Sperm cryopreservation is a powerful tool to preserve threatened animal species or for livestock breeding. However, this process is not free of disadvantages. Thus, during the cryopreservation process a significant amount of sperm suffers considerable cryodamage, which may affect sperm quality and fertility. Recently, the use of different “omics” technologies in sperm cryobiology, especially proteomics studies, has led to a better understanding of the molecular modifications induced by sperm cryopreservation, facilitating the identification of different freezability biomarkers and certain proteins that can be added before cryopreservation to enhance sperm cryosurvival. This entreviewy provides an updated overview of the molecular mechanism involved in sperm cryodamage, as well as the molecular aspects of those novel strategies that have been developed to reduce sperm cryodamage, including including new cryoprotectants, antioxidants, proteins, nanoparticles and vitrification.
- cryopreservation
- sperm
- proteomics
- molecular
- ruminants
1. Molecular dDamaged cCaused by the fFreezing-tThawing pProcess
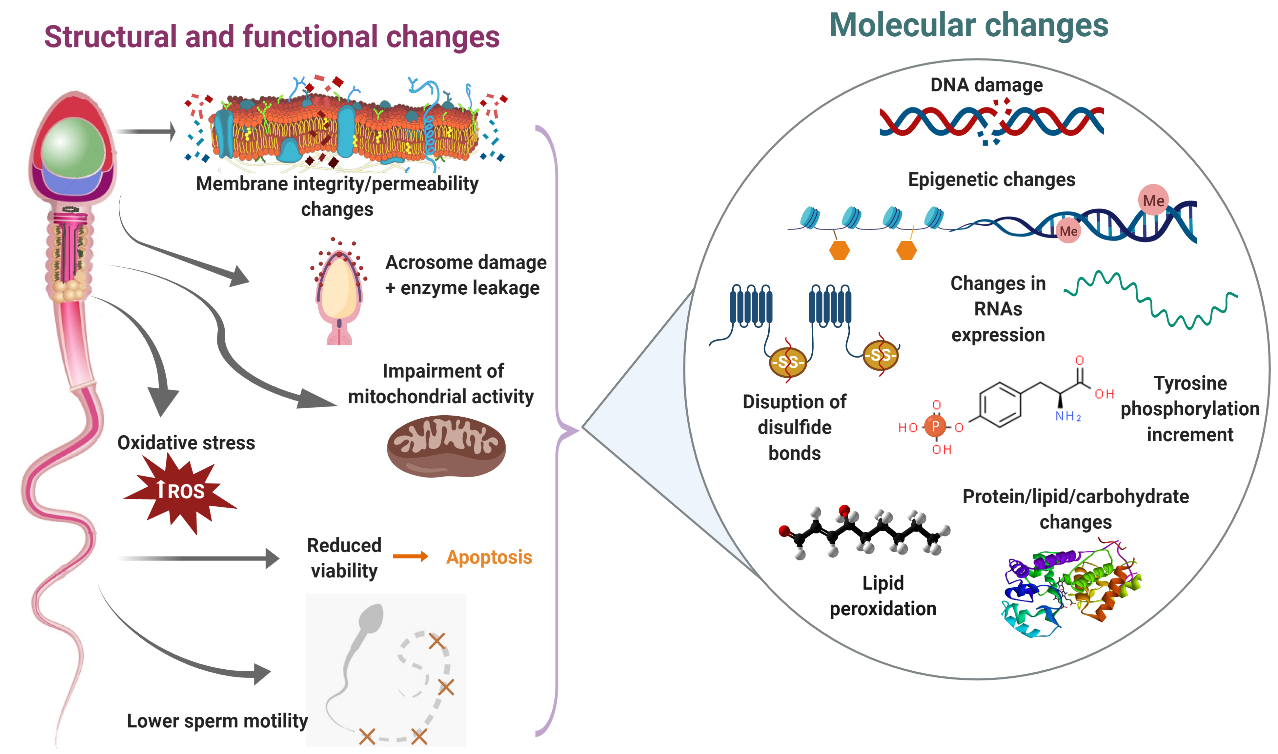
Sperm cryopreservation has been reported to induce an increase in plasma membrane fluidity–permeability, overproduction of reactive oxygen species (ROS), reduction of acrosome integrity, impairment of mitochondrial membrane potential and lower sperm motility in bull [1–5][1][2][3][4][5], buffalo [6–8][6][7][8], buck [9–11][9][10][11], ram[12][13][14][15][16] [12-16] and red deer [17][17]. Molecular studies during sperm cryopreservation offer the possibility of recognizing those specific elements (proteins, lipids, ions, carbohydrates, etc.) altered by the freezing–thawing process that are in part responsible for the structural and functional changes observed in cryopreserved sperm (Figure 1).
In this sense, understanding the molecular modifications inflicted by the freezing–thawing process is essential to diminish or prevent cryodamage. Owing to the reduced [18][18], if not seemingly absent,[19][20] [19,20] transcriptional and translational activity in mature sperm, proteomics studies represent the best option for investigating the molecular mechanisms regulating sperm functionality [21][21]. Moreover, it is also important to study the impact of cryopreservation on sperm RNAs transcripts since some of them are delivered to the oocyte participating in fertilization and embryo development, while others are involved in capacitation, motility, metabolism and other relevant sperm functions [22][22].
Figure 1. Main consequences of sperm cryodamage in ruminants. During the cryopreservation process, ruminant sperm suffer several structural and functional damages, which are probably the result of different molecular changes. This figure summarizes those structural, functional and molecular changes produced during the freezing–thawing procedure.
