34, 3493–3503.
- Ueda, T.; Shimada, E.; Urakawa, T. Serum levels of cytokines in patients with colorectal cancer: Possible involvement of interleukin-6 and interleukin-8 in hematogenous metastasis. J. Gastroenterol. 1994, 29, 423–429.
- Jin, W.J.; Xu, J.M.; Xu, W.L.; Gu, D.H.; Li, P.W. Diagnostic value of interleukin-8 in colorectal cancer: A case-control study and meta-analysis. World J. Gastroenterol. 2014, 20, 16334–16342.
- Sun, Q.; Sun, F.; Wang, B.; Liu, S.; Niu, W.; Liu, E.; Peng, C.; Wang, J.; Gao, H.; Liang, B.; et al. Interleukin-8 promotes cell migration through integrin alphavbeta6 upregulation in colorectal cancer. Cancer Lett. 2014, 354, 245–253.
- Xia, W.; Chen, W.; Zhang, Z.; Wu, D.; Wu, P.; Chen, Z.; Li, C.; Huang, J. Prognostic value, clinicopathologic features and diagnostic accuracy of interleukin-8 in colorectal cancer: A meta-analysis. PLoS ONE 2015, 10, e0123484.
- Li, B.; Wang, F.; Ma, C.; Hao, T.; Geng, L.; Jiang, H. Predictive value of IL-18 and IL-10 in the prognosis of patients with colorectal cancer. Oncol. Lett. 2019, 18, 713–719.
- Connolly, E.C.; Freimuth, J.; Akhurst, R.J. Complexities of TGF-beta targeted cancer therapy. Int. J. Biol. Sci. 2012, 8, 964–978.
- Gotzmann, J.; Mikula, M.; Eger, A.; Schulte-Hermann, R.; Foisner, R.; Beug, H.; Mikulits, W. Molecular aspects of epithelial cell plasticity: Implications for local tumor invasion and metastasis. Mutat. Res. 2004, 566, 9–20.
- Shalapour, S.; Karin, M. Immunity, inflammation, and cancer: An eternal fight between good and evil. J. Clin. Investig. 2015, 125, 3347–3355.
- Sabeh, F.; Ota, I.; Holmbeck, K.; Birkedal-Hansen, H.; Soloway, P.; Balbin, M.; Lopez-Otin, C.; Shapiro, S.; Inada, M.; Krane, S.; et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J. Cell Biol. 2004, 167, 769–781.
- Wautier, J.L.; Wautier, M.P. Vascular Permeability in Diseases. Int. J. Mol. Sci. 2022, 23, 3645.
- Gold, P.; Freedman, S.O. Demonstration of Tumor-Specific Antigens in Human Colonic Carcinomata by Immunological Tolerance and Absorption Techniques. J. Exp. Med. 1965, 121, 439–462.
- Beauchemin, N.; Arabzadeh, A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev. 2013, 32, 643–671.
- Kuroki, M.; Matsuo, Y.; Kuroki, M.; Matsuoka, Y. Nonspecific cross-reacting antigen (NCA) expressed by human granulocytes: Six species with different peptide sizes and membrane anchoring forms. Biochem. Biophys. Res. Commun. 1990, 166, 701–708.
- van Gisbergen, K.P.; Aarnoudse, C.A.; Meijer, G.A.; Geijtenbeek, T.B.; van Kooyk, Y. Dendritic cells recognize tumor-specific glycosylation of carcinoembryonic antigen on colorectal cancer cells through dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin. Cancer Res. 2005, 65, 5935–5944.
- Zid, M.; Drouin, G. Gene conversions are under purifying selection in the carcinoembryonic antigen immunoglobulin gene families of primates. Genomics 2013, 102, 301–309.
- Beauchemin, N.; Draber, P.; Dveksler, G.; Gold, P.; Gray-Owen, S.; Grunert, F.; Hammarstrom, S.; Holmes, K.V.; Karlsson, A.; Kuroki, M.; et al. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp. Cell Res. 1999, 252, 243–249.
- Yago, T.; Leppanen, A.; Qiu, H.; Marcus, W.D.; Nollert, M.U.; Zhu, C.; Cummings, R.D.; McEver, R.P. Distinct molecular and cellular contributions to stabilizing selectin-mediated rolling under flow. J. Cell Biol. 2002, 158, 787–799.
- Borsig, L. Selectins in cancer immunity. Glycobiology 2018, 28, 648–655.
- Bonsor, D.A.; Zhao, Q.; Schmidinger, B.; Weiss, E.; Wang, J.; Deredge, D.; Beadenkopf, R.; Dow, B.; Fischer, W.; Beckett, D.; et al. The Helicobacter pylori adhesin protein HopQ exploits the dimer interface of human CEACAMs to facilitate translocation of the oncoprotein CagA. EMBO J. 2018, 37, e98664.
