Members in the polypeptide N-acetylgalactosaminyltransferase (GALNT) family function as the initiating enzymes to catalyze mucin-type O-glycosylation of proteins, of which dysregulated expression can alter cancer cell behaviors such as de novo occurrence, proliferation, migration, metastasis and drug resistance. One of its members, GALNT14, is aberrantly expressed in multiple cancers and involved in a variety of biological functions. Moreover, the single nucleotide polymorphisms (SNPs) of
GALNT14
-rs9679162 has been shown to predict the therapeutic outcomes in patients with hepatocellular carcinoma as well as several other different types of gastrointestinal cancers.
- polypeptide N-acetylgalactosaminyltransferase
- single nucleotide polymorphism
- biomarker
- cancer
1. Introduction
Glycosylation, an enzymatic process that attaches glycans to proteins or other organic molecules, is essential for multicellular life. Among several types of glycosylation, addition of a sugar to the oxygen atom of amino acid residues (O-glycosylation) occurs on thousands of secreted and cell surface proteins, altering their structures, functionalities and subcellular distributions
. O-glycans are built by adding sugar molecules to protein sequentially. The members in N-acetylgalactosaminyltransferase (GALNT) family are enzymes that initiate O-glycosylation by addition of an N-Acetylgalactosamine (GalNAc) to a Serine or Threonine residue of mucin-type protein
[5]
. This process plays a pivotal role in the synthesis of Thomsen-nouvelle (Tn) antigens, which are well-characterized tumor-associated molecules
[6]
. The GALNT families contain 20 members, from GALNT1 to 14 and from GANLTL1 to L6
[5]
. In cancer, the altered O-glycosylation by GALNTs may affect a variety of biological processes, including tumor progression, proliferation and migration
[7]
.
GALNT14
was firstly cloned and identified from gastric cancer cell line MKN45 in 2003
[8]
. The
GALNT14
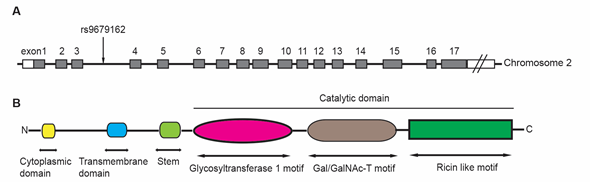
gene is located at chromosome 2p23.1, spanning over 228kb and containing at least 17 exons (Figure 1A). It has been found in many human tissues and highly expressed in skin and kidney
[8]
.
The GALNT14 protein is a 552-amino acid, type-II membrane protein that consists of a N-terminal cytoplasmic domain, followed by a transmembrane domain, a stem region, and a catalytic domain (Figure 1B)
[6]
. The catalytic domain includes a glycosyltransferase 1 motif, a Gal/GalNAc transferase motif, and a ricin-like lectin motif, which are commonly observed in the GALNT family proteins
[9]
. The glycosyltransferase 1 motif appears to be responsible for Mn
2+
coordination and binding both GalNAc and ribose of the sugar nucleotide donor
[10]
. The Gal/GalNAc transferase motif contains the catalytic general base of the β1,4-galactosyltransferase family. The ricin-like lectin motif contains three homologous repeats (α, β and γ) and may function to modulate and improve the catalytic efficiency of Gal/GalNAc transferase
[5]
.
The functions of GALNT14 in various cancers have been studied, including alteration of apoptotic signaling
[11]
, change of tissue invasiveness, and modulation of migration properties
. GALNT14 expression also regulates multi-drug resistance in breast cancer cells
[16]
. Clinically, GALNT14 level serves as a prognostic marker for neuroblastoma
[17]
and non-small cell lung cancer (NSCLC)
[15]
. It has been proposed as a predictive marker for Apo2L/TRAIL-based anticancer therapy
[11]
. Finally, a single nucleotide polymorphism (SNP) in
GALNT14
gene, rs9679162, has been identified as a prognostic marker in several gastrointestinal cancers. This review summarizes our current knowledge of GALNT14 functions in various tumors, so as to provide insights into its predictive values in the therapeutic outcomes of cancers.
Figure 1. The structure of
GALNT14
gene, including the location of rs9679162 (A), and the common functional domains of GALNT proteins (B). The
GALNT14
gene is located on chromosome 2 and includes at least 17 exons. The rs9679162 is located between exon 3 and 4 (A). The members in GALNT family possess several common functional domains (B). The N-terminal cytoplasmic domain contains 4 to 22 amino acids. The transmembrane domain contains 15 to 25 amino acids. The catalytic region contains more than 450 amino acids, which can be divided into three parts: a glycosyltransferase 1 motif, a Gal/GalNAc-T motif and a ricin like motif.
2. Discovery of a GALNT14 SNP, capable of predicting therapeutic outcomes for hepatocellular carcinoma (HCC) patients receiving systemic chemotherapy, transcatheter arterial chemoembolization (TACE), hepatic arterial infusion chemotherapy (HAIC), and sorafenib treatment.
The predictive value of GANT14-rs9679162 SNP was firstly discovered in patients with advanced HCC receiving systemic chemotherapy. Subsequently, this SNP was found to associate with several other anti-HCC treatments, including TACE, HAIC and sorafenib. These results were summarized in Table 1.
2.1. GALNT14-rs9679162 SNP discovery and its association with HCC under chemotherapy
The predictive value of GALNT14-rs9679162 SNP in cancer was first discovered in a study attempting to identify germline SNP markers capable of predicting the treatment responses of advanced HCC patients receiving chemotherapy using 5-fluorouracil, mitoxantrone and cisplatin (FMP)
[18]
. Two steps of experiments were conducted in this study. Firstly, a genome-wide association study (GWAS) was performed in a cohort of 16 patients who either suffered from rapid disease progression (n = 9), or had partial (n = 5) or complete (n = 2) treatment responses, retrospectively. Among 16 SNPs on chromosomes 2, 6, 15 and X achieving allelic Chi-suqare test p-values smaller than 0.0001, GALNT14-rs9679162 had the lowest p-value (p = 0.000017) by using the kernel-based association test. Secondly, validation of SNP rs9679162 in association with therapeutic responses was performed in an independent cohort of 41 patients prospectively. In this validation cohort, significant association was found again between rs9679162 and the therapeutic responses (p = 0.006326). A follow-up survival analysis demonstrated that patients with "TT" genotype had better progressive-free survival (PFS) in both retrospective and prospective cohorts (p=0.00041 and 0.01485, respectively) than "non-TT" ("GT" + "GG") genotypes. However, the overall survival (OS) was only significantly different in the retrospective cohort (p = 0.00622) but not in the prospective cohort. This study demonstrated firstly that GALNT14-rs9679162 genotypes is potentially associated with the responses of the first course of FMP chemotherapy in patients with advanced HCC and could serve as a predictor marker in HCC.
