The major goal of liver tissue engineering is to reproduce the phenotype and functions of liver cells, especially primary hepatocytes ex vivo. Several strategies have been explored in the recent past for culturing the liver cells in the most apt environment using biological scaffolds supporting hepatocyte growth and differentiation. Nanofibrous scaffolds have been widely used in the field of tissue engineering for their increased surface-to-volume ratio and increased porosity, and their close resemblance with the native tissue extracellular matrix (ECM) environment. Electrospinning is one of the most preferred techniques to produce nanofiber scaffolds. TIn the current review, we have discussed the various technical aspects of electrospinning that have been employed for scaffold development for different types of liver cells were discussed. T. We have highlighted the use of synthetic and natural electrospun polymers along with liver ECM in the fabrication of these scaffolds was highlighted. N. We have also described novel strategies that include modifications, such as galactosylation, matrix protein incorporation, etc., in the electrospun scaffolds that have evolved to support the long-term growth and viability of the primary hepatocytes were also described .
- liver tissue engineering
- electrospinning
- nanofibers
- natural and synthetic polymers
1. Electrospinning
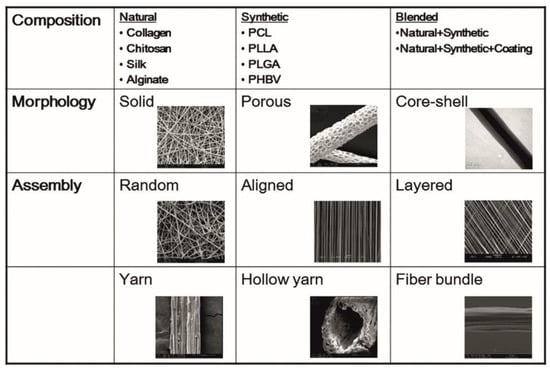
2. Hepatic Cell Types on Electrospun Nanofiber Scaffolds
3. Recent Innovative Approaches in Electrospinning for Liver Tissue Engineering
For prolonged cultures of hepatocytes, studies have now reported the use of hepatic cells in the form of 3D spheroids. Innovative approaches have been employed by researchers to incorporate the hepatocytes as spheroids, which is discussed in the following section. Wei et al. modified the conventional electrospinning method and came up with the idea of short fibers to support culture of hepatic spheroids without the need for surface modifications. They reported that the length of the fibers can be modified according to the length of the spheroids and showed that spheroids cultured on the PSMA (Poly(styrene-co-methyl acrylate) fibers of about 50µm length have improved drug metabolism and drug clearance [59]. Carbon nanotubes (CNTs) in the form of nanofibrous mats are known to provide electrically conductive surfaces and have been used for prolonged 3D spheroid cultures [60]. Koga et al. have reported that CNTs have the ability to induce the formation of hepatocyte spheroids [61]. Wei et al. have also used multiwalled CNTs functionalized with galactose moieties on the surface for efficient hepatic spheroid cultures. They demonstrated that hepatocytes cultured on these functionalized fibrous mats showed better functions, namely, better drug clearance and increased expression of drug metabolizing genes [62]. Besides in vitro cultures, electrospun liver scaffolds are also being used for in vivo applications. The scaffolds can be fabricated as patches containing nano-fibrous mesh that can then be implanted at the site of injury. Kim et al. have recently shown that electrospun scaffold patches can be used to deliver healthy hepatic cells in toxin-induced liver injury mouse models. In this study, they used PCL for fabricating electrospun scaffolds/sheets and seeded them with patient-derived primary hepatocytes in a stacking manner by 3D bioprinting to mimic the native liver environment. The survival of the animals with the hepatic sheet transplant was 70% as compared to that of the control group without the scaffold. This study has opened the doors of using electrospun liver cell scaffolds for liver transplantation and in vivo therapy [63]. Another study by Salerno et al. has also proved the potential of electrospinning in mimicking the native liver tissue architecture with multiple cell types. They used the dry jet–wet electrospinning method to prepare hollow PCL fibers, and placed them in a bioreactor which contained an outer luminal segment where primary human hepatocytes were cultured and an inner luminal segment where endothelial cells were seeded in a hexagonal manner, thereby mimicking the native liver architecture [64]. The authors observed improved hepatic functions such as glucose consumption and albumin secretion for up to 18 days in the perfusion bioreactor. The recent studies on the electrospun scaffolds show the potential of them on the clinical front, an overview of the recent strategies employed for the fabrication of electrospun nanofiber scaffolds for liver cells is given schematically in Figure 2. Patient-specific scaffolds made out of this technique would give us the edge of replacing liver transplantation treatment with tissue engineering.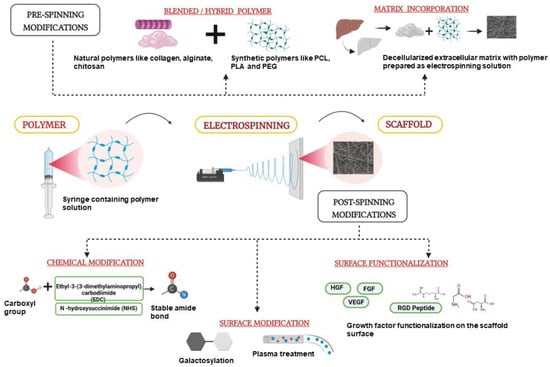
References
- Tillman, B.W.; Yazdani, S.K.; Lee, S.J.; Geary, R.L.; Atala, A.; Yoo, J.J. The in vivo stability of electrospun polycaprolactone-collagen scaffolds in vascular reconstruction. Biomaterials 2009, 30, 583–588.
