There is an increasing interest in the use of beneficial microorganisms as alternatives to chemically synthesized or plant-derived molecules to produce therapeutic agents. Bacterial endophytes are plant-associated microorganisms that can colonize different parts of living plants without causing any diseases. Diverse endophytic bacteria possess the ability to synthesize a wide range of secondary metabolites with unique chemical structures that have been exploited for their anti-microbial, antiviral, anti-cancer, and anti-inflammatory properties. Additionally, production of these bioactive compounds can also benefit the host plant as they may play a significant role in a plant’s interaction with the environment for adaptation and defense.
- therapeutic applications
- secondary metabolites
- plant growth promoting bacteria
- endophytes
- mechanisms
1. Introduction
2. Plant Bacterial Endophytes
2.1. An Overview of Plant Growth-Promoting Bacterial Endophytes
Plant growth-promoting bacterial endophytes are plant growth-promoting bacteria (PGPB) that are typically present as free-living bacteria in the soil immediately around the plant’s roots (the rhizosphere). From the rhizosphere, these bacteria generally enter a plant through root wounds and cracks [16,17][16][17]. It has been estimated that nearly all the world’s ~300,00 plant species [18] contain several different endophytic bacteria (~105 – 108 cells per gram of plant tissue) as well as numerous fungal endophytes. Importantly, bacterial endophytes can grow inside plant tissues in a mutualistic relationship with the plant without harming or inhibiting the growth of the plant. As depicted schematically in Figure 1, endophytic bacteria are mostly found between plant cells (i.e., intercellularly), whereas fungal endophytes (not shown in this figure) are typically found inside of plant cells (i.e., intracellularly). Moreover, while endophytic PGPB are attracted to a specific plant’s root exudates [19,20][19][20] and enter the plant through the roots, many of these bacteria are motile and can travel through the plant to other tissues such as leaves and stems (where they are generally found in lower concentration than in the plant roots). In addition, various plant species and subspecies, plant organs and different stages of plant growth exude a different range of small organic molecules, and therefore, attract different bacteria [21]. Consequently, different tissues within the same plant may contain different groups of bacterial (and fungal) endophytes [22].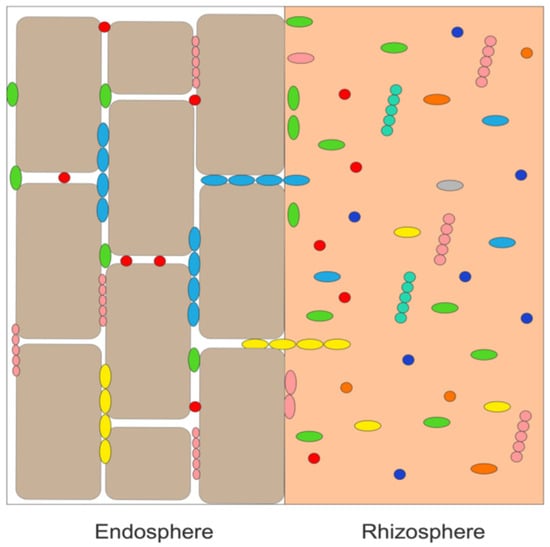
2.2. Isolation of Bacterial Endophytes
Since endophytic PGPB have been found to colonize nearly all plant species [18,25][18][25] they can be isolated directly from various plant tissues. This is done by harvesting plant tissue (most commonly this is either roots, stems or leaves) and then sterilizing the outer surface of that tissue with a 10% bleach (sodium hypochlorite) solution and then with ~70% ethanol; surface sterilization of plant tissues is the most critical step in this process. Tween-20, Tween-80 and Triton X-100 have also been used to facilitate the surface sterilization process [26]. The surface-sterilized tissue is treated with sodium bicarbonate to inhibit fungal growth, then macerated, followed by removal of the solid material, and then dilutions of the plant sap are plated onto selective solid media, keeping in mind that a large portion (estimated to be >90%) of environmental bacterial samples are recalcitrant to growing in laboratory culture. The individual colonies that form on selective media are then characterized for a variety of traits. A variation of this approach includes collecting soil samples from a range of selected relevant environments and then planting sterilized seeds in those soil samples. Many of the endophytic PGPB that are present in the soil samples will be taken up into the roots (and perhaps into the shoots and leaves as well) of the growing plant and may be isolated as indicated above from specific pieces of plant tissue [27,28,29][27][28][29]. Initial characterization of bacterial endophytes typically includes sequencing the DNA of their 16S rRNA genes [30].2.3. Endophytic PGPB Mechanisms That Directly Promote Plant Growth