One of the first structures affected by the cryopreservation is the sperm plasma membrane[23]. (23).During the freezing some sperm surface proteins as well as membrane proteins are lost or translocated with the consequent loss of their function. For example, proteins involved in capacitation, sperm–oocyte interaction and gamete fusion, such as TCP1, LOC101123268, RPN1, P25b, HEXB, CSNK1G2, ICA, LOC101123216, ADAM2 and TIMP-2, decreased in abundance in ram, gazelle and bull sperm after cryopreservation [24–27][24][25][26][27], while another protein associated with fertilization, HSP70, was lost in buffalo sperm [28][28]. Other proteins involved in transport, membrane stabilization and protection against lipid peroxidation or cold-shock, such as GLUT, CLU, BSP5, BSP1, aSFP, HSPA4L, TRAP1, GPX4 and GPX5 also decreased in abundance in these species along with antiapoptotic and decapacitating proteins (CSNK2A2 and Spermadhesin Z13) [24,26,29,30][24][26][29][30].
Cryopreservation also induces significant changes in the distribution or abundance of those proteins that act as ROS scavengers. Relevant antioxidant enzymes such as glutathione peroxidase (GPx), glutathione reductase (GR) and superoxide dismutase (SOD) were redistributed on ram sperm surface following cryopreservation [30][31]. These findings, together with the reduced antioxidant activity of SOD and reduced glutathione (GSH) observed in bull and ram sperm after cryopreservation, could explain in part the increased susceptibility of frozen–thawed sperm to suffer lipid peroxidation and oxidative damage [30,31][30][31].
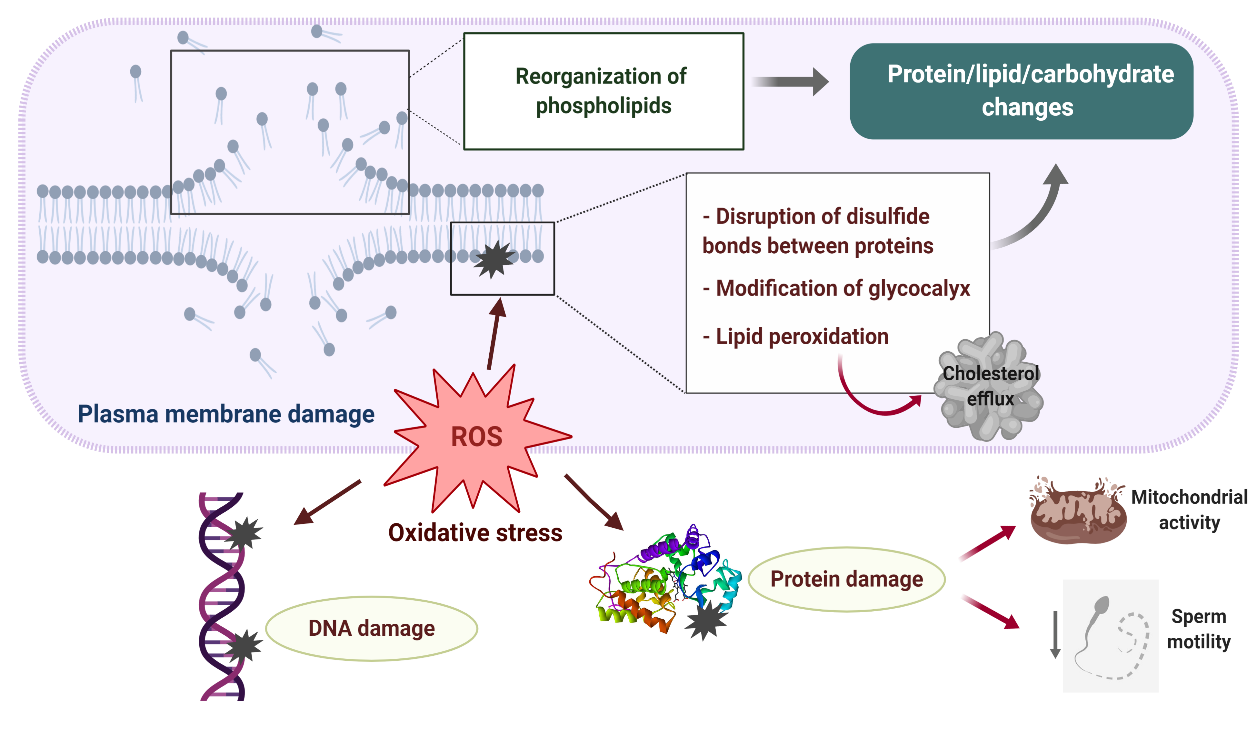
Disturbances in the sperm antioxidant system during cryopreservation and the activation of L-Amino acid oxidase in dead or defective cryopreserved sperm significantly contribute to the increased ROS production detected in ruminant sperm after freezing–thawing, the sperm plasma membrane being the primary site where ROS-induced damage is manifested (Figure 2) [32,3,14,33][3][14][32][33]. Excessive generation of ROS during cryopreservation leads to major protein, lipid and carbohydrate changes in the sperm membrane due to the reduction of disulfide bonds between membrane proteins [34][34], peroxidation of membrane phospholipids and modifications of the sperm glycocalyx [35][35].
Figure 2. Plasma membrane damage during sperm cryopreservation and its relationship with oxidative stress. A reorganization of sperm membrane phospholipids takes places during freezing–thawing, altering lipid–protein, lipid–carbohydrate and protein–carbohydrate interactions which are necessary for proper membrane activity. Excessive production of reactive oxygen species (ROS) leads to major protein, lipid and carbohydrate changes in the sperm membrane due to the reduction of disulfide bonds between membrane proteins, peroxidation of membrane phospholipids and modifications of the sperm glycocalyx. As a result, the sperm membrane becomes fragile and its semipermeable property is lost. Overproduction of ROS during sperm cryopreservation may also cause DNA damage and impair several axonemal and mitochondrial proteins, which negatively affect mitochondrial activity and axonemal integrity, resulting in the loss of sperm motility.