- Kumbar, S.G.; Nukavarapu, S.P.; James, R.; Hogan, M.V.; Laurencin, C.T. Recent patents on electrospun biomedical nanostructures: An overview. Recent Pat. Biomed. Eng. 2008, 1, 68–78.
- Shahriar, S.M.; Mondal, J.; Hasan, M.N.; Revuri, V.; Lee, D.Y.; Lee, Y.K. Electrospinning nanofibers for therapeutics delivery. Nanomaterials 2019, 9, 532.
- Sayin, S.; Tufani, A.; Emanet, M.; Genchi, G.G.; Sen, O.; Shemshad, S.; Ozaydin Ince, G. Electrospun nanofibers with pH-responsive coatings for control of release kinetics. Front. Bioeng. Biotechnol. 2019, 7, 309.
- Volpato, F.Z.; Almodóvar, J.; Erickson, K.; Popat, K.C.; Migliaresi, C.; Kipper, M.J. Preservation of FGF-2 bioactivity using heparin-based nanoparticles, and their delivery from electrospun chitosan fibers. Acta Biomater. 2012, 8, 1551–1559.
- Almodóvar, J.; Kipper, M.J. Coating electrospun chitosan nanofibers with polyelectrolyte multilayers using the polysaccharides heparin and N, N, N-trimethyl chitosan. Macromol. Biosci. 2011, 11, 72–76.
- Luong-Van, E.; Grøndahl, L.; Chua, K.N.; Leong, K.W.; Nurcombe, V.; Cool, S.M. Controlled release of heparin from poly (ε-caprolactone) electrospun fibers. Biomaterials 2006, 27, 2042–2050.
- Karpov, T.E.; Peltek, O.O.; Muslimov, A.R.; Tarakanchikova, Y.V.; Grunina, T.M.; Poponova, M.S.; Surmenev, R.A. Development of optimized strategies for growth factor incorporation onto electrospun fibrous scaffolds to promote prolonged release. ACS Appl. Mater. Interfaces 2019, 12, 5578–5592.
- Liu, C.; Wang, C.; Zhao, Q.; Li, X.; Xu, F.; Yao, X.; Wang, M. Incorporation and release of dual growth factors for nerve tissue engineering using nanofibrous bicomponent scaffolds. Biomed. Mater. 2018, 13, 044107.
- Sahoo, S.; Ang, L.T.; Goh JC, H.; Toh, S.L. Growth factor delivery through electrospun nanofibers in scaffolds for tissue engineering applications. J. Biomed. Mater. Res. Part A 2010, 93, 1539–1550.
- Yin, Z.; Chen, X.; Song, H.X.; Hu, J.J.; Tang, Q.M.; Zhu, T.; Shen, W.L.; Chen, J.L.; Liu, H.; Heng, B.C.; et al. Electrospun scaffolds for multiple tissues regeneration in vivo through topography dependent induction of lineage specific differentiation. Biomaterials 2015, 44, 173–185.
- Blackwood, K.A.; McKean, R.; Canton, I.; Freeman, C.O.; Franklin, K.L.; Cole, D.; MacNeil, S. Development of biodegradable electrospun scaffolds for dermal replacement. Biomaterials 2008, 29, 3091–3104.