To confirm the predive value of GALNT14-rs9679162, a prospective study was conducted in advanced HCC patients receiving a less toxic, split-dose FMP protocol
[19]
. 107 patients with advanced HCC stage (Bacelona Clinical Liver Cancer stage C with either main portal vein thrombosis and/or distal metastasis) were enrolled and treated by split-dose FMP therapy. Of these patients, 28 (26.2%) were "TT" and 79 (73.8%) were "non-TT" genotype. The patients with "TT" genotype, when compared to "non-TT", had better prognosis, including longer OS (6.8 vs. 3.9 months, p < 0.001) and PFS (3.9 vs. 2.1 months p < 0.001), and better response rate (28.6% vs. 10.1%, p = 0.029) and disease control rate (35.7% vs. 15.2%, p = 0.030). Further multivariate analysis confirmed that rs9679162 genotype was an independent predictor for OS (p = 0.002). Cateogrical analysis showed a subroup of patients with "TT" genotype, tumor size < 10cm and neutrophils < 74% had the best outcome (median OS 25.5 months, therapeutic response rate 47.1%). This prospective study confirmed that GALNT14-rs9679162 SNP is an enffective predictor for therapeutic outcome in advanced HCC patients receiving split-dose FMP chemotherapy.
To evaluate whether combination of the SNP and other clinical parameters together with on-treatment side effects serves as an effective predictor for favorable outcome, 118 advanced HCC patients receiving split-dose FMP were retrospectively enrolled for the study
[20]
. Results showed that pretreatment α‑fetoprotein (AFP) ≤ 2,800 ng/mL (median level), GALNT14 "TT" genotype, on‑treatment leukopenia and absence of vomiting were independent predictors for favorable PFS (p = 0.001, 0.035, 0.008 and 0.009, respectively) and OS (p = 0.028, 0.006, 0.027 and 0.013, respectively). A total of 30 patients (25.4%) with both AFP ≤ 2,800 ng/mL and GLANT14 "TT" genotype exhibited longer median PFS and OS (3.11 vs. 2.11 months, p = 0.014; and 5.75 vs. 3.93 months, p = 0.001, respectively), and 9 patients (7.6%) with all four favorable factors exhibited the longest median PFS and OS (10.64 vs. 2.07 months, p = 0.002; and 25.50 vs. 4.50 months, p < 0.001, respectively). This results suggested that lower AFP level in combination with GALNT14 "TT" genotype could serve as favorable pre‑therapeutic predictors for advanced HCC patients receiving FMP chemotherapy.
The distribution of different GALNT14 genotypes in HCC has been demonstrated to be related to HCC etiologies and ethnicities
[21]
. The "TT" genotype was lower in percentage among patients with virus-originated HCC compared with those with non-viral HCC (22.57% vs 47.06%, p = 0.023). The proportion of the "TT" genotype in Chinese population ranged from 24.18% to 30.15% in different cohorts. It was significantly higher in Japanese and African population (42.11-54.55%, p < 0.0001), but significantly lower in an Italian cohort (7.84%, p = 0.004).
2.2. GALNT14-rs9679162 SNP association with HCC patients receving TACE
TACE is currently the standard treatment in HCC patients with Barcelona Clinic Liver Cancer stage B. A cohort of 327 HCC patients treated by TACE was enrolled to investigate the prognosis predictive value of GALNT14-rs9679162 genotype
[22]
. Again, the "TT" genotype was associated with better prognosis, including shorter time-to-response (HR = 2.362, p < 0.001), time-to-complete response (HR = 1.947, p = 0.004) and longer PFS (p < 0.001), when compared with the "non-TT" genotype. In patients with albumin < 3.5 g/dL, the "TT" genotype was associated with longer OS (p = 0.027). This study showed that GALNT14 genotypes were also significantly associated with clinical outcomes of HCC patients receiving TACE.
2.3. The prediction value of combination of GALNT14-rs9679162 and other SNPs in advanced HCC receiving chemotherapy
The GALNT14-rs9679162 has been demonstrated to be capable of predicting chemotherapy responses in advanced HCC
[18]
. However, the GWAS also indentified several other candidate SNP markers, including rs6025211, rs715171 (x-linked), LOC105369482-rs1955024 and WWOX-rs13338697. The prognostic value of these candidate markers were evaluated in an independent cohort of 116 advanced HCC patients receiving split-dose FMP chomotherapy
[23]
. It was shown that "TT" genotype of GALNT14 remained an effective predictor for favorable time-to-tumor progression (TTP) and OS (p = 0.012 and 0.002). Moreover, WWOX-rs13338697 "CT" genotype was associated with unfavorable TTP (p = 0.031) and rs6025211"CT" genotype was associated with unfavorable OS (p = 0.014). When these three SNPs were combined, patients with GALNT14-rs9679162 "TT"/WWOX-rs13338697 "non-CT" genotypes achieved the most favorable treatment outcomes (n = 19; median TTP, median OS and response rate were 3.9 months, 6.8 months and 4/19 [21.1%], respectively); whereas patients with GALNT14-rs9679162 "non- TT"/rs6025211 "CT" genotypes associated with the most unfavorable treatment outcomes (n = 40; median TTP, median OS and response rate were 1.9 months, 3.5 months and 1/40 [2.5%], respectively). The remaining patients had an intermediate clincal outcome. This study suggests that pretreatment genotyping of these SNPs helps to decide whether these patients should receive chemotherapy or other treatments.