Bacterial endophytes employ a wide range of mechanisms, following their interaction with plants, where they directly promote plant growth and development. Endophytic PGPB appear to use a similar, if not identical, repertoire of mechanisms to directly promote plant growth as rhizospheric PGPB [17,31,32,33,34,35][17][31][32][33][34][35]. These mechanisms include (but may not be limited to) the production of molecules involved in inorganic phosphate and potassium solubilization (e.g., various low molecular weight organic acids); synthesis of siderophores (chelating agents) that sequester iron from the soil and provide it to plants; synthesis of gibberelins and cytokinins (phytohormones that regulate various plant developmental processes); synthesis of auxins (such as indole 3-acetic acid, the most common auxin) which are phytohormones that promote plant cell elongation and proliferation; synthesis of the unusual and highly stable water-structuring sugar molecule trehalose which can help the plant to lower (overcome) salt and drought stress; the ability to fix atmospheric nitrogen into the ammonia which is necessary to synthesize proteins and nucleic acids; and synthesis of the enzyme 1-aminocyclopropane-1-caroxylate (ACC) deaminase which lowers plant ethylene levels, thereby decreasing the inhibitory effects of various abiotic stresses. There are often genes encoding the biosynthesis of plant hormones including auxin [36], cytokinin [37] and gibberellin [38] found within the microbiome of endophytic communities (although not necessarily within the same bacterium). While all these mechanisms may be involved in promoting plant growth and development, the synthesis of ACC deaminase is arguably the key mechanism in the promotion of plant growth by PGPB [39].2.4. Endophytic PGPB Mechanisms That Indirectly Promote Plant Growth
The indirect promotion of plant growth occurs when a PGPB prevents or lessens plant growth inhibition that is caused by plant pathogens. These pathogens are most often fungi but also include some bacteria, insects, and nematodes. Some endophytic PGPB utilize (biocontrol) mechanisms that thwart the functioning of various phytopathogens. However, these endophytic PGPB do not necessarily stimulate the growth of the plant directly. These indirect mechanisms include the synthesis of (i) antibiotics, (ii) hydrogen cyanide, (iii) fungal cell wall hydrolyzing enzymes, (iv) siderophores (which deprive phytopathogens of sufficient iron for their proliferation), (v) phytopathogen inhibiting volatile organic compounds (VOCs), (vi) chemical compounds that induce systemic resistance (ISR) within target plants, and (vii) ACC deaminase (which lowers the plant’s level of growth inhibiting stress ethylene) [17]. Below are a few recent examples of endophytic PGPB indirectly promoting plant growth. (i) da Siveira et al. [40] isolated endophytic PGPB from the roots of sugarcane plants and found that several bacterial strains that produced siderophores, hydrogen cyanide, and VOCs inhibited the proliferation of the fungal phytopathogens Bipolaris sacchari and Ceratocystis paradoxa. (ii) Worsley et al. [41] reported isolating an endophytic strain of Streptomyces that demonstrated broad-spectrum antimicrobial activity and synthesized the compound 14-hydroxyisochainin which inhibited the proliferation of the pathogenic fungus, Gaeumannomyces graminis var. tritici (wheat take-all fungus). (iii) Gupta et al. [42] found a large decrease in the disease mortality of pea plants infected with the fungal phytopathogen Fusarium oxysporum when they were treated with a consortium of endophytic PGPB that produced VOCs and elicited ISR. (iv) Hamaoka et al. [43] noted that the endophytic PGPB Bacillus velezensis KOF112, originally isolated from Japanese wine grapes, inhibited the mycelial growth of the fungal phytopathogens Botrytis cinerea, Colletotrichum gloeosporioides, and Phytophthora infestans (where strain KOF112 synthesized antibiotics and elicited ISR in treated plants). (v) Uwaremwe et al. [44] discovered that an endophytic strain of suppressed root rot of Chinese wolfberry (Lycium barbarum) caused by Fusarium oxysporum functioned by modifying the amounts of various wolfberry rhizospheric bacterial taxa, each employing different mechanisms. These recent examples of the effectiveness of endophytic PGPB in indirectly promoting the growth of different plants are consistent with the successful employment of a wide variety of strategies used by these bacteria in thwarting phytopathogen inhibition of plant growth.2.5. Endophytic PGPB Protect Plants against Abiotic Stresses
Most of the mechanisms that endophytic PGPB use to promote plant growth help (at least to some extent) to protect plants against various abiotic stresses including high salt, flooding, drought, the presence of inhibitory organic compounds in the soil, and temperature extremes. Since all these abiotic stresses (as well as biotic stresses such as the presence of various phytopathogens) result in the synthesis of growth-inhibiting levels of stress ethylene by the plant subjected to these stresses [45], one of the major mechanisms that endophytic PGPB use to protect stressed plants from abiotic (and biotic) stress is the synthesis of the enzyme ACC deaminase [46]. Moreover, endophytic PGPB that synthesize both ACC deaminase and indole 3-acetic acid are most efficient at enabling plants subject to different types of stress to grow normally. Recently, several studies have reported that endophytic PGPB with the ability to directly promote plant growth are successful in helping plants to overcome salt stress (a major environmental/abiotic stress worldwide). These reported studies of overcoming salt stress have included tomato [47]; sorghum, cucumber, and tomato [48]; chickpea [49]; and peanut [50]. Moreover, the approach of using endophytic PGPB to overcome abiotic stress has been very recently reviewed [51,52][51][52].3. Production of Secondary Metabolites