- Klumpp, D.; Rudisile, M.; Kühnle, R.I.; Hess, A.; Bitto, F.F.; Arkudas, A.; Bleiziffer, O.; Boos, A.M.; Kneser, U.; Horch, R.E.; et al. Three-dimensional vascularization of electrospun PCL/collagen- blend nanofibrous scaffolds in vivo. J. Biomed. Mater. Res. Part A 2012, 100A, 2302–2311.
- Yu, D.G.; Chian, W.; Wang, X.; Li, X.Y.; Li, Y.; Liao, Y.Z. Linear drug release membrane prepared by a modified coaxial electrospinning process. J. Membr. Sci. 2013, 428, 150–156.
- Yang, J.M.; Zha, L.S.; Yu, D.G.; Liu, J. Coaxial electrospinning with acetic acid for preparing ferulic acid/zein composite fibers with improved drug release profiles. Colloids Surf. B Biointerfaces 2013, 102, 737–743.
- Place, L.W.; Sekyi, M.; Taussig, J.; Kipper, M.J. Two-Phase Electrospinning to Incorporate Polyelectrolyte Complexes and Growth Factors into Electrospun Chitosan Nanofibers. Macromol. Biosci. 2016, 16, 371–380.
- Wang, C.; Hou, W.; Guo, X.; Li, J.; Hu, T.; Qiu, M.; Liu, X. Two-phase electrospinning to incorporate growth factors loaded chitosan nanoparticles into electrospun fibrous scaffolds for bioactivity retention and cartilage regeneration. Mater. Sci. Eng. C 2017, 79, 507–515.
- Zhang, L.H.; Duan, X.P.; Yan, X.; Yu, M.; Ning, X.; Zhao, Y.; Long, Y.Z. Recent advances in melt electrospinning. RSC Adv. 2016, 6, 53400–53414.
- Qin, C.C.; Duan, X.P.; Wang, L.; Zhang, L.H.; Yu, M.; Dong, R.H.; Long, Y.Z. Melt electrospinning of poly (lactic acid) and polycaprolactone microfibers by using a hand-operated Wimshurst generator. Nanoscale 2015, 7, 16611–16615.
- Das, P.; DiVito, M.D.; Wertheim, J.A.; Tan, L.P. Collagen-I and fibronectin modified three-dimensional electrospun PLGA scaffolds for long-term in vitro maintenance of functional hepatocytes. Mater. Sci. Eng. C 2020, 111, 110723.
- Ding, Y.; Xu, W.; Xu, T.; Zhu, Z.; Fong, H. Theories and principles behind electrospinning. In Advanced Nanofibrous Materials Manufacture Technology Based on Electrospinning; CRC Press: Boca Raton, FL, USA, 2019; pp. 22–51.
- Al-Dhahebi, A.M.; Ling, J.; Krishnan, S.G.; Yousefzadeh, M.; Elumalai, N.K.; Saheed, M.S.; Ramakrishna, S.; Jose, R. Electrospinning research and products: The road and the way forward. Appl. Phys. Rev. 2022, 9, 011319.
- Bate, T.S.; Gadd, V.L.; Forbes, S.J.; Callanan, A. Response differences of HepG2 and Primary Mouse Hepatocytes to morphological changes in electrospun PCL scaffolds. Sci. Rep. 2021, 11, 3059.
- Liu, X.; Ma, P.X. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004, 32, 477.
- Kang, B.J.; Kim, H.; Lee, S.K.; Kim, J.; Shen, Y.; Jung, S.; Cho, J.Y. Umbilical-cord-blood-derived mesenchymal stem cells seeded onto fibronectin-immobilized polycaprolactone nanofiber improve cardiac function. Acta Biomater. 2014, 10, 3007–3017.
- Jiang, X.; Cao, H.Q.; Shi, L.Y.; Ng, S.Y.; Stanton, L.W.; Chew, S.Y. Nanofiber topography and sustained biochemical signaling enhance human mesenchymal stem cell neural commitment. Acta Biomater. 2012, 8, 1290–1302.
- Nguyen, L.T.; Liao, S.; Ramakrishna, S.; Chan, C.K. The role of nanofibrous structure in osteogenic differentiation of human mesenchymal stem cells with serial passage. Nanomedicine 2011, 6, 961–974.
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. Tissue Eng. 2006, 12, 1197–1211.
- Garg, K.; Bowlin, G.L. Electrospinning jets and nanofibrous structures. Biomicrofluidics 2011, 5, 013403.
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for tissue engineering applications. Prog. Mater. Sci. 2020, 117, 100721.