2.4. A GALNT14 -rs9679162 genotype-guided therapeutic strategy for advanced HCC
Although targeted agents are recommended as the first-line treatment for advanced HCC, it could not significantly prolong survival in certain advanced HCC patients with distal metastasis. On the other hand, systemic chemotherapy or hepatic arterial infusion chemotherapy (HAIC) could achieve complete response in a small proportion of patients. In order to identify the subgroups of patients with the best outcome in advanced HCC patients receving either chemotherapy, HAIC or targeted agents, previously identifiend SNP predictors (GALNT14-rs9679162, WWOX-rs13338697, and rs6025211) have been tested in a real-world cohort of 237 advanced HCC patients (171 receiving systemic FMP chemotherapy followed by various anticancer treatments including sorafenib; 66 receiving HAIC)
[24]
. The results showed that patients with all three SNPs with favorable chemotherapy outcome (GALNT14-rs9679162 "TT", WWOX-rs13338697 "non-CT" and rs6025211"non-CT") had the best complete response rate and median OS (35.3%, 17.8 vs. 5.3 months, p = 0.024), compared with remaining patients. When the three favorable SNP markers were combined with two clinical criteria (tumor diameter < 8cm, neutrophil < 80%), the complete response rate to chemotherapy reached to 60%. Subsequent sorafenib for chemotherapy non-responders was associated with longer OS (p < 0.01). Suprisingly, GALNT14 "GG" genotype was associated with longer OS (p < 0.001, median OS > 10.5 months) in HAIC treated patients.
To evaluate the predictive role of GALNT14-rs9679162 in patients receiving sorafenib treatment, a cohort of 81 HCC patients who was treated by sorafenib as the first-line therapy was studied
[24]
. The results showed that "TT" genotype was not associated with OS when all patients were included. However, it was found that "TT" genotype was associated with a longer OS (p = 0.027) and "GG" genotype was associated with a shorter OS (p = 0.006) in patients with positive anti-hepatitis C virus antibody. In patients with positive hepatitis B virus surface antigen, no significant association was found between the genotypes and prognosis (p> 0.05 for all comparisons).
Table 1.
GALNT14 SNPs in different HCC stages and treatments
.
HCC stage and treatment | ||||
Case number | ||||
Favorable | ||||
Genotype | ||||
(% of total patient) | ||||
Prognostic value | ||||
Reference | ||||
Advanced HCC receiving FMP C/T | ||||
16 patients for SNP discovery (retrospective cohort) | ||||
41 patients for confirmation (prospective cohort) | ||||
"TT" (37.5% in retrospective cohort; 43.9% in prospective cohort) | ||||
1.In retrospective cohort, longer PFS (p = 0.00041) and OS (p = 0.0062) | ||||
2. In prospective cohort, longer PFS in prospective cohort (p = 0.01485) | ||||
[ | ] | |||
Advanced HCC receiving split-dose FMP C/T | ||||
107 patients (prospective cohort) | ||||
"TT" (26.2%) | ||||
1. Favorable OS (HR = 0.385, p = 0.002) | ||||
2. Favorable one-year survival rate (32.1% vs. 11.4%, p = 0.018) | ||||
3. Favorable PFS (HR = 0.431, p = 0.001) | ||||
4. Favorable CT response (28.6% vs. 10.1%, p = 0.029) | ||||
5. Favorable disease control rate (35.7% vs. 15.2%, p = 0.030) | ||||
[ | ] | |||
Advanced HCC receiving split-dose FMP C/T | ||||
118 patients (retrospective cohort) | ||||
"TT" (25.4%) | ||||
1. Combination of AFP £ 2800ng/mL, longer PFS (3.11 vs. 2.11 months, p = 0.014); longer OS (5.75 vs. 3.93 months, p = 0.001) | ||||
2. Combination of AFP £ 2800ng/mL, leukopenia, absence of vomiting, longest median PFS (10.64 vs. 2.07 months, p = 0.002); longest OS (25.5 vs. 4.5 months, p < 0.001) | ||||
[ | ] | |||
Advanced HCC receiving TACE | ||||
327 patients (retrospective cohort) | ||||
"TT" (29.1%) | ||||
1. Shorter time-to-response (HR = 2.362, p < 0.001) | ||||
2. Shorter time-to-complete response (HR = 1.947, p = 0.004) | ||||
3. Longer PFS (p < 0.001) | ||||
4. In patients with albumin < 3.5g/dL, longer OS (p = 0.027) | ||||
[ | ] | |||
Advanced HCC receiving split-dose FMP C/T | ||||
171 patients (retrospective cohort) | ||||
GALNT14 "TT", WWOX-rs13338697 "non-CT" rs6025211"non-CT" | ||||
(9.9%) | ||||
1. Favorable CR rate (35.3%); | ||||
2. Longer OS (p = 0.024) | ||||
3. Combined with tumor diameter < 8cm and neutrophil < 80%, best CR rate (60%) | ||||
4. For chemotherapy non-responder, soranefib has longer OS (p < 0.01) | ||||
[ | ] | |||
Advanced HCC receiving HAIC | ||||
66 patients (retrospective cohort) | ||||
"GG" (16.7%) | ||||
1. Longer OS (p < 0.001) | ||||
2. Median OS > 10.5 months | ||||
[ | ] | |||
Advanced HCC receiving sorafenib as first-line therapy | ||||
81 patients (retrospective cohort) | ||||
"TT" in patients with positive anti-HCV | ||||
1. Longer OS (p = 0.027) | ||||
[ | ] | |||
Abbreviations: AFP, α-fetoprotein; C/T, chemotherapy; HR, hazard ratio; OS, overall survival; PFS, progressive free survival; FMP, 5-fluorouracil, mitoxantrone, cisplatin; SNP, single nucleotide polymorphism; TACE, transcatheter arterial chemoembolization; GALNT14, N-acetylgalactosaminyltransferase14; HAIC, hepatic arterial infusion chemotherapy; HCC, hepatocellular carcinoma | ||||
3.
3.
GALNT14-rs9679162
SNP association with different GI cancers
The predictive value of
GALNT14-
rs9679162 SNP has been studied in various gastrointestinal (GI) cancers, including esophageal squamous cell carcinoma, gastric signet ring cell carcinoma, colorectal adenocarcinoma, pancreatic adenocarcinoma and cholangiocarcinoma (Table 2).
3.1. Esophageal squamous cell carcinoma
Concurrent chemoradiotherapy (CCRT) is the most common treatment for patients with locally advanced, unresected esophageal squamous cell carcinoma (ESCC) to prolong patient survival
. However, CCRT has a high-toxicity profile which restricts its clinical use. In order to select suitable patient group for CCRT, GALNT14-rs9679162 SNP has been examined as a therapeutic response predictor in ESCC
[27]
. A cohort of 108 patients with ESCC receving CCRT was recuited. Among these patients, the GALNT14-rs9679162 "TT", "TG" and "GG" genotypes were 28 (25.9%), 51 (47.2%) and 29 (26.9%) respectively. While the genotypes were not associated with the OS, the "GG" genotype was associated with a lower rate of CCRT response (24.1% vs 50.6%, p = 0.014). Further multivariate Cox-proportional hazard model analysis showed that the "GG" genotype was assocated with a longer time to complete/partial response (HR = 0.385, p = 0.022). Since the presence of a complete/partial response to CCRT was critical for advanced ESCC patients to achieve better outcome, the "GG" genotype can be used as a unfavorable predictor for CCRT in advanced CCRT.