Besides sperm membrane damage, oxidative stress disrupts mitochondrial activity with the consequent loss of sperm motility [36][36]. In ruminants, two main metabolic pathways, oxidative phosphorylation and glycolysis, produce the energy required to maintain sperm motility in the form of ATP [37][37]. Comparative proteomics studies between fresh and cryopreserved sperm revealed that freezing–thawing procedures alter the abundance of several enzymes implicated in oxidative phosphorylation and glycolysis in ram, bull and gazelle sperm [24,29,38,39][24][29][38][39]. Among them, different ATP synthases, COX5B, AK1, NDUFV2, ODPB2, ACO2 and NDPK7 were some of those proteins related to oxidative phosphorylation, while different hexokinases, GPI, ALDOA, GAPDH5, PGK2, PGAM2, PKM2 and TPI were some of those proteins related to glycolysis.
Sperm with damaged DNA can complete the fertilization process; however, embryo development can be seriously interrupted or altered once the embryo genome is activated at the 4- or 8-cell stage due to the transcription of damaged paternal genes [40,41][40][41]. Moreover, several coding and non-coding RNAs, nuclear proteins and other epigenetics marks from sperm are delivered to the offspring together with the paternal genome [42][42]. In consequence, aside from DNA damage, changes in the relative abundance of RNAs, aberrant DNA methylation, abnormal histone modifications or improper chromatin compaction in sperm due to alterations in the nucleoprotein structure could have a severe impact on fertilization or embryogenesis [40,43,44][40][43][44]. While the effect of freezing–thawing on sperm DNA stability has been widely investigated, few studies in ruminants explored the influence of freezing–thawing on sperm epigenome. Messenger RNA (mRNA) carries the genetic code to translate proteins, but there are other RNAs termed non-coding RNAs (ncRNAs) that do not code for proteins. Both types of RNAs (mRNA and ncRNAs) have been found to modulate a variety of biological functions in sperm [22][22]. In addition, some ncRNAs are also involved in epigenetic regulation [45][45]. In consequence, variations in RNA transcripts during cryopreservation could adversely affect sperm integrity, functionality and its fertilizing potential or make the sperm vulnerable to epigenetic errors. Chen et al.[46] [46] reported that cryopreservation modified in bull sperm the relative abundance of four ncRNAs involved in embryo development. Moreover, in horses, sperm cryopreservation increased global DNA methylation [47][47], whereas in boar sperm, freezing–thawing decreased the relative abundance of mRNAs as well as the protein levels of some genes associated with DNA methylation (DNMT3A, DNMT3B), histone modifications (JHDM2A, KAT8) and genomic imprinting (IGF2) [48][48].
2. Molecular Aspects of those Novel Strategies to Reduce Sperm Cryodamage
Currently, there is a wide variety of extenders that can be used during sperm cryopreservation in different ruminant species (reviewed by [49,50-54][49][50][51][52][53][54]); however, not all of them offer the same protection against sperm cryodamage. Extenders usually contain various components (buffers, antibiotics, sugars, fatty acids, cryoprotectants, antioxidants and other substances) to efficiently protect sperm viability and fertility during cryopreservation [53][53].
Cryoprotectants protect sperm from ice crystal formation, osmotic and chemical stress. Such components can be classified into permeating and non-permeating, and both types of cryoprotectants are usually included in the extenders. Glycerol is the permeating cryoprotectant most commonly used in ruminants during sperm cryopreservation, while egg yolk is the non-permeating cryoprotectant. The former is cytotoxic beyond certain concentration and has been shown to alter in bull sperm some proteins associated with sperm–oocyte binding (IZUMO4), energy metabolism (PDB1, NUDFV2, NDPK7), cytoskeleton organization (CAPZB, ODF2) and ROS metabolism (SOD2), which may negatively affect sperm function [55][55]. Recently, a novel cryoprotective agent, carboxylated poly-L-lysine, has been used to reduce glycerol concentration in the freezing medium, enhancing in vivo fertility of cryopreserved buffalo and bull sperm [56,57][56][57]. Regarding non-permeating cryoprotectants, it has been reported that egg yolk also alters the proteome of ram sperm before cryopreservation [26][26]. Therefore, special attention should be payed to sperm-cryoprotectant interactions since these interactions may affect sperm cryopreservation outcomes. Additional studies should be conducted to elucidate whether glycerol and egg yolk exert the same impact on the sperm proteome of other ruminant species.
Another strategy for protecting sperm against cryodamage is the increment of the cholesterol membrane content prior to cryopreservation by adding cholesterol-loaded cyclodextrins (CLC) to the freezing medium. This treatment improves sperm membrane stability after incorporating exogenous cholesterol to the plasma membrane, which in turn enhances sperm cryosurvival, motility, mitochondrial activity and the number of sperm attached to zona pellucida, reducing at the same time cryo-capacitation and premature tyrosine phosphorylation [58-60][58][59][60]. The beneficial effects of CLC seem to be greater in those ejaculates with low freezability, at least in ram sperm [61][61]. Moreover, the addition of CLC to the extender attenuated in gazelle sperm the degradation of three proteins related to energy metabolism and cytoskeletal organization (CAPZB, HSP90A, PAGM2) during the freezing–thawing process compared to untreated sperm, which may explain the increased motility observed in CLC treated sperm [24][24].
Supplementation of the freezing medium with antioxidants reduces the negative effects generated by the excessive ROS production during cryopreservation, which improves sperm cryosurvival. Antioxidants can be classified into enzymatic and non-enzymatic, and both types can be added to the freezing medium, yielding different results [62,63][62][63]. The former includes superoxide dismutase (SOD), glutathione reductase (GR), glutathione peroxidase (GPx) and catalase (CAT), while the latter includes reduced glutathione (GSH), vitamins, plant extracts (e.g., cinnamtannin B-1), minerals, amino acids, proteins and other exogenous compounds (e.g., resveratrol or quercetin) with antioxidant properties [53,64][53][64].