3.1. Antibiotics
Human and animal pathogen antibiotic resistance and the emergence of multi-resistant bacterial strains is a current problem of clinical relevance and represents a serious threat to human and animal health worldwide [53]. As a result, there is need to discover new novel antibiotics. Bacterial endophytes are one of the untapped potential sources of novel antibiotics. With high species diversity and adaptation to various environments, endophytes represent a rich source of metabolites [54,55][54][55]. Endophytes may have an edge over other microorganisms because of their capacity to defend, communicate with and colonize their plant host, resulting in the production of a large number of structurally diverse secondary metabolites compared with epiphytes or soil microbes [56]. Moreover, because they are symbiotically associated with plants, endophyte-derived antibiotics are likely to be less toxic to humans, which may be of critical importance to the medical community, as potential antibiotics isolated from endophytes may not adversely affect human cells [57]. Antibiotics secreted by endophytes can protect the plant hosts from attack by various phytopathogens [56,58][56][58] or prevent insects [59] and nematodes [60] from infecting plants. Other anti-microbial agents are also produced by endophytes that help the host plant to develop systemic resistance against pathogens [61,62][61][62]. Additionally, antimicrobials synthesized by microbial endophytes kill or inhibit the growth of plant pathogens including bacteria, fungi, viruses and protozoans that also cause human and animal diseases [63,64][63][64]. Some new antibiotics have recently been discovered in endophytes that colonize different plant species [65].3.2. Anti-Cancer Compounds
Cancer is a severe disease characterized by uncontrolled cell growth. According to a recent report, cancer is the leading cause of death worldwide, accounting for nearly 10 million deaths in 2020 [105][66]. The drugs used in the treatment of various cancers show non-specific toxicity for normal cells, have negative side effects, and many are still not active in the treatment of some cancer forms [106][67]. The discovery of secondary metabolites with cytotoxic properties has provided new insights in anti-cancer treatments [107][68].3.2.1. Cyclic Analogs
Numerous bioactive anti-cancer compounds belonging to different classes such as anthracyclines, glycopeptides, aureolic acids, anthraquinones, enediynes, polysachharides, carzinophilin, mitomycins, alnumycin, pterocidin, napthomycin and alkyl salicylic acids (salaceyins) are reportedly produced by many endophytic bacteria [108][69]. The anti-cancer potential of endophytic actinomycetes bacteria is evidenced in many studies. Streptomyces from the Brazilian medicinal plant Lychnophora ericoides showed strong cytotoxic activity against human cancer cell lines [109][70]. The majority of secondary metabolites produced by endophytic bacteria have been characterized after growing them in vitro. Kim et al. [110][71] grew endophytic Streptomyces lacey MS53 in vitro and detected two new anti-cancer agents, salaceyins (A and B), which were cytotoxic to human breast cancer line SKBR3. Streptomyces sp. strain DSM11575 isolated from root nodules of Alnus glutinosa produced the compound alnumycin, which inhibited growth of K562 human leukemia cells [111][72]. Studies have shown that acquisition of secondary metabolites with diverse structural compositions from endophytes is affected by the plant’s adaption to a specific niche. This is emphasized by recently described endophytes isolated from plants growing in the tropical wetlands of the Pantanal region of Brazil. Crude extracts of isolates of Streptomyces albidoflavus CMRP4852 and Verrucosispora sp. CMR P4860 demonstrated anti-melanoma activities with no effect on normal non-cancerous cells [112][73]. Consequently, the natural products synthesized by endophytic bacteria have attracted enormous interest and research on these strains [113][74]. The endophytic actinomycete strain YBQ59 isolated from a Chinese cinnamon plant produced metabolites effective against human lung cancer cells [114][75]. Additionally, Igarishi et