3.2. Gastric signet ring cell carcinoma
Gastric signet ring cell carcinoma (SRCC) is one type of gastric cancer carrying a poorer prognosis compared with other types of gastric cancer
[28]
. However, the prognostic factors for gastric SRCC itself have seldom been delineated. To investigate whether GALNT14-rs9679162 SNP can be used as a prognostic marker in gastric SRCC, a cohort of 347 patients with gastric SRCC receiving surgical resection was recuited for the study
[29]
. The "TT", "TG" and "GG" genotypes of these patients were 23.34%, 59.65% and 17% respectively. The results showed that "TT" genotype was independently associated with unfavorable OS (HR = 1.550, p = 0.048) in SRCC with advaced stage (TNM stage IIB to IV). The subgroup analysis further revealed that the "TT" genotype was associated with unfavorable OS in SRCCs harboring more aggressive phenotypes such as node status > 0 (HR = 1.808, p = 0.0013), lymphatic invasion (HR = 1.587, p = 0.021), vascular invasion (HR = 3.389, p = 0.0076) and perineural invasion (HR = 1.604, p = 0.0161). It suggested that gastric SRCC could be stratified into different prognostic subgroups by the combination of cliniopathological factors and GALNT14 genotype. The poorest prognosis subgroup included patients having aggressive phenotype together with the GALNT14 "TT" genotype.
3.3. Colorectal adenocarcinoma
Adjuvant oxaliplatin-based chemotherapy has been suggested as a standard treatment for patients with stage III colorectal adenocarcinoma following surgical resection. Although the adjuvant chemotherapy could reduce around 30% of disease recurrence and 22% to 32% of motarlity, not all patients benefit from the adjuvant therapy
. Therefore, the predictive role of GALNT14-rs9679162 SNP has been investigated in this group of patients
[33]
. A cohort of 300 patients with stage III colorectal adenocarcinoma receiving curative resection followed by oxaliplatin-based chemotherapy was retrospectively recruited. Of these patients, 18% patients had "TT" genotype and harbored an unfavorable OS (HR = 5.406, p = 0.019). Subgroup analysis further showed that the "TT" genotype was associated with unfavorable OS in the following subgroups: age £ 65 years, male, left side CRC, N2 stage, carcinoembryonic antigen > 5 ng/mL, and mucinous histology (p = 0.012, 0.011, 0.009, 0.025, 0.013, and 0.007, respectively). This study concluded that "TT" genotype is an unfavorable prognostic marker in stage III colorectal adenocarcinoma receiving oxaliplatin-based adjuvant chemotherapy.
3.4. Pancreatic ductal adenocarcinoma
Pancreatic ductal adenocarcinoma (PDA) is one of the most aggressive cancers with poor prognosis due to advanced presentation when diagnosed and limited therapeutic options. The lack of validated predictive markers further complicates this situation
[34]
. Again, the predictive value of GALNT14-rs9679162 SNP genotype was examined in PDA patients receiving surgical resection
[35]. A cohort of 103 patients with PDA patients was enrolled for analysis. The GALNT14 genotype analysis revealed that 19.4%, 60.2% and 20.4% of patients had the "TT", “TG” and “GG” genotypes, respectively. Patients with the "GG" genotype had a longer mean OS time compared with that of the "non-GG" genotype (37.1 vs 16.1 months, p = 0.005). Further univariate followed by multivariate Cox proportional hazard analysis identified the“GG” genotype, negative resection margin, and locoregional disease as independent predictors for favorable OS (p = 0.003, p = 0.037, p = 0.021, respectively). The study suggested that "GG" genotype could serve as a favorable prognostic marker in patients with resected PDA.
. A cohort of 103 patients with PDA patients was enrolled for analysis. The GALNT14 genotype analysis revealed that 19.4%, 60.2% and 20.4% of patients had the "TT", "TG" and "GG" genotypes, respectively. Patients with the "GG" genotype had a longer mean OS time compared with that of the "non-GG" genotype (37.1 vs 16.1 months, p = 0.005). Further univariate followed by multivariate Cox proportional hazard analysis identified the"GG" genotype, negative resection margin, and locoregional disease as independent predictors for favorable OS (p = 0.003, p = 0.037, p = 0.021, respectively). The study suggested that "GG" genotype could serve as a favorable prognostic marker in patients with resected PDA.
3.5. Cholangiocarcinoma
Cholangiocarcinoma emerges from dysregulated proliferation of cholangiocytes, and is notorious for its poor prognosis and response to chemotherapy
[36]
. The association between the prognosis of patients with resected cholangiocarcinoma and the GALNT14-rs9679162 SNP genotype was examined
[37]
. A cohort of patients with cholangiocarcinoma (n = 112) were retrospectively recruited. Of these patients, 31.3, 49.1 and 19.6% had "TT", "TG" and "GG" genotypes, respectively. The "TT" genotype was associated with unfavorable OS in univariate analysis (HR = 2.282, p = 0.023). Furthermore, two tumor characteristics, perineural and vascular invasion, were independently associated with unfavorable OS (p = 0.001 and p = 0.002, respectively). In a multivariate linear analysis, the "TT" genotypes were independently associated with two known predictors of unfavorable prognosis, perineural invasion (p = 0.035) and lymph node metastasis (p = 0.005). This study concluded that the "TT" genotype was associated with perineural invasion and lymph node metastasis, as well as unfavorable OS in patients with resected cholangiocarcinoma.