Recent studies investigated the addition of different nanoparticles to the freezing medium to overcome the main drawbacks that conventional antioxidants could present, like the low durability to harsh conditions [65–68][65][66][67][68]. Nanotechnology advances have contributed to the design of novel nano-compounds that possess antioxidant properties, such as selenium, zinc oxide and apoferritin containing gold-silver nanoparticles. Addition of selenium nanoparticles to semen extender enhanced viability, motility and chromatin integrity of cryopreserved bull sperm, obtaining greater in vivo fertility results [65][65]. Similar results were reported in cryopreserved ram sperm when selenium particles were added to the freezing medium [66][66].
Melatonin is another potential candidate to include in the freezing medium due to its protective effect against oxidative stress, which is dose-dependent [69][69]. The beneficial effects of melatonin on sperm cryopreservation rely on its powerful antioxidant property and its ability to stimulate the enzymatic activity of SOD, GPx and CAT [70][70]. Moreover, addition of melatonin to the freezing medium prevents a prolonged opening of MPTP during cryopreservation, which in turn increases ATP production, improving post-thaw sperm motility [71][71].
Proteomics studies on seminal plasma have greatly contributed to identifying those proteins with beneficial effects on sperm cryopreservation, facilitating the generation of recombinant proteins as a promising strategy for sperm cryopreservation. Recently, supplementation of the extender with recombinant seminal plasma proteins such as regucalcin (RGN), a recombinant peptide containing four FNII domains (TrxA-FNIIx4-His6) and serine protease inhibitor kazal-type 3 (SPINK3) have been shown to exert a cryoprotective effect on sperm [72-74][72][73][74].
Antifreeze proteins and glycoproteins are other cryoprotective elements that deserve special attention. These proteins, which are produced by some insects, Antarctic fishes, crustaceans, bacteria, fungi and microalgae, have the capacity to protect sperm membrane from cryodamage by preventing ice crystal formation [75][75]. Addition of antifreeze protein and glycoprotein type I to semen extender significantly increased post-thaw motility in ram sperm [76][76], whereas in bull, supplementation with antifreeze protein type I only improved the osmotic resistance of sperm during cryopreservation [77][77].
3. Future Directions
Cryopreservation alters a variety of proteins and ARNs transcripts involved in relevant sperm functions, such as sperm motility, capacitation, fertilization and embryo development. Understanding the molecular damages caused by the freezing–thawing process is fundamental to protect these molecular elements and prevent or reduce those changes in sperm structure or function that negatively affect the reproductive performance. Moreover, supplementation of the freezing medium with novel cryoprotectants, antioxidants and other new components such as proteins or nanoparticles requires a further optimization to be an effective alternative to the commercial extenders currently used for cryopreservation of ruminant sperm.
Abbreviations
|
TCP1 |
T-complex protein 1 subunit alpha |
|
LOC101123268 |
Dolichyl-diphosphooligosaccharide-protein glycosyltransferase |
|
RPN1 |
Dolichyl-diphosphooligosaccharide-protein glycosyltransferase subunit 1 |
|
HEXB |
Beta-hexosaminidase subunit beta-like isoform X1 |
|
CSNK1G2 |
Casein kinase I isoform gamma-2 isoform X2 |
|
ICA |
Inhibitor of carbonic anhydrase-like isoform X3 |
|
LOC101123216 |
Disintegrin and metalloproteinase domain-containing protein 20 |
|
ADAM2 |
Fertilin beta |
|
TIMP-2 |
Tissue inhibitor of metalloproteinases 2 |
|
HSP70 |
Heat shock 70 kDa protein |
|
GLUT |
Glucose transporter |
|
CLU |
Clusterin |
|
BSP5 |
Binder of sperm protein 5 |
|
BSP1 |
Binder of sperm protein 1 |
|
aSFP |
Acidic seminal fluid protein |
|
HSP4AL |
Heat shock 70 kDa protein 4 L isoform C1 |
|
TRAP1 |
Heat shock protein 75 kDa, mitochondrial isoform X3 |
|
GPX4 |
Phospholipid hydroperoxide glutathione peroxidase |
|
GPX5 |
Epididymal secretory glutathione peroxidase |
|
CSNK2A2 |
Casein kinase II subunit alpha |
|
SOD2 |
Superoxide dismutase 2 |
|
COX5B |
Cytochrome c oxidase subunit 5B, mitochondrial |
|
AK1 |
Adenylate kinase isoenzyme 1 |
|
NUDFV2 |
NADH dehydrogenase flavoprotein 2 |
|
ODPB2 |
Pyruvate dehydrogenase E1 component subunit beta, mitochondrial isoform 2 |
|
ACO2 |
Aconitate hydratase, mitochondrial |
|
NDPK7 |
Nucleoside diphosphate kinase 7 |
|
GPI |
Glucose-6-phosphate isomerase |
|
ALDOA |
Fructose-bisphosphate aldolase |
|
GAPDH5 |
Glyceraldehyde-3-phosphate dehydrogenase, testis-specific |
|
PGK2 |
Phosphoglycerate kinase 2 |
|
PGAM2 |
Phosphoglycerate mutase 2 |
|
PKM2 |
Pyruvate kinase M2 |
|
TPI |
Triosephosphate isomerase |
|
HSP90 |
Heat shock 90 kDa protein |
|
DNMT3A |
DNA (cytosine-5-)-methyltransferase 3 alpha |
|
DNMT3B |
DNA (cytosine-5-)-methyltransferase 3 beta |
|
JHDM2A |
JmjC domain-containing histone demethylation protein 2A |
|
KAT8 |
K(lysine) acetyltransferase 8 |
|
IGF2 |
Insulin-like growth factor 2 |
|
IZUMO4 |
Izumo sperm–egg fusion protein 4 |
|
PDB1 |
Pyruvate dehydrogenase E1 component subunit beta, mitochondrial precursor |
|
HSP90A |
Heat shock 90 kDa protein alpha |
References:
- Khalil, W.A.; El-harairy, M.A.; Zeidan, A.E.B.; Hassan, M.A.E.; Mohey-Elsaeed, O. Evaluation of bull spermatozoa during and after cryopreservation: Structural and ultrastructural insights. J. Vet. Sci. Med. 2018, 6, S49–S56.