al. [115][76] reported that pterocidins produced by Streptomyces hygroscopicus TP-A0451 isolated from Pteridium aquilinum exhibited cytotoxic activity to human cancer cell lines NCI-H522, OVCAR-3, SF539, and LOX-IMVI. Similarly, Streptomyces sp. CS isolated from Maytenus hookeri produced the compound napthomycin, which is effective against P388 and A549 human tumor cells [116][77]. Sebola et al. [117][78] tested the anti-cancer activity of crude extracts of bacterial endophytes isolated from Crinum macowanii baker bulbs. In this study, the authors observed that Acinetobacter guillouiae dramatically reduced growth of the U87MG brain cancer cell line; whereas Raoultella ornithinolytica strongly inhibited lung carcinoma cells (62% reduction in cell growth).3.2.2. Maytansinoids
Given the role of chemical communication in plants and endophytes, it is understood that certain compounds formerly believed to be synthesized by plants or exclusively considered to be plant metabolites may be produced by endophytes. For instance, Kusari et al. [118][79] studied the root endophytic communities of Putterlickia verrucosa and Putterlickia retrospinosa and concluded that maytansine, an anti-cancer agent effective against breast cancer and previously thought to be produced by plants, was in fact synthesized by an endophytic bacterium colonizing the plant roots. Interestingly, the shoot bacterial community did not produce any maytansine. Indeed, the roots may represent a metabolic sink from which to explore bacteria with therapeutic potential. Zhao et al. [119][80] isolated maytansine producing Streptomyces sp. Is9131 from the medicinal plant Maytenus hookeri. An extracellular extract of this endophyte was inhibitory to human cell lines implicated in various cancers including leukemia, lung, gastric and liver cancers. Since maytansinoids are an important class of drugs, reportedly more cytotoxic than many anti-cancer drugs, isolation of maytansine-producing bacteria represents an opportunity to discover novel drugs and offers a renewable source of natural products.3.2.3. Extracellular Metabolites
Exopolysaccharides (EPS) may play a significant role as anti-cancer agents. An endophytic Bacillus amyloliquefaciens strain isolated from Ophiopogon japonicus, a Chinese medicinal plant, produced EPS that inhibited the growth of human gastric cancer cell lines MC-4 and SGC-7901. EPS-treated cells had abnormal cell morphology and cell death, possibly caused by a mitochondrial dysfunction [120][81]. This study is a good example of the therapeutic potential of such compounds in anti-cancer applications. Phenolic compounds have also been reported to be involved in various bioactive properties, including anti-cancer activity. For example, two biphenyl producing Streptomyces sp. isolated from the root tissue of Boesenbergia rotunda (L.) Mansf A. showed strong cytotoxicity against three cancer cell lines (HeLa, HepG2, and Huh7) and less toxicity towards normal cells (L929) [121][82].References
- Vargiu, A.V.; Pos, K.M.; Poole, K.; Nikaido, H. Editorial: Bad Bugs in the XXIst Century: Resistance Mediated by Multi-Drug Efflux Pumps in Gram-Negative Bacteria. Front. Microbiol. 2016, 7, 833.
- Gakuubi, M.M.; Munusamy, M.; Liang, Z.X.; Ng, S.B. Fungal Endophytes: A. Promising Frontier for Discovery of Novel Bioactive Compounds. J. Fungi 2021, 7, 786.
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.-S.; Patra, J.K. Endophytes: A Treasure House of Bioactive Compounds of Medicinal Importance. Front. Microbiol. 2016, 7, 1538.
- Petrini, O.; Sieber, T.N.; Toti, L.; Viret, O. Ecology, Metabolite Production, and Substrate Utilization in Endophytic Fungi. Nat. Toxins 1992, 1, 185–196.
- Khan, Z.; Guelich, G.; Phan, H.; Redman, R.; Doty, S. Bacterial and Yeast Endophytes FromPoplar and Willow Promote Growth in Crop Plants and Grasses. ISRN Agron. 2012, 2012, 890280.
- Khan, Z.; Roman, D.; Kintz, T.; delas Alas, M.; Yap, R.; Doty, S. Degradation, Phytoprotection and Phytoremediation of Phenanthrene by Endophyte Pseudomonas Putida, PD1. Environ. Sci. Technol. 2014, 48, 12221–12228.
- Khan, Z.; Kandel, S.L.; Ramos, D.N.; Ettl, G.J.; Kim, S.-H.; Doty, S.L. Increased Biomass of Nursery-Grown Douglas-Fir Seedlings upon Inoculation with Diazotrophic Endophytic Consortia. Forests 2015, 6, 3582–3593.
- Khan, Z.; Rho, H.; Firrincieli, A.; Hung, S.H.; Luna, V.; Masciarelli, O.; Kim, S.-H.; Doty, S.L. Growth Enhancement and Drought Tolerance of Hybrid Poplar upon Inoculation with Endophyte Consortia. Curr. Plant Biol. 2016, 6, 38–47.
- Kandel, S.L.; Firrincieli, A.; Joubert, P.M.; Okubara, P.A.; Leston, N.D.; McGeorge, K.M.; Mugnozza, G.S.; Harfouche, A.; Kim, S.-H.; Doty, S.L. An In Vitro Study of Bio-Control and Plant Growth Promotion Potential of Salicaceae Endophytes. Front. Microbiol. 2017, 8, 386.