Table 2. GALNT14-rs9679162
SNP in different GI cancers.
|
Type of GI cancers |
Case number and treatment |
Unfavorable Genotype (% of total patient) |
Prognostic value |
Reference |
|
Esophageal squamous cell carcinoma |
108 patients with advanced esophageal squamous cell carcinoma with CCRT |
"GG" (26.1%) |
1. Lower rate of CCRT response (24.1% vs. 50.6%, p = 0.014) 2. Longer time to complete/partial response (HR 0.385, p = 0.022) |
[27] |
|
Gastric signet ring cell carcinoma |
347 patients with gastric signet ring cell carcinoma receiving surgical resection |
"TT" (23.3%) |
1. In advaced stage (IIB to IV) group, unfavorable OS (HR = 1.550, p = 0.048) 2. In N > 0 subgroup, unfavorable OS (HR 1.808, p = 0.0013) 3. In lymphatic invasion subgroup, unfavorable OS (HR 1.587, p = 0.0021) 4. In vascular invasion subgroup, unfavorable OS (HR 3.389, p = 0.0076) 5. In perineural invasion subgroup, unfavorable OS (HR 1.604, p = 0.0161) |
[29] |
|
Colorectal adenocarcinoma |
300 patients with stage III colorectal cancer with Oxaliplatin based adjuvant CT |
"TT" (18.1%) |
1. Unfavorable OS (HR = 5.406, p = 0.019) 2. In age £ 65y subgroup, unfavorable OS (HR = 5.051, p = 0.024) 3. In male subgroup, unfavorable OS (HR = 7.337, p = 0.030) 4. In left side colorectal cancer group, unfavorable OS (HR = 7.857, p = 0.026) 5. In N2 stage subgroup, unfavorable OS (HR = 6.017, p = 0.049) 6. In CEA > 5ng/mL subgroup, unfavorable OS (HR = 11.295, p = 0.049) 7. In mucinous histology subgroup, unfavorable OS (HR = 13.296, p = 0.037) |
[33] |
|
Pancreatic ductal adenocarcinoma |
103 patients with pancreatic adenocarcinoma receiving surgical resection |
"Non-GG (TG+TT)" (80.6%) |
1. Lower mean OS time (16.1 vs. 37.1 months, p = 0.005) 2. Unfavorable OS (HR = 3.663, p = 0.003) |
[35] |
|
Cholangiocarcinoma |
112 patients with cholangiocarcinoma receiving surgical resection |
"TT" (31.3%) |
1. Unfavorable OS (HR = 2.282, p = 0.023) 2. Associated with perineural invasion (p = 0.035) 3. Associated with LN metastasis (p = 0.005) |
[37] |
|
Abbreviations: CCRT, concurrent chemoradiotherapy; HR, hazard ratio; OS, overall survival; CEA, carcinoembryonic antigen; LN, lymph node; C/T, chemotherapy; GALNT14, N-acetylgalactosaminyltransferase14 |
||||
4. GALNT14 enzyme level is associated with
4. GALNT14 enzyme level is associated with
GALNT14
SNP genotype
Current studies of the predictive role of
GALNT14-rs9679162 SNP showed that in HCC and esophageal cancer (“carcinoma”), patients with the “TT” genotype had a better prognosis, whereas in other cancers (“adenocarcinoma”), patients with the “GG” genotype had a better outcome
-rs9679162 SNP showed that in HCC and esophageal cancer ("carcinoma"), patients with the "TT" genotype had a better prognosis, whereas in other cancers ("adenocarcinoma"), patients with the "GG" genotype had a better outcome
. Since GALNT14 enzyme levels have been associated with cancer characteristics, it can be speculated that GALNT14 enzyme expression levels were associated with different SNP genotypes. This hypothesis has been examined in HCC
[22]
and PDA
[35]
tissues. The protein levels of GALNT14 and cFLIP-S, an inhibitor of the apoptotic signal transduction, in relation to the
GALNT14
genotype were invested in a cohort of 44 patients with surgical resection of HCC
[22]. Analysis of quantitative protein levels on the genotype-stratified patient subgroups showed: (i) the cancer parts had higher GALNT14 protein levels than those of the noncancer parts (p = 0.008 and < 0.001 for "TT" and "non-TT" genotypes, respectively); and (ii) a significantly higher cancer to noncancer (C/non-C) ratio of GALNT14 expression was noted in the "TT" genotype compared with the "non- TT" genotype (p = 0.001). Additionally, the C/non-C ratios of cFLIP-S were significantly lower in the "TT" genotype than those in the "non-TT" genotype (p = 0.014). Glycoproteomic analysis showed that the glycosylated residues of death receptor 5 (DR5) in the "TT" patients-derived HCC tissues were clustered in the two major glycosylation sites, in contrast to a scattered glycosylation pattern in the "non-TT" liver tumor tissues. These molecular finding suggested that the cancerous tissues from HCC patients with "TT" genotype were more sensitive to extrinsic apoptosis signaling compared with those with “non-TT” genotype.
. Analysis of quantitative protein levels on the genotype-stratified patient subgroups showed: (i) the cancer parts had higher GALNT14 protein levels than those of the noncancer parts (p = 0.008 and < 0.001 for "TT" and "non-TT" genotypes, respectively); and (ii) a significantly higher cancer to noncancer (C/non-C) ratio of GALNT14 expression was noted in the "TT" genotype compared with the "non- TT" genotype (p = 0.001). Additionally, the C/non-C ratios of cFLIP-S were significantly lower in the "TT" genotype than those in the "non-TT" genotype (p = 0.014). Glycoproteomic analysis showed that the glycosylated residues of death receptor 5 (DR5) in the "TT" patients-derived HCC tissues were clustered in the two major glycosylation sites, in contrast to a scattered glycosylation pattern in the "non-TT" liver tumor tissues. These molecular finding suggested that the cancerous tissues from HCC patients with "TT" genotype were more sensitive to extrinsic apoptosis signaling compared with those with "non-TT" genotype.
In PDA, a preliminary assessment of the GALNT14 enzyme expressions from 20 patients (10 with "TT" and 10 with "GG" genotype) was performed
[35]
. Among them, patients with "TT" genotype had higher expression levels of GALNT14 while those with "GG" genotype exhibited lower GALNT14 levels in the cancerous cells, albeit the pattern was reversed in the islet cells. This finding suggested that GALNT14 protein in PDA had an oncogene-like function, similar to what was reported in breast and ovarian cancers
.