- Gürler, H.; Malama, E.; Heppelmann, M.; Calisici, O.; Leiding, C.; Kastelic, J.P.; Bollwein, H. Effects of cryopreservation on sperm viability, synthesis of reactive oxygen species, and DNA damage of bovine sperm. Theriogenology 2016, 86, 562–571.
- Chatterjee, S.; Gagnon, C. Production of reactive oxygen species by spermatozoa undergoing cooling, freezing, and thawing. Reprod. Dev. 2001, 59, 451–458.
- Pons-Rejraji, H.; Bailey, J.L.; Leclerc, P. Cryopreservation affects bovine sperm intracellular parameters associated with capacitation and acrosome exocytosis. Fertil. Dev. 2009, 21, 525–537.
- Yoon, S.J.; Kwon, W.S.; Rahman, M.S.; Lee, J.S.; Pang, M.G. A novel approach to identifying physical markers of cryo-damage in bull spermatozoa. PLoS ONE 2015, 10, e0126232.
- Rasul, Z.; Ahmad, N.; Anzar, M. Changes in motion characteristics, plasma membrane integrity, and acrosome morphology during cryopreservation of buffalo spermatozoa. Androl. 2001, 22, 278–283.
- Kadirvel, G.; Kumar, S.; Kumaresan, A. Lipid peroxidation, mitochondrial membrane potential and DNA integrity of spermatozoa in relation to intracellular reactive oxygen species in liquid and frozen-thawed buffalo semen. Animal Reprod. Sci. 2009, 114, 125–134.
- Kumar, D.; Kumar, P.; Singh, P.; Yadav, S.P.; Yadav, P.S. Assessment of sperm damages during different stages of cryopreservation in water buffalo by fluorescent probes. Cytotechnology 2016, 68, 451–458.
- Ahmad, M.; Nasrullah, R.; Riaz, H.; Sattar, A.; Ahmad, N. Changes in motility, morphology, plasma membrane and acrosome integrity during stages of cryopreservation of buck sperm. S. Afr. Vet. Assoc. 2014, 85, 1–5.
- Dorado, J.; Hidalgo, M.; Muñoz, A.; Rodríguez, I. Assessment of goat semen freezability according to the spermatozoa characteristics from fresh and frozen samples. Animal Reprod. Sci. 2009, 112, 150–157.
- Chauhan, M.S.; Kapila, R.; Gandhi, K.K.; Anand, S.R. Acrosome damage and enzyme leakage of goat spermatozoa during dilution, cooling and freezing. Andrologia 1994, 26, 21–26.
- Nur, Z.; Zik, B.; Ustuner, B.; Sagirkaya, H.; Ozguden, C.G. Effects of different cryoprotective agents on ram sperm morphology and DNA integrity. Theriogenology 2010, 73, 1267–1275.
- Peris-Frau, P.; Martín-Maestro, A.; Iniesta-Cuerda, M.; Sánchez-Ajofrín, I.; Cesari, A.; Garde, J.J.; Villar, M.; Soler, A.J. Cryopreservation of ram sperm alters the dynamic changes associated with in vitro capacitation. Theriogenology 2020, 145, 100–108.
- Da Silva Maia, M.; Bicudo, S.D.; Sicherle, C.C.; Rodello, L.; Gallego, I.C.S. Lipid peroxidation and generation of hydrogen peroxide in frozen-thawed ram semen cryopreserved in extenders with antioxidants. Animal Reprod. Sci. 2010, 122, 118–123.
- Peris, S.I.; Morrier, A.; Dufour, M.; Bailey, J.L. Cryopreservation of ram semen facilitates sperm DNA damage: Relationship between sperm andrological parameters and the sperm chromatin structure Assay. Androl. 2004, 25, 224–233.
- García-Álvarez, O.; Maroto-Morales, A.; Martínez-Pastor, F.; Garde, J.J.; Ramón, M.; Fernández-Santos, M.R.; Esteso, M.C.; Pérez-Guzmán, M.D.; Soler, A. J. Sperm characteristics and in vitro fertilization ability of thawed spermatozoa from black manchega ram: Electroejaculation and postmortem collection. Theriogenology 2009, 72, 160–168.
- Fernández-Santos, M.R.; Esteso, M.C.; Montoro, V.; Soler, A.J.; Garde, J.J. Cryopreservation of Iberian red deer (Cervus elaphus hispanicus) epididymal spermatozoa: Effects of egg yolk, glycerol and cooling rate. Theriogenology 2006, 66, 1931–1942.
- Gur, Y.; Breitbart, H. Mammalian sperm translate nuclear-encoded proteins by mitochondrial-type ribosomes. Genes Dev. 2006, 20, 411–416.
- Baker, M. a.; Nixon, B.; Naumovski, N.; Aitken, R.J. Proteomic insights into the maturation and capacitation of mammalian spermatozoa. Biol. Reprod. Med. 2012, 58, 211–217.