- Aghai, M.M.; Khan, Z.; Joseph, M.R.; Stoda, A.M.; Sher, A.W.; Ettl, G.J.; Doty, S.L. The Effect of Microbial Endophyte Consortia on Pseudotsuga Menziesii and Thuja Plicata Survival, Growth, and Physiology Across Edaphic Gradients. Front. Microbiol. 2019, 10, 1353.
- Doty, S.L.; Freeman, J.L.; Cohu, C.M.; Burken, J.G.; Firrincieli, A.; Simon, A.; Khan, Z.; Isebrands, J.G.; Lukas, J.; Blaylock, M.J. Enhanced Degradation of TCE on a Superfund Site Using Endophyte-Assisted Poplar Tree Phytoremediation. Environ. Sci. Technol. 2017, 51, 10050–10058.
- Gupta, S.; Chaturvedi, P.; Kulkarni, M.G.; Van Staden, J. A Critical Review on Exploiting the Pharmaceutical Potential of Plant Endophytic Fungi. Biotechnol. Adv. 2020, 39, 107462.
- Haque, M.A.; Lee, J.H.; Cho, K.M. Endophytic Bacterial Diversity in Korean Kimchi Made of Chinese Cabbage Leaves and Their Antimicrobial Activity against Pathogens. Food Control 2015, 56, 24–33.
- Mishra, S.; Bhardwaj, P.; Sharma, S. Metabolomic Insights into Endophyte-Derived Bioactive Compounds. Front. Microbiol. 2022, 13, 835931.
- Segers, K.; Declerck, S.; Mangelings, D.; Heyden, Y.V.; Eeckhaut, A.V. Analytical Techniques for Metabolomic Studies: A Review. Bioanalysis 2019, 11, 2297–2318.
- Zhang, H.W.; Song, Y.C.; Tan, R.X. Biology and Chemistry of Endophytes. Nat. Prod. Rep. 2006, 23, 753–771.
- Glick, B.R. Beneficial Plant-Bacterial Interactions, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 383–398.
- Strobel, G.; Daisy, B. Bioprospecting for Microbial Endophytes and Their Natural Products. Microbiol. Mol. Biol. Rev. 2003, 67, 491–502.
- Reinhold-Hurek, B.; Bünger, W.; Burbano, C.S.; Sabale, M.; Hurek, T. Roots Shaping Their Microbiome: Global Hotspots for Microbial Activity. Annu. Rev. Phytopathol. 2015, 53, 403–424.
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends Plant Sci. 2018, 23, 25–41.
- Dwibedi, V.; Rath, S.K.; Joshi, M.; Kaur, R.; Kaur, G.; Singh, D.; Kaur, G.; Kaur, S. Microbial Endophytes: Application towards Sustainable Agriculture and Food Security. Appl. Microbiol. Biotechnol. 2022, 106, 5359–5384.
- Johnston-Monje, D.; Raizada, M.N. Conservation and Diversity of Seed Associated Endophytes in Zea across Boundaries of Evolution, Ethnography and Ecology. PLoS ONE 2011, 6, e20396.
- Glick, B.R.; Gamalero, E. Recent Developments in the Study of Plant Microbiomes. Microorganisms 2021, 9, 1533.
- Santoyo, G.; Guzmán-Guzmán, P.; Parra-Cota, F.I.; Santos-Villalobos, S.D.L.; Orozco-Mosqueda, M.D.C.; Glick, B.R. Plant Growth Stimulation by Microbial Consortia. Agronomy 2021, 11, 219.
- Bacon, C.W.; White, J. Microbial Endophytes; CRC Press: Boca Raton, FL, USA, 2000; p. 500.
- Hallmann, J.; Schulz, B.; Berg, G. Isolation procedures for endophytic microorganisms. In Microbial Root Endophytes, 1st ed.; Springer: New York, NY, USA, 2006; pp. 299–314.
- Sturz, A.V.; Christie, B.R.; Matheson, B.G. Associations of Bacterial Endophyte Populations from Red Clover and Potato Crops with Potential for Beneficial Allelopathy. Can. J. Microbiol. 1998, 44, 162–167.
- Surette, M.A.; Sturz, A.V.; Lada, R.R.; Nowak, J. Bacterial Endophytes in Processing Carrots (Daucus Carota L. Var. Sativus): Their Localization, Population Density, Biodiversity and Their Effects on Plant Growth. Plant Soil 2003, 253, 381–390.
- Shimaila Rashid. Isolation and Characterization of New Plant Growth-Promoting Bacterial Endophytes. Appl. Soil Ecol. 2012, 61, 217–224.