- Bonsignore, P.; Kuiper, J.W.P.; Adrian, J.; Goob, G.; Hauck, C.R. CEACAM3-A Prim(at)e Invention for Opsonin-Independent Phagocytosis of Bacteria. Front. Immunol. 2019, 10, 3160.
- Ordonez, C.; Screaton, R.A.; Ilantzis, C.; Stanners, C.P. Human carcinoembryonic antigen functions as a general inhibitor of anoikis. Cancer Res. 2000, 60, 3419–3424.
- Samara, R.N.; Laguinge, L.M.; Jessup, J.M. Carcinoembryonic antigen inhibits anoikis in colorectal carcinoma cells by interfering with TRAIL-R2 (DR5) signaling. Cancer Res. 2007, 67, 4774–4782.
- Zheng, J.; Miller, K.K.; Yang, T.; Hildebrand, M.S.; Shearer, A.E.; DeLuca, A.P.; Scheetz, T.E.; Drummond, J.; Scherer, S.E.; Legan, P.K.; et al. Carcinoembryonic antigen-related cell adhesion molecule 16 interacts with alpha-tectorin and is mutated in autosomal dominant hearing loss (DFNA4). Proc. Natl. Acad. Sci. USA 2011, 108, 4218–4223.
- Kuespert, K.; Pils, S.; Hauck, C.R. CEACAMs: Their role in physiology and pathophysiology. Curr. Opin. Cell Biol. 2006, 18, 565–571.
- Skubitz, K.M.; Skubitz, A.P. Interdependency of CEACAM-1, -3, -6, and -8 induced human neutrophil adhesion to endothelial cells. J. Transl. Med. 2008, 6, 78.
- Hastings, W.D.; Anderson, D.E.; Kassam, N.; Koguchi, K.; Greenfield, E.A.; Kent, S.C.; Zheng, X.X.; Strom, T.B.; Hafler, D.A.; Kuchroo, V.K. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur. J. Immunol. 2009, 39, 2492–2501.
- Iijima, H.; Neurath, M.F.; Nagaishi, T.; Glickman, J.N.; Nieuwenhuis, E.E.; Nakajima, A.; Chen, D.; Fuss, I.J.; Utku, N.; Lewicki, D.N.; et al. Specific regulation of T helper cell 1-mediated murine colitis by CEACAM1. J. Exp. Med. 2004, 199, 471–482.
- Zhang, Y.; Cai, P.; Li, L.; Shi, L.; Chang, P.; Liang, T.; Yang, Q.; Liu, Y.; Wang, L.; Hu, L. Co-expression of TIM-3 and CEACAM1 promotes T cell exhaustion in colorectal cancer patients. Int. Immunopharmacol. 2017, 43, 210–218.
- Piancone, F.; Saresella, M.; Marventano, I.; La Rosa, F.; Caputo, D.; Mendozzi, L.; Rovaris, M.; Clerici, M. A Deficit of CEACAM-1-Expressing T Lymphocytes Supports Inflammation in Primary Progressive Multiple Sclerosis. J. Immunol. 2019, 203, 76–83.
- Ma, Y.; Zhang, P.; Yang, J.; Liu, Z.; Yang, Z.; Qin, H. Candidate microRNA biomarkers in human colorectal cancer: Systematic review profiling studies and experimental validation. Int. J. Cancer 2012, 130, 2077–2087.
- Diaz, L.A., Jr.; Bardelli, A. Liquid biopsies: Genotyping circulating tumor DNA. J. Clin. Oncol. 2014, 32, 579–586.
- Heitzer, E.; Auer, M.; Ulz, P.; Geigl, J.B.; Speicher, M.R. Circulating tumor cells and DNA as liquid biopsies. Genome Med. 2013, 5, 73.
- Jo, P.; Jung, K.; Grade, M.; Conradi, L.C.; Wolff, H.A.; Kitz, J.; Becker, H.; Ruschoff, J.; Hartmann, A.; Beissbarth, T.; et al. CpG island methylator phenotype infers a poor disease-free survival in locally advanced rectal cancer. Surgery 2012, 151, 564–570.
- Wang, C.; Fakih, M. Targeting KRAS in Colorectal Cancer. Curr. Oncol. Rep. 2021, 23, 28.
- Duffy, M.J.; van Dalen, A.; Haglund, C.; Hansson, L.; Klapdor, R.; Lamerz, R.; Nilsson, O.; Sturgeon, C.; Topolcan, O. Clinical utility of biochemical markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines. Eur. J. Cancer 2003, 39, 718–727.