- Oliva, R.; De Mateo, S.; Estanyol, J.M. Sperm cell proteomics. Proteomics 2009, 9, 1004–1017.
- Mohanty, G.; Swain, N.; Samanta, L. Sperm proteome: What is on the horizon? Sci. 2014, 22, 638–653.
- Dai, D.; Qazi, I.H.; Ran, M.; Liang, K.; Zhang, Y. Exploration of miRNA and mRNA profiles in fresh and frozen-thawed boar sperm by transcriptome and small RNA sequencing. J. Mol. Sci. 2019, 20, 802.
- Bailey, J.L.; Bilodeau, J.F.; Cormier, N. Semen cryopreservation in domestic animals: A damaging and capacitating phenomenon. Androl. 2000, 21, 1–7.
- Wojtusik, J.; Wang, Y.; Pukazhenthi, B.S. Pretreatment with cholesterol-loaded cyclodextrins prevents loss of motility associated proteins during cryopreservation of addra gazelle (Nanger dama ruficollis) spermatozoa. Cryobiology 2018, 81, 74–80.
- Lessard, C.; Parent, S.; Leclerc, P.; Bailey, J.L.; Sullivan, R. Cryopreservation alters the levels of the bull sperm surface protein P25b. Androl. 2000, 21, 700–707.
- Pini, T.; Rickard, J.P.; Leahy, T.; Crossett, B.; Druart, X.; De Graaf, S.P. Cryopreservation and egg yolk medium alter the proteome of ram spermatozoa. Proteom. 2018, 181, 73–82.
- Peris-Frau, P.; Martín-Maestro, A.; Iniesta-Cuerda, M.; Sánchez-Ajofrín, I.; Mateos-Hernández, L.; Garde, J.J.; Villar, M.; Soler, A.J. Freezing—Thawing procedures remodel the proteome of ram sperm before and after in vitro capacitation. J. Mol. Sci. 2019, 20, 4596.
- Varghese, T.; Divyashree, B.C.; Roy, S.C.; Roy, K.S. Loss of heat shock protein 70 from apical region of buffalo (Bubalus bubalis) sperm head after freezing and thawing. Theriogenology 2016, 85, 828–834.
- Westfalewicz, B.; Dietrich, M.A.; Ciereszko, A. Impact of cryopreservation on bull (Bos taurus) semen proteome. Animal Sci. 2015, 93, 5240–5253.
- Marti, E.; Marti, J.I.; Muiño-Blanco, T.; Cebrián-Pérez, J.A. Effect of the cryopreservation process on the activity and immunolocalization of antioxidant enzymes in ram spermatozoa. Androl. 2008, 29, 459–467.
- Bilodeau, J.-F.; Chatterjee, S.; Sirard, M.A.; Gagnon, C. Levels of antioxidant defenses are decreased in bovine spermatozoa after a cycle of freezing. Hum. Reprod. 2000, 55, 282–288.
- Kumar, A.; Prasad, J.K.; Srivastava, N.; Ghosh, S.K. Strategies to minimize various stress-related freeze–thaw damages during conventional cryopreservation of mammalian spermatozoa. Biobank. 2019, 17, 603–612.
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Reprod. Dev. 2017, 84, 1039–1052.
- Chatterjee, S.; De Lamirande, E.; Gagnon, C. Cryopreservation alters membrane sulfhydryl status of bull spermatozoa: Protection by oxidized glutathione. Reprod. Dev. 2001, 60, 498–506.
- Pini, T.; Leahy, T.; de Graaf, S.P. Sublethal sperm freezing damage: Manifestations and solutions. Theriogenology 2018, 118, 172–181.
- Aitken, R.J. Free radicals, lipid peroxidation and sperm function. Fertil. Dev. 1995, 7, 659–658.
- Losano, J.; Angrimani, D.; Dalmazzo, A.; Rui, B.; Brito, M.; Mendes, C.; Kawai, G.; Vannucchi, C.; Assumpção, M.; Barnabe, V.; et al. Effect of mitochondrial uncoupling and glycolysis inhibition on ram sperm functionality. Domest. Animals 2017, 52, 289–297.
- He, Y.; Wang, K.; Zhao, X.; Zhang, Y.; Ma, Y.; Hu, J. Differential proteome association study of freeze-thaw damage in ram sperm. Cryobiology 2016, 72, 60–68.
- Yoon, S.J.; Rahman, M.S.; Kwon, W.S.; Ryu, D.Y.; Park, Y.J.; Pang, M.G. Proteomic identification of cryostress in epididymal spermatozoa. Animal Sci. Biotechnol. 2016, 7, 1–12.
- Kumar, M.; Kumar, K.; Jain, S.; Hassan, T.; Dada, R. Novel insights into the genetic and epigenetic paternal contribution to the human embryo. Clinics 2013, 68, 5–14.
- Lewis, S.E.M.; Aitken, R.J. DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res. 2005, 322, 33–41.
- Yamauchi, Y.; Shaman, J.A.; Ward, W.S. Non-genetic contributions of the sperm nucleus to embryonic development. Asian J. Androl. 2011, 13, 31–35.
- Verma, A.; Rajput, S.; De, S.; Kumar, R.; Chakravarty, Atish Kumar Datta, T.K. Genome-wide pro fi ling of sperm DNA methylation in relation to buffalo (Bubalus bubalis) bull fertility. Theriogenology 2014, 82, 750–759.
- Ge, S.; Lin, S.; Zhao, Z.; Sun, Q. Epigenetic dynamics and interplay during spermatogenesis and embryogenesis: Implications for male fertility and offspring health. Oncotarget 2017, 8, 53804–53818.
- Collins, L.J.; Schönfeld, B.; Xiaowei, S.C. The epigenetics of non-coding RNA. In Handbook of Epigenetics; Elsevier Inc.: Amsterdam, The Netherlands, 2011; pp. 49–61.