- Liu, W.; Li, L.; Khan, M.A.; Zhu, F. Popular Molecular Markers in Bacteria. Mol. Genet. Microbiol. Virol. 2012, 27, 103–107.
- Glick, B.R. The Enhancement of Plant Growth by Free-Living Bacteria. Can. J. Microbiol. 1995, 41, 109–117.
- Gamalero, E.; Glick, B. Mechanisms Used by Plant Growth-Promoting Bacteria. In Bacteria in Agrobiology: Plant Nutrient Management; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; pp. 17–46.
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401.
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of Action of Plant Growth Promoting Bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197.
- Orozco-Mosqueda, M.D.C.; Rocha-Granados, M.D.C.; Glick, B.R.; Santoyo, G. Microbiome Engineering to Improve Biocontrol and Plant Growth-Promoting Mechanisms. Microbiol. Res. 2018, 208, 25–31.
- Zúñiga, A.; Poupin, M.J.; Donoso, R.; Ledger, T.; Guiliani, N.; Gutiérrez, R.A.; González, B. Quorum Sensing and Indole-3-Acetic Acid Degradation Play a Role in Colonization and Plant Growth Promotion of Arabidopsis Thaliana by Burkholderia Phytofirmans PsJN. Mol. Plant-Microbe Interact. 2013, 26, 546–553.
- Bhore, S.J.; Ravichantar, N.; Loh, C.Y. Screening of Endophytic Bacteria Isolated from Leaves of Sambung Nyawa for Cytokinin-like Compounds. Bioinformation 2010, 5, 191–197.
- Shahzad, R.; Waqas, M.; Khan, A.L.; Asaf, S.; Khan, M.A.; Kang, S.-M.; Yun, B.-W.; Lee, I.-J. Seed-Borne Endophytic Bacillus Amyloliquefaciens RWL-1 Produces Gibberellins and Regulates Endogenous Phytohormones of Oryza Sativa. Plant Physiol. Biochem. 2016, 106, 236–243.
- Glick, B.R. Bacteria with ACC Deaminase Can Promote Plant Growth and Help to Feed the World. Microbiol. Res. 2014, 169, 30–39.
- da Silveira, A.P.D.; Iório, R.D.P.F.; Marcos, F.C.C.; Fernandes, A.O.; de Souza, S.A.C.D.; Kuramae, E.E.; Cipriano, M.A.P. Exploitation of New Endophytic Bacteria and Their Ability to Promote Sugarcane Growth and Nitrogen Nutrition. Antonie Leeuwenhoek 2019, 112, 283–295.
- Worsley, S.F.; Newitt, J.; Rassbach, J.; Batey, S.F.D.; Holmes, N.A.; Murrell, J.C.; Wilkinson, B.; Hutchings, M.I. Streptomyces Endophytes Promote Host Health and Enhance Growth across Plant Species. Appl. Environ. Microbiol. 2020, 86, e01053-20.
- Gupta, S.; Pandey, S.; Sharma, S. Decoding the Plant Growth Promotion and Antagonistic Potential of Bacterial Endophytes from Ocimum Sanctum Linn. Against Root Rot Pathogen Fusarium Oxysporum in Pisum Sativum. Front. Plant Sci. 2022, 13, 813686.
- Hamaoka, K.; Aoki, Y.; Suzuki, S. Isolation and Characterization of Endophyte Bacillus Velezensis KOF112 from Grapevine Shoot Xylem as Biological Control Agent for Fungal Diseases. Plants 2021, 10, 1815.
- Uwaremwe, C.; Yue, L.; Wang, Y.; Tian, Y.; Zhao, X.; Liu, Y.; Zhou, Q.; Zhang, Y.; Wang, R. An Endophytic Strain of Bacillus Amyloliquefaciens Suppresses Fusarium Oxysporum Infection of Chinese Wolfberry by Altering Its Rhizosphere Bacterial Community. Front. Microbiol. 2022, 12, 782523.
- Abeles, F.B.; Morgan, P.W.; Saltveit, M.E. Preface. In Ethylene in Plant Biology, 2nd ed.; Academic Press: New York, NY, USA, 1992; pp. xi–xii.
- Gamalero, E.; Glick, B.R. Ethylene and Abiotic Stress Tolerance in Plants. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 395–412.
- Ali, S.; Charles, T.C.; Glick, B.R. Amelioration of High Salinity Stress Damage by Plant Growth-Promoting Bacterial Endophytes That Contain ACC Deaminase. Plant Physiol. Biochem. 2014, 80, 160–167.
- Gamalero, E.; Favale, N.; Bona, E.; Novello, G.; Cesaro, P.; Massa, N.; Glick, B.R.; Orozco-Mosqueda, M.D.C.; Berta, G.; Lingua, G. Screening of Bacterial Endophytes Able to Promote Plant Growth and Increase Salinity Tolerance. Appl. Sci. 2020, 10, 5767.