5. GALNT14 enzyme levels were associated with aggressiveness of breast, ovarian cancer, NSCLC and neuroblastoma
5.1 Breast cancer
The association between cancer characteristics and GALNT14 protein expression has been studied in breast cancer
[38]
. The GALNT14 was over-expressed in most breast cancer tissues (47/56, 83.9%), but in only 7/48 (14.6%) non-malignant breast tissues. Higher histological grade of invasive ductal carcinoma was correspondent to lower expression level of GALNT14. Moreover, Song
et al.
reported that GALNT14 expression in breast cancer was linked to lung metastasis
[13]
. High expression of GALNT14 in advanced breast cancer was associated with shorter lung metastasis-free survival (p = 0.0015). GALNT14 promotes breast cancer metastasis to lung by enhancing the initiation of metastatic colonies and their subsequent growth into overt metastases. The inhibitory effect of lung-derived bone morphogenetic proteins (BMPs) on cancer self-renewal was overcome by GALNT14, which facilitated metastasis initiation within the lung microenvironment. In addition, GALNT14 not only enhanced the recruitment of macrophages to the site of metastases, but also exploited macrophage-derived fibroblast growth factors (FGFs). Moreover, the KRAS-PI3K-c-JUN signaling was identified as an upstream pathway that accounted for the elevated expression of GALNT14 in lung-metastatic breast cancer. In a study conducted with MCF-7 breast cancer cell line, overexpression of GALNT14 significantly enhanced cell proliferation, migration and tumor invasion, while knockdown of GALNT14 reduced clonogenicity and attenuated cell migration and cell invasion
[12]
. Moreover, GALNT14 mediated O-glycosylation of EGF-containing fibulin-like extracellular matrix protein 2 (EFEMP2) which significantly increased the invasion ability of breast cancer cell lines (MCF-7 and MBA-MD-231)
[39]
. The chemosensitivity of breast cancer was also associated with GALNT14. Osterix decreased the chemosensitivity and enhanced the anti-apoptosis by upregulating
GALNT14
[40]
. Further analysis of 129 breast cancer patients showed that high expression of GALNT14 in breast cancer tissues was associated with higher HER2 (76.7% vs. 58.1%, p = 0.038), higher clinical stages (p < 0.0001) and shorter DFS (p = 0.029). Another study showed that
GALNT14
regulated the stability of P-pg, the efflux pump localized on the cell membrane, which further promoted multidrug resistance
[16]
. These studies suggested GALNT14 might play an important role in modulating breast cancer aggressiveness and could be considered as a therapeutic target for treatment.
5.2 Ovarian cancer
Overexpression of GALNT14 was found in ovarian cancer
. Knockdown of
GALNT14
significantly suppressed cell migration and cellular morphology change through aberrant glycosylation of transmembrane mucin 13
[14]
. Yang
et al.
demonstrated that the expression levels of miR-125a were downregulated and negatively related to GALNT14 expression in ovarian cancer tissues
[42]
. Moreover, luciferase reporter assay identified
GALNT14
as a direct target of miR-125a, and overexpression of miR-125a markedly reduced the expression of GALNT14. Both miR-125a mimics and
GALNT14
siRNA suppressed the activity of matrix metalloproteinase (MMP)-2 and MMP-9, further inhibited the extracellular matrix degradation. A recent study showed that aberrant expression of BORIS (Brother of the Regulator of Imprinted Sites) altered cell migration and invasion via upregulation of
GALNT14
, suggesting BORIS as a potential therapeutic target in ovarian high-grade serous carcinoma
[43]
.
5.3 NSCLC
The expression of GALNT14 was highly associated with shorter RFS in a clinicogenomics study including 138 NSCLC patients
[44]
. In PDA, melanoma and NSCLC cell lines, GALNT14 expression was correlated with Apo2L/TRAIL sensitivity
[11]
. Overexpression of
GALNT14
increased cellular Apo2L/TRAIL sensitivity, whereas RNA interference of
GALNT14
expression reduced responsiveness. Through modulation of extracellular DR4 and DR5 O-glycosylation sites, GALNT14 enhanced apoptotic signaling. Immunohistochemistry assays that measured GALNT14 expression had been developed to select NSCLC patients who might be more sensitive to the proapoptotic receptor agonists dulanermin (rhApo2L/TRAIL) and drozitumab (DR5-agonist antibody)
[45]
. Moreover, GALNT14 increased the sensitivity of the WNT signaling and increased the stability of the β-catenin protein, leading to induced HOXB9 expression and acquisition of an invasive phenotype
[15]
. A meta-analysis of clinical genomics data showed that overexpression of GALNT14 or HOXB9 was strongly correlated with reduced RFS and increased HR, suggesting that targeting the GALNT14/WNT/HOXB9 axis might be a novel therapeutic approach to inhibit NSCLC metastasis.
5.4 Neuroblastoma
A
GALNT14
mutation (c.802C > T) was identified as a neuroblastoma predisposition gene and predicted as functionally damaging by the PolyPhen2 (Polymorphism Phenotyping v2) and SIFT (Sorting Intolerant From Tolerant) scoring methods
[17]
. Furthermore, high expression of GALNT14 was associated with a worse OS in a public dataset of 88 neuroblastoma samples.
GALNT14
is located closely to
ALK
on 2p23.1, a region previously discovered in linkage with neuroblastoma.
6. Conclusions
GALN14, an initiating enzyme of O-glycosylation, has been demonstrated to play a pivotal role in cancer cell proliferation, migration and metastases. The expression level of
GALNT14
as well as its SNP genotype can be used to predict clinical outcomes in various types of cancer. However, the relationships between the
GALNT14
SNPs, the enzyme expressions, and the tumor behaviors have not been fully unveiled. Here, we summarized the current knowledge of GALNT14 in various cancers, particularly focusing on its role as a biomaker. Further studies are still needed to provide the missing mechanisms regarding the links between the genotypes and expression levels, as well as other GALNT14-involved microenvironmental elements, which can modulate cancer behaviors. Hopefully, with these knowledges, new therapeutic strategies can be devised in the future.
References
- Moremen, K.W.; Tiemeyer, M.; Nairn, A.V. Vertebrate protein glycosylation: Diversity, synthesis and function. Nat Rev Mol Cell Biol 2012, 13, 448-462.
- Tarp, M.A.; Clausen, H. Mucin-type o-glycosylation and its potential use in drug and vaccine development. Biochim Biophys Acta 2008, 1780, 546-563.
- Tian, E.; Ten Hagen, K.G. Recent insights into the biological roles of mucin-type o-glycosylation. Glycoconj J 2009, 26, 325-334.
- Tran, D.T.; Ten Hagen, K.G. Mucin-type o-glycosylation during development. J Biol Chem 2013, 288, 6921-6929.