- Chen, X.; Wang, Y.; Zhu, H.; Hao, H.; Zhao, X.; Qin, T.; Wang, D. Comparative transcript profiling of gene expression of fresh and frozen-thawed bull sperm. Theriogenology 2015, 83, 504–511.
- Aurich, C.; Schreiner, B.; Ille, N.; Alvarenga, M.; Scarlet, D. Cytosine methylation of sperm DNA in horse semen after cryopreservation. Theriogenology 2016, 86, 1347–1352.
- Zeng, C.; Peng, W.; Ding, L.; He, L.; Zhang, Y.; Fang, D.; Tang, K. A preliminary study on epigenetic changes during boar spermatozoa cryopreservation. Cryobiology 2014, 69, 119–127.
- Lv, C.; Wu, G.; Hong, Q.; Quan, G. Spermatozoa cryopreservation: State of art and future in small ruminants. Biobank. 2018, 17, 171–182.
- Ugur, M.R.; Abdelrahman, A.S.; Evans, H.C.; Gilmore, A.A.; Hitit, M.; Arifiantini, R.I.; Purwantara, B.; Kaya, A.; Memili, E. Advances in cryopreservation of bull sperm. Vet. Sci. 2019, 6, 268.
- Layek, S.S.; Mohanty, T.K.; Kumaresan, A.; Parks, J.E. Cryopreservation of bull semen: Evolution from egg yolk based to soybean based extenders. Animal Reprod. Sci. 2016, 172, 1–9.
- Barbas, J.P.; Mascarenhas, R.D. Cryopreservation of domestic animal sperm cells. Cell Tissue Bank 2009, 10, 49–62.
- Allai, L.; Benmoula, A.; Marciane da Silva, M.; Nasser, B.; El Amiri, B. Supplementation of ram semen extender to improve seminal quality and fertility rate. Animal Reprod. Sci. 2018, 192, 6–17.
- Purdy, P.H. A review on goat sperm cryopreservation. Small Rumin. Res. 2006, 63, 215–225.
- Yoon, S.J.; Rahman, M.S.; Kwon, W.S.; Park, Y.J.; Pang, M.G. Addition of cryoprotectant significantly alters the epididymal sperm proteome. PLoS ONE 2016, 11, e0152690.
- Fujikawa, T.; Imamura, S.; Tokumaru, M.; Ando, T.; Gen, Y.; Hyon, S.H.; Kubota, C. Cryoprotective effect of antifreeze polyamino-acid (Carboxylated Poly-L-Lysine) on bovine sperm: A technical note. Cryobiology 2018, 82, 159–162.
- Tariq, A.; Ahmad, M.; Iqbal, S.; Riaz, M.I.; Tahir, M.Z.; Ghafoor, A.; Riaz, A. Effect of carboxylated poly L-Lysine as a cryoprotectant on post-thaw quality and in vivo fertility of Nili Ravi buffalo (Bubalus bubalis) bull semen. Theriogenology 2020, 144, 8–15.
- Longobardi, V.; Albero, G.; Canditiis, C. De; Salzano, A.; Natale, A.; Balestrieri, A.; Neglia, G.; Campanile, G.; Gasparrini, B. Cholesterol-loaded cyclodextrins prevent cryocapacitation damages in buffalo (Bubalus bubalis) cryopreserved sperm. Theriogenology 2017, 89, 359–364.
- Mocé, E.; Purdy, P.H.; Graham, J.K. Treating ram sperm with cholesterol-loaded cyclodextrins improves cryosurvival. Animal Reprod. Sci. 2010, 118, 236–247.
- Purdy, P.H.; Graham, J.K. Effect of cholesterol-loaded cyclodextrin on the cryosurvival of bull sperm. Cryobiology 2004, 48, 36–45.
- Batissaco, L.; De Arruda, R.P.; Alves, M.B.R.; Torres, M.A.; Lemes, K.M.; Prado-Filho, R.R.; De Almeida, T.G.; De Andrade, A.F.C.; Celeghini, E.C.C. Cholesterol-loaded cyclodextrin is efficient in preserving sperm quality of cryopreserved ram semen with low freezability. Biol. 2020, 20, 14–24.
- Amidi, F.; Pazhohan, A.; Shabani Nashtaei, M.; Khodarahmian, M.; Nekoonam, S. The role of antioxidants in sperm freezing: A review. Cell Tissue Bank. 2016, 17, 745–756.
- Fernández-Santos, M.R.; Martínez-Pastor, F.; García-Macías, V.; Esteso, M.C.; Soler, A.J.; Paz, P.; Anel, L.; Garde, J.J. Sperm characteristics and DNA integrity of Iberian red deer (Cervus elaphus hispanicus) epididymal spermatozoa frozen in the presence of enzymatic and nonenzymatic antioxidants. Androl. 2007, 28, 294–305.
- Sánchez-Rubio, F.; Fernández-Santos, M.R.; Castro-Vázquez, L.; García-Álvarez, O.; Maroto-Morales, A.; Soler, A.J.; Martínez-Pastor, F.; Garde, J.J. Cinnamtannin B-1, a novel antioxidant for sperm in red deer. Animal Reprod. Sci. 2018, 195, 44–52.
- Khalil, W.A.; El-Harairy, M.A.; Zeidan, A.E.B.; Hassan, M.A.E. Impact of selenium nano-particles in semen extender on bull sperm quality after cryopreservation. Theriogenology 2019, 126, 121–127.
- Hozyen, H.F.; Shamy, A.A.E.; Farghali, A.A. In vitro Supplementation of nano selenium minimizes freeze-thaw induced damage to ram spermatozoa. J. Vet. Sci. 2019, 8, 249–254.