- Egamberdieva, D.; Wirth, S.J.; Shurigin, V.V.; Hashem, A.; Abd Allah, E.F. Endophytic Bacteria Improve Plant Growth, Symbiotic Performance of Chickpea (Cicer Arietinum L.) and Induce Suppression of Root Rot Caused by Fusarium Solani under Salt Stress. Front. Microbiol. 2017, 8, 1887.
- Pal, K.K.; Dey, R.; Sherathia, D.N.; Devidayal; Mangalassery, S.; Kumar, A.; Rupapara, R.B.; Mandaliya, M.; Rawal, P.; Bhadania, R.A.; et al. Alleviation of Salinity Stress in Peanut by Application of Endophytic Bacteria. Front. Microbiol. 2021, 12, 650771.
- Verma, H.; Kumar, D.; Kumar, V.; Kumari, M.; Singh, S.K.; Sharma, V.K.; Droby, S.; Santoyo, G.; White, J.F.; Kumar, A. The Potential Application of Endophytes in Management of Stress from Drought and Salinity in Crop Plants. Microorganisms 2021, 9, 1729.
- Tyagi, J.; Chaudhary, P.; Mishra, A.; Khatwani, M.; Dey, S.; Varma, A. Role of Endophytes in Abiotic Stress Tolerance: With Special Emphasis on Serendipita Indica. Int. J. Environ. Res. 2022, 16, 62.
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309–318.
- Matjaz, R.; Matic, T.; Damjan, J.; Borut, S.; Samo, K.; Ravnikar, M.; Tercelj, M.; Janes, D.; Strukelj, B.; Kreft, S. Antibacterial activity of endophytic fungi isolated from conifer needles. Afr. J. Biotechnol. 2015, 14, 867–871.
- Singh, M.; Kumar, A.; Singh, R.; Pandey, K.D. Endophytic Bacteria: A New Source of Bioactive Compounds. 3 Biotech 2017, 7, 315.
- Strobel, G.A. Endophytes as Sources of Bioactive Products. Microbes Infect. 2003, 5, 535–544.
- Zin, N.M.; Baba, M.S.; Zainal-Abidin, A.H.; Latip, J.; Mazlan, N.W.; Edrada-Ebel, R. Gancidin W, a Potential Low-Toxicity Antimalarial Agent Isolated from an Endophytic Streptomyces SUK10. Drug Des. Dev. Ther. 2017, 11, 351–363.
- Berg, G.; Hallmann, J. Control of Plant Pathogenic Fungi with Bacterial Endophytes. In Microbial Root Endophytes; Schulz, B.J.E., Boyle, C.J.C., Sieber, T.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 53–69.
- Azevedo, J.L.; Maccheroni, W., Jr.; Pereira, J.O.; de Araújo, W.L. Endophytic microorganisms: A review on insect control and recent advances on tropical plants. Electron. J. Biotechnol. 2000, 3.
- Gamalero, E.-G.; Bernard, R. TI-The Use of Plant Growth-Promoting Bacteria to Prevent Nematode Damage to Plants. Biology 2020, 9, 381.
- Kloepper, J.W.; Ryu, C.-M. Bacterial Endophytes as Elicitors of Induced Systemic Resistance. In Microbial Root Endophytes; Schulz, B.J.E., Boyle, C.J.C., Sieber, T.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 33–52.
- Pandey, P.K.; Samanta, R.; Yadav, R.N.S. Inside the Plant: Addressing Bacterial Endophytes in Biotic Stress Alleviation. Arch. Microbiol. 2019, 201, 415–429.
- Demain, A.L. Industrial Microbiology. Science 1981, 214, 987–995.
- Tripathi, V.C.; Satish, S.; Horam, S.; Raj, S.; Lal, A.; Arockiaraj, J.; Pasupuleti, M.; Dikshit, D.K. Natural Products from Polar Organisms: Structural Diversity, Bioactivities and Potential Pharmaceutical Applications. Polar Sci. 2018, 18, 147–166.
- Martinez-Klimova, E.; Rodríguez-Peña, K.; Sánchez, S. Endophytes as Sources of Antibiotics. Biochem. Pharmacol. 2017, 134, 1–17.
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789.
- Pasut, G.; Veronese, F.M. PEG Conjugates in Clinical Development or Use as Anticancer Agents: An Overview. Adv. Drug Deliv. Rev. 2009, 61, 1177–1188.
- Pimentel, M.R.; Molina, G.; Dionísio, A.P.; Maróstica Junior, M.R.; Pastore, G.M. The Use of Endophytes to Obtain Bioactive Compounds and Their Application in Biotransformation Process. Biotechnol. Res. Int. 2011, 2011, 576286.