- Sukanya, V.S.; Mohanan, P.V. Degradation of Poly (ε- caprolactone) and bio-interactions with mouse bone marrow mesenchymal stem cells. Colloids Surf. B Biointerfaces 2018, 163, 107–118.
- Semnani, D.; Naghashzargar, E.; Hadjianfar, M.; Dehghan Manshadi, F.; Mohammadi, S.; Karbasi, S.; Effaty, F. Evaluation of PCL/chitosan electrospun nanofibers for liver tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 149–157.
- Wang, Y.K.; Yong, T.; Ramakrishna, S. Nanofibres and their influence on cells for tissue regeneration. Aust. J. Chem. 2005, 58, 704–712.
- Gluck, J.M. Electrospun Poly (E-Caprolactone) (PCL) Nanofibrous Scaffolds for Liver Tissue Engineering. Master’s Thesis, North Carolina State University, Raleigh, NC, USA, 18 July 2007.
- Feng, Z.Q.; Chu, X.; Huang, N.P.; Wang, T.; Wang, Y.; Shi, X.; Gu, Z.Z. The effect of nanofibrous galactosylated chitosan scaffolds on the formation of rat primary hepatocyte aggregates and the maintenance of liver function. Biomaterials 2009, 30, 2753–2763.
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of Collagen Nanofibers. Biomacromolecules 2002, 3, 232–238.
- Ishibashi, H.; Nakamura, M.; Komori, A.; Migita, K.; Shimoda, S. Liver architecture, cell function, and disease. Semin. Immunopathol. 2009, 31, 399–409.
- Feng, Z.-Q.; Chu, X.-H.; Huang, N.-P.; Leach, M.K.; Wang, G.; Wang, Y.-C.; Ding, Y.-T.; Gu, Z.-Z. Rat hepatocyte aggregate formation on discrete aligned nanofibers of type-I collagen-coated poly (L-lactic acid). Biomaterials 2010, 31, 3604–3612.
- Chu, X.-H.; Shi, X.-L.; Feng, Z.-Q.; Gu, Z.-Z.; Ding, Y.-T. Chitosan nanofiber scaffold enhances hepatocyte adhesion and function. Biotechnol. Lett. 2009, 31, 347–352.
- Kasoju, N.; Bora, U. Silk fibroin in tissue engineering. Adv. Healthc. Mater. 2012, 1, 393–412.
- Liu, X.; Zhou, L.; Heng, P.; Xiao, J.; Lv, J.; Zhang, Q.; Wang, J. Lecithin doped electrospun poly (lactic acid)-thermoplastic polyurethane fibers for hepatocyte viability improvement. Colloids Surf. B Biointerfaces 2019, 175, 264–271.
- Silnutzer, J.E.; Barnes, D.W. Effects of fibronectin-related peptides on cell spreading. Vitr. Cell. Dev. Biol. 1995, 21, 73–78.
- Rajendran, D.; Hussain, A.; Yip, D.; Parekh, A.; Shrirao, A.; Cho, C.H. Long-term liver-specific functions of hepatocytes in electrospun chitosan nanofiber scaffolds coated with fibronectin. J. Biomed. Mater. Res. Part A 2017, 105, 2119–2128.
- Bell, C.C.; Dankers, A.C.A.; Lauschke, V.M.; Sison-Young, R.; Jenkins, R.; Rowe, C.; Goldring, C.E.; Park, K.; Regan, S.L.; Walker, T.; et al. Comparison of Hepatic 2D Sandwich Cultures and 3D Spheroids for Long-term Toxicity Applications: A Multicenter Study. Toxicol. Sci. 2018, 162, 655–666.
- Chua, K.-N. Surface-aminated electrospun nanofibers enhance adhesion and expansion of human umbilical cord blood hematopoietic stem/progenitor cells. Biomaterials 2006, 27, 6043–6051.
- Hamilton, G.A.; Jolley, S.L.; Gilbert, D.; Coon, D.J.; Barros, S.; LeCluyse, E.L. Regulation of cell morphology and cytochrome P450 expression in human hepatocytes by extracellular matrix and cell–cell interactions. Cell Tissue Res. 2001, 306, 85–99.
- Richert, L.; Binda, D.; Hamilton, G.; Viollon-Abadie, C.; Alexandre, E.; Bigot-Lasserre, D.; Bars, R.; Coassolo, P.; LeCluyse, E. Evaluation of the effect of culture configuration on morphology, survival time, antioxidant status and metabolic capacities of cultured rat hepatocytes. Toxicol. Vitr. 2002, 16, 89–99.