- Bennett, E.P.; Mandel, U.; Clausen, H.; Gerken, T.A.; Fritz, T.A.; Tabak, L.A. Control of mucin-type o-glycosylation: A classification of the polypeptide galnac-transferase gene family. Glycobiology 2012, 22, 736-756.
- Ten Hagen, K.G.; Fritz, T.A.; Tabak, L.A. All in the family: The udp-galnac:Polypeptide n-acetylgalactosaminyltransferases. Glycobiology 2003, 13, 1R-16R.
- Beaman, E.M.; Brooks, S.A. The extended ppgalnac-t family and their functional involvement in the metastatic cascade. Histol Histopathol 2014, 29, 293-304.
- Wang, H.; Tachibana, K.; Zhang, Y.; Iwasaki, H.; Kameyama, A.; Cheng, L.; Guo, J.; Hiruma, T.; Togayachi, A.; Kudo, T., et al. Cloning and characterization of a novel udp-galnac:Polypeptide n-acetylgalactosaminyltransferase, pp-galnac-t14. Biochem Biophys Res Commun 2003, 300, 738-744.
- Hagen, F.K.; Hazes, B.; Raffo, R.; deSa, D.; Tabak, L.A. Structure-function analysis of the udp-n-acetyl-d-galactosamine:Polypeptide n-acetylgalactosaminyltransferase. Essential residues lie in a predicted active site cleft resembling a lactose repressor fold. J Biol Chem 1999, 274, 6797-6803.
- Unligil, U.M.; Zhou, S.; Yuwaraj, S.; Sarkar, M.; Schachter, H.; Rini, J.M. X-ray crystal structure of rabbit n-acetylglucosaminyltransferase i: Catalytic mechanism and a new protein superfamily. EMBO J 2000, 19, 5269-5280.
- Wagner, K.W.; Punnoose, E.A.; Januario, T.; Lawrence, D.A.; Pitti, R.M.; Lancaster, K.; Lee, D.; von Goetz, M.; Yee, S.F.; Totpal, K., et al. Death-receptor o-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand apo2l/trail. Nat Med 2007, 13, 1070-1077.
- Huanna, T.; Tao, Z.; Xiangfei, W.; Longfei, A.; Yuanyuan, X.; Jianhua, W.; Cuifang, Z.; Manjing, J.; Wenjing, C.; Shaochuan, Q., et al. Galnt14 mediates tumor invasion and migration in breast cancer cell mcf-7. Mol Carcinog 2015, 54, 1159-1171.
- Song, K.H.; Park, M.S.; Nandu, T.S.; Gadad, S.; Kim, S.C.; Kim, M.Y. Galnt14 promotes lung-specific breast cancer metastasis by modulating self-renewal and interaction with the lung microenvironment. Nat Commun 2016, 7, 13796.
- Wang, R.; Yu, C.; Zhao, D.; Wu, M.; Yang, Z. The mucin-type glycosylating enzyme polypeptide n-acetylgalactosaminyltransferase 14 promotes the migration of ovarian cancer by modifying mucin 13. Oncol Rep 2013, 30, 667-676.
- Kwon, O.S.; Oh, E.; Park, J.R.; Lee, J.S.; Bae, G.Y.; Koo, J.H.; Kim, H.; Choi, Y.L.; Choi, Y.S.; Kim, J., et al. Galnac-t14 promotes metastasis through wnt dependent hoxb9 expression in lung adenocarcinoma. Oncotarget 2015, 6, 41916-41928.
- Shan, J.; Liu, Y.; Wang, Y.; Li, Y.; Yu, X.; Wu, C. Galnt14 involves the regulation of multidrug resistance in breast cancer cells. Transl Oncol 2018, 11, 786-793.
- De Mariano, M.; Gallesio, R.; Chierici, M.; Furlanello, C.; Conte, M.; Garaventa, A.; Croce, M.; Ferrini, S.; Tonini, G.P.; Longo, L. Identification of galnt14 as a novel neuroblastoma predisposition gene. Oncotarget 2015, 6, 26335-26346.
- Liang, K.H.; Lin, C.C.; Yeh, C.T. Galnt14 snp as a potential predictor of response to combination chemotherapy using 5-fu, mitoxantrone and cisplatin in advanced hcc. Pharmacogenomics 2011, 12, 1061-1073.
- Yeh, C.T.; Liang, K.H.; Lin, C.C.; Chang, M.L.; Hsu, C.L.; Hung, C.F. A single nucleotide polymorphism on the galnt14 gene as an effective predictor of response to chemotherapy in advanced hepatocellular carcinoma. Int J Cancer 2014, 134, 1214-1224.
- Lin, W.R.; Hsu, C.W.; Chen, Y.C.; Chang, M.L.; Liang, K.H.; Huang, Y.H.; Yeh, C.T. Galnt14 genotype, alpha-fetoprotein and therapeutic side effects predict post-chemotherapy survival in patients with advanced hepatocellular carcinoma. Mol Clin Oncol 2014, 2, 630-640.
- Liang, K.H.; Yang, P.C.; Yeh, C.T. Genotyping the galnt14 gene by joint analysis of two linked single nucleotide polymorphisms using liver tissues for clinical and geographical comparisons. Oncol Lett 2014, 8, 2215-2220.
- Liang, K.H.; Lin, C.L.; Chen, S.F.; Chiu, C.W.; Yang, P.C.; Chang, M.L.; Lin, C.C.; Sung, K.F.; Yeh, C.; Hung, C.F., et al. Galnt14 genotype effectively predicts the therapeutic response in unresectable hepatocellular carcinoma treated with transcatheter arterial chemoembolization. Pharmacogenomics 2016, 17, 353-366.
- Lin, W.R.; Hsu, C.W.; Yeh, C.S.; Chen, Y.C.; Chang, M.L.; Liang, K.H.; Lin, C.C.; Chu, Y.D.; Yeh, C.T. Combinations of single nucleotide polymorphisms wwox-rs13338697, galnt14-rs9679162 and rs6025211 effectively stratify outcomes of chemotherapy in advanced hepatocellular carcinoma. Asia Pac J Clin Oncol 2018, 14, e54-e63.
- Lin, C.C.; Hsu, C.W.; Chen, Y.C.; Chang, M.L.; Liang, K.H.; Lai, M.W.; Lin, C.L.; Chien, R.N.; Lin, K.H.; Yeh, C.T. A galnt14 rs9679162 genotype-guided therapeutic strategy for advanced hepatocellular carcinoma: Systemic or hepatic arterial infusion chemotherapy. Pharmacogenomics J 2019.