- Ek-Ramos, M.J.; Gomez-Flores, R.; Orozco-Flores, A.A.; Rodríguez-Padilla, C.; González-Ochoa, G.; Tamez-Guerra, P. Bioactive Products from Plant-Endophytic Gram-Positive Bacteria. Front. Microbiol. 2019, 10, 463.
- Conti, R.; Chagas, F.O.; Caraballo-Rodriguez, A.M.; Melo, W.G.D.P.; do Nascimento, A.M.; Cavalcanti, B.C.; de Moraes, M.O.; Pessoa, C.; Costa-Lotufo, L.V.; Krogh, R.; et al. Endophytic Actinobacteria from the Brazilian Medicinal Plant Lychnophora Ericoides Mart. and the Biological Potential of Their Secondary Metabolites. Chem. Biodivers. 2016, 13, 727–736.
- Kim, N.; Shin, J.C.; Kim, W.; Hwang, B.Y.; Kim, B.S.; Hong, Y.-S.; Lee, D. Cytotoxic 6-Alkylsalicylic Acids from the Endophytic Streptomyces Laceyi. J. Antibiot. 2006, 59, 797–800.
- Bieber, B.; Nüske, J.; Ritzau, M.; Gräfe, U. Alnumycin a New Naphthoquinone Antibiotic Produced by an Endophytic Streptomyces Sp. J. Antibiot. 1998, 51, 381–382.
- Assad, B.M.; Savi, D.C.; Biscaia, S.M.; Mayrhofer, B.F.; Iantas, J.; Mews, M.; de Oliveira, J.C.; Trindade, E.S.; Glienke, C. Endophytic Actinobacteria of Hymenachne Amplexicaulis from the Brazilian Pantanal Wetland Produce Compounds with Antibacterial and Antitumor Activities. Microbiol. Res. 2021, 248, 126768.
- Cardoso-Filho, J.A. Endophytic Microbes as a Novel Source for Producing Anticancer Compounds as Multidrug Resistance Modulators, Anticancer Plants: Natural Products and Biotechnological Implements; Akhtar, M., Swamy, M., Eds.; Springer: Singapore, 2008; pp. 343–381.
- Vu, H.-N.T.; Nguyen, D.T.; Nguyen, H.Q.; Chu, H.H.; Chu, S.K.; Chau, M.V.; Phi, Q.-T. Antimicrobial and Cytotoxic Properties of Bioactive Metabolites Produced by Streptomyces Cavourensis YBQ59 Isolated from Cinnamomum Cassia Prels in Yen Bai Province of Vietnam. Curr. Microbiol. 2018, 75, 1247–1255.
- Igarashi, Y.; Miura, S.-S.; Fujita, T.; Furumai, T. Pterocidin, a Cytotoxic Compound from the Endophytic Streptomyces Hygroscopicus. J. Antibiot. 2006, 59, 193–195.
- Lu, C.; Shen, Y. A New Macrolide Antibiotic with Antitumor Activity Produced by Streptomyces Sp. CS, a Commensal Microbe of Maytenus Hookeri. J. Antibiot. 2003, 56, 415–418.
- Sebola, T.E.; Uche-Okereafor, N.C.; Tapfuma, K.I.; Mekuto, L.; Green, E.; Mavumengwana, V. Evaluating Antibacterial and Anticancer Activity of Crude Extracts of Bacterial Endophytes from Crinum Macowanii Baker Bulbs. MicrobiologyOpen 2019, 8, e914.
- Kusari, S.; Lamshöft, M.; Kusari, P.; Gottfried, S.; Zühlke, S.; Louven, K.; Hentschel, U.; Kayser, O.; Spiteller, M. Endophytes Are Hidden Producers of Maytansine in Putterlickia Roots. J. Nat. Prod. 2014, 77, 2577–2584.
- Zhao, P.-J.; Fan, L.-M.; Li, G.-H.; Zhu, N.; Shen, Y.-M. Antibacterial and Antitumor Macrolides from Streptomyces Sp. Is9131. Arch. Pharm. Res. 2005, 28, 1228–1232.
- Chen, Y.-T.; Yuan, Q.; Shan, L.-T.; Lin, M.-A.; Cheng, D.-Q.; Li, C.-Y. Antitumor Activity of Bacterial Exopolysaccharides from the Endophyte Bacillus Amyloliquefaciens Sp. Isolated from Ophiopogon Japonicus. Oncol. Lett. 2013, 5, 1787–1792.
- Taechowisan, T.; Chaisaeng, S.; Phutdhawong, W.S. Antibacterial, Antioxidant and Anticancer Activities of Biphenyls from Streptomyces Sp. BO-07: An Endophyte in Boesenbergia Rotunda (L.) Mansf A. Food Agric. Immunol. 2017, 28, 1330–1346.