- Lu, H.F.; Chua, K.N.; Zhang, P.C.; Lim, W.S.; Ramakrishna, S.; Leong, K.W.; Mao, H.Q. Three-dimensional co-culture of rat hepatocyte spheroids and NIH/3T3 fibroblasts enhances hepatocyte functional maintenance. Acta Biomater. 2005, 1, 399–410.
- Chua, K.N.; Lim, W.S.; Zhang, P.; Lu, H.; Wen, J.; Ramakrishna, S.; Mao, H.Q. Stable immobilization of rat hepatocyte spheroids on galactosylated nanofiber scaffold. Biomaterials 2005, 26, 2537–2547.
- Chien, H.W.; Lai, J.Y.; Tsai, W.B. Galactosylated electrospun membranes for hepatocyte sandwich culture. Colloids Surf. B Biointerfaces 2014, 116, 576–581.
- Ghahremanzadeh, F.; Alihosseini, F.; Semnani, D. Investigation and comparison of new galactosylation methods on PCL/chitosan scaffolds for enhanced liver tissue engineering. Int. J. Biol. Macromol. 2021, 174, 278–288.
- Liang, D.; Hsiao, B.S.; Chu, B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv. Drug Deliv. Rev. 2007, 59, 1392–1412.
- Fan, J.; Shang, Y.; Yuan, Y.; Yang, J. Preparation and characterization of chitosan/galactosylated hyaluronic acid scaffolds for primary hepatocytes culture. J. Mater. Sci. Mater. Med. 2010, 21, 319–327.
- Chua, K.N.; Tang, Y.N.; Quek, C.H.; Ramakrishna, S.; Leong, K.W.; Mao, H.Q. A dual-functional fibrous scaffold enhances P450 activity of cultured primary rat hepatocytes. Acta Biomater. 2007, 3, 643–650.
- Bishi, D.K.; Mathapati, S.; Venugopal, J.R.; Guhathakurta, S.; Cherian, K.M.; Verma, R.S.; Ramakrishna, S.A. Patient-Inspired Ex Vivo Liver Tissue Engineering Approach with Autologous Mesenchymal Stem Cells and Hepatogenic Serum. Adv Healthc. Mater. 2016, 5, 1058–1070.
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current applications of poly (lactic acid) composites in tissue engineering and drug delivery. Compos. Part B Eng. 2020, 199, 108238.
- Lv, Q.; Hu, K.; Feng, Q.; Cui, F.; Cao, C. Preparation and characterization of PLA/fibroin composite and culture of HepG2 (human hepatocellular liver carcinoma cell line) cells. Compos. Sci. Technol. 2007, 67, 3023–3030.
- Ghaedi, M.; Soleimani, M.; Shabani, I.; Duan, Y.; Lotfi, A. Hepatic differentiation from human mesenchymal stem cells on a novel nanofiber scaffold. Cell. Mol. Biol. Lett. 2012, 17, 89–106.
- Wei, J.; Lu, J.; Liu, Y.; Yan, S.; Li, X. Spheroid culture of primary hepatocytes with short fibers as a predictable in vitro model for drug screening. J. Mater. Chem. B 2016, 4, 7155–7167.
- Abdullah CA, C.; Albert, E.L. Carbon nanotubes as biological transporters and tissue-engineering scaffolds. In Synthesis, Technology and Applications of Carbon Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–156.
- Koga, H.; Fujigaya, T.; Nakashima, N.; Nakazawa, K. Morphological and functional behaviors of rat hepatocytes cultured on single-walled carbon nanotubes. J. Mater. Sci. Mater. Med. 2011, 22, 2071–2078.
- Wei, J.; Lu, J.; Chen, M.; Xie, S.; Wang, T.; Li, X. 3D spheroids generated on carbon nanotube-functionalized fibrous scaffolds for drug metabolism and toxicity screening. Biomater. Sci. 2020, 8, 426–437.
- Kim, Y.; Kim, Y.W.; Lee, S.B.; Kang, K.; Yoon, S.; Choi, D.; Jeong, J. Hepatic patch by stacking patient-specific liver progenitor cell sheets formed on multiscale electrospun fibers promotes regenerative therapy for liver injury. Biomaterials 2021, 274, 120899.
- Salerno, S.; Tasselli, F.; Drioli, E.; De Bartolo, L. Poly (ε-caprolactone) hollow fiber membranes for the biofabrication of a vascularized human liver tissue. Membranes 2020, 10, 112.