- Honing, J.; Smit, J.K.; Muijs, C.T.; Burgerhof, J.G.; de Groot, J.W.; Paardekooper, G.; Muller, K.; Woutersen, D.; Legdeur, M.J.; Fiets, W.E., et al. A comparison of carboplatin and paclitaxel with cisplatinum and 5-fluorouracil in definitive chemoradiation in esophageal cancer patients. Ann Oncol 2014, 25, 638-643.
- Stahl, M.; Budach, W.; Meyer, H.J.; Cervantes, A.; Group, E.G.W. Esophageal cancer: Clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010, 21 Suppl 5, v46-49.
- Tsou, Y.K.; Liang, K.H.; Lin, W.R.; Chang, H.K.; Tseng, C.K.; Yeh, C.T. Galnt14 genotype as a response predictor for concurrent chemoradiotherapy in advanced esophageal squamous cell carcinoma. Oncotarget 2017, 8, 29151-29160.
- Piessen, G.; Messager, M.; Leteurtre, E.; Jean-Pierre, T.; Mariette, C. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg 2009, 250, 878-887.
- Chen, T.H.; Lin, W.R.; Lee, C.; Chiu, C.T.; Hsu, J.T.; Yeh, T.S.; Lin, K.H.; Le, P.H.; Yeh, C.T. Prognostic stratification of advanced gastric signet ring cell carcinoma by clinicopathological factors and galnt14 genotype. J Cancer 2018, 9, 3540-3547.
- Andre, T.; Boni, C.; Mounedji-Boudiaf, L.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Zaninelli, M.; Clingan, P.; Bridgewater, J., et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004, 350, 2343-2351.
- Kuebler, J.P.; Wieand, H.S.; O'Connell, M.J.; Smith, R.E.; Colangelo, L.H.; Yothers, G.; Petrelli, N.J.; Findlay, M.P.; Seay, T.E.; Atkins, J.N., et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage ii and iii colon cancer: Results from nsabp c-07. J Clin Oncol 2007, 25, 2198-2204.
- Sanoff, H.K.; Carpenter, W.R.; Martin, C.F.; Sargent, D.J.; Meyerhardt, J.A.; Sturmer, T.; Fine, J.P.; Weeks, J.; Niland, J.; Kahn, K.L., et al. Comparative effectiveness of oxaliplatin vs non-oxaliplatin-containing adjuvant chemotherapy for stage iii colon cancer. J Natl Cancer Inst 2012, 104, 211-227.
- Lin, W.R.; Chiang, J.M.; Liang, K.H.; Lim, S.N.; Lai, M.W.; Tsou, Y.K.; Hsieh, T.Y.; Hsu, C.K.; Yeh, C.T. Galnt14 genotype predicts postoperative outcome of stage iii colorectal cancer with oxaliplatin as adjuvant chemotherapy. Medicine (Baltimore) 2016, 95, e3487.
- Hasan, S.; Jacob, R.; Manne, U.; Paluri, R. Advances in pancreatic cancer biomarkers. Oncol Rev 2019, 13, 410.
- Chiang, C.C.; Yeh, C.T.; Hwang, T.L.; Chu, Y.D.; Lim, S.N.; Chen, C.W.; Kuo, C.J.; Le, P.H.; Chen, T.H.; Lin, W.R. The galnt14 genotype predicts postoperative outcome of pancreatic ductal adenocarcinoma. J Clin Med 2019, 8.
- Blechacz, B.; Gores, G.J. Cholangiocarcinoma: Advances in pathogenesis, diagnosis, and treatment. Hepatology 2008, 48, 308-321.
- Liang, K.H.; Yeh, T.S.; Wu, R.C.; Yeh, C.N.; Yeh, C.T. Galnt14 genotype is associated with perineural invasion, lymph node metastasis and overall survival in resected cholangiocarcinoma. Oncol Lett 2017, 13, 4215-4223.
- Wu, C.; Guo, X.; Wang, W.; Wang, Y.; Shan, Y.; Zhang, B.; Song, W.; Ma, S.; Ge, J.; Deng, H., et al. N-acetylgalactosaminyltransferase-14 as a potential biomarker for breast cancer by immunohistochemistry. BMC Cancer 2010, 10, 123.
- Zuo, T.; Shan, J.; Liu, Y.; Xie, R.; Yu, X.; Wu, C. Efemp2 mediates galnt14-dependent breast cancer cell invasion. Transl Oncol 2018, 11, 346-352.
- Wu, J.; Chen, X.; Bao, Q.; Duan, R.; Jin, Y.; Shui, Y.; Yao, B.; Lu, X.; Wang, Y.; Cui, H., et al. Osterix decreases the chemosensitivity of breast cancer cells by upregulating galnt14. Cell Physiol Biochem 2017, 44, 998-1010.
- Sheta, R.; Bachvarova, M.; Plante, M.; Gregoire, J.; Renaud, M.C.; Sebastianelli, A.; Popa, I.; Bachvarov, D. Altered expression of different galnactransferases is associated with disease progression and poor prognosis in women with high-grade serous ovarian cancer. Int J Oncol 2017, 51, 1887-1897.
- Yang, J.; Li, G.; Zhang, K. Mir-125a regulates ovarian cancer proliferation and invasion by repressing galnt14 expression. Biomed Pharmacother 2016, 80, 381-387.
- Hillman, J.C.; Pugacheva, E.M.; Barger, C.J.; Sribenja, S.; Rosario, S.; Albahrani, M.; Truskinovsky, A.M.; Stablewski, A.; Liu, S.; Loukinov, D.I., et al. Boris expression in ovarian cancer precursor cells alters the ctcf cistrome and enhances invasiveness through galnt14. Mol Cancer Res 2019, 17, 2051-2062.
- Lee, E.S.; Son, D.S.; Kim, S.H.; Lee, J.; Jo, J.; Han, J.; Kim, H.; Lee, H.J.; Choi, H.Y.; Jung, Y., et al. Prediction of recurrence-free survival in postoperative non-small cell lung cancer patients by using an integrated model of clinical information and gene expression. Clin Cancer Res 2008, 14, 7397-7404.
- Stern, H.M.; Padilla, M.; Wagner, K.; Amler, L.; Ashkenazi, A. Development of immunohistochemistry assays to assess galnt14 and fut3/6 in clinical trials of dulanermin and drozitumab. Clin Cancer Res 2010, 16, 1587-1596.