- Shahin, M.A.; Khalil, W.A.; Saadeldin, I.M.; Swelum, A.A.-A.; El-harairy, M.A. Comparison between the effects of adding vitamins, trace elements, and nanoparticles to SHOTOR extender on the cryopreservation of dromedary camel epididymal spermatozoa. Animals 2020, 10, 78.
- Dashtestani, F.; Ghourchian, H.; Najafi, A. Silver-gold-apoferritin nanozyme for suppressing oxidative stress during cryopreservation. Sci. Eng. C 2019, 94, 831–840.
- Cebrián-Pérez, J.A.; González-Arto, M.; Dos Santos Hamilton, T.R.; Pérez-Pé, R.; Muiño-Blanco, T. Melatonin in sperm biology: Breaking paradigms. Domest. Animals 2014, 49, 11–21.
- Ashrafi, I.; Kohram, H.; Ardabili, F.F. Antioxidative effects of melatonin on kinetics, microscopic and oxidative parameters of cryopreserved bull spermatozoa. Animal Reprod. Sci. 2013, 139, 25–30.
- Fang, Y.; Zhao, C.; Xiang, H.; Zhao, X.; Zhong, R. Melatonin inhibits formation of mitochondrial permeability transition pores and improves oxidative phosphorylation of frozen-thawed ram sperm. Endocrinol. 2020, 10, 896.
- Pillai, H.; Parmar, M.S.; Shende, A.M.; Thomas, J.; Kartha, H.S.; Sharma, T.; Ghosh, G.S.K.; Kumaresan, A.; Bhure, S.K. Effect of supplementation of recombinant regucalcin in extender on cryopreservation of spermatozoa of water buffalo (Bubalus bubalis). Reprod. Dev. 2017, 84, 1133–1139.
- Ledesma, A.; Zalazar, L.; Buchelly, F.; Ignacio, J.; Brown, P.; Mitch, E.; Hozbor, F.; Cesari, A. Recombinant peptide reverses cryo-capacitation in ram sperm and improves in vitro fertilization. Animal Reprod. Sci. 2019, 207, 61–72.
- Zalazar, L.; Iniesta-Cuerda, M.; Sánchez-Ajofrín, I.; Soler, A.J.; Garde, J.J.; Cesari, A. Recombinant SPINK3 improves ram sperm quality and in vitro fertility after cryopreservation. Theriogenology 2020, 144, 45–55.
- Robles, V.; Valcarce, D.G.; Riesco, M.F. The use of antifreeze proteins in the cryopreservation of gametes and embryos. Biomolecules 2019, 9, 1–12.
- Payne, S.R.; Oliver, J.E.; Upreti, G.C. Effect of antifreeze proteins on the motility of ram spermatozoa. Cryobiology 1994, 31, 180–184.
- Prathalingam, N.S.; Holt, W.V.; Revell, S.G.; Mirczuk, S.; Fleck, R.A.; Watson, P.F. Impact of antifreeze proteins and antifreeze glycoproteins on bovine sperm during freeze-thaw. Theriogenology 2006, 66, 1894–1900.
References
- Khalil, W.A.; El-harairy, M.A.; Zeidan, A.E.B.; Hassan, M.A.E.; Mohey-Elsaeed, O. Evaluation of bull spermatozoa during and after cryopreservation: Structural and ultrastructural insights. J. Vet. Sci. Med. 2018, 6, S49–S56.
- Gürler, H.; Malama, E.; Heppelmann, M.; Calisici, O.; Leiding, C.; Kastelic, J.P.; Bollwein, H. Effects of cryopreservation on sperm viability, synthesis of reactive oxygen species, and DNA damage of bovine sperm. Theriogenology 2016, 86, 562–571.
- Chatterjee, S.; Gagnon, C. Production of reactive oxygen species by spermatozoa undergoing cooling, freezing, and thawing. Reprod. Dev. 2001, 59, 451–458.
- Pons-Rejraji, H.; Bailey, J.L.; Leclerc, P. Cryopreservation affects bovine sperm intracellular parameters associated with capacitation and acrosome exocytosis. Fertil. Dev. 2009, 21, 525–537.
- Yoon, S.J.; Kwon, W.S.; Rahman, M.S.; Lee, J.S.; Pang, M.G. A novel approach to identifying physical markers of cryo-damage in bull spermatozoa. PLoS ONE 2015, 10, e0126232.
- Rasul, Z.; Ahmad, N.; Anzar, M. Changes in motion characteristics, plasma membrane integrity, and acrosome morphology during cryopreservation of buffalo spermatozoa. Androl. 2001, 22, 278–283.
- Kadirvel, G.; Kumar, S.; Kumaresan, A. Lipid peroxidation, mitochondrial membrane potential and DNA integrity of spermatozoa in relation to intracellular reactive oxygen species in liquid and frozen-thawed buffalo semen. Animal Reprod. Sci. 2009, 114, 125–134.
- Kumar, D.; Kumar, P.; Singh, P.; Yadav, S.P.; Yadav, P.S. Assessment of sperm damages during different stages of cryopreservation in water buffalo by fluorescent probes. Cytotechnology 2016, 68, 451–458.
- Ahmad, M.; Nasrullah, R.; Riaz, H.; Sattar, A.; Ahmad, N. Changes in motility, morphology, plasma membrane and acrosome integrity during stages of cryopreservation of buck sperm. S. Afr. Vet. Assoc. 2014, 85, 1–5.
- Dorado, J.; Hidalgo, M.; Muñoz, A.; Rodríguez, I. Assessment of goat semen freezability according to the spermatozoa characteristics from fresh and frozen samples. Animal Reprod. Sci. 2009, 112, 150–157.
- Chauhan, M.S.; Kapila, R.; Gandhi, K.K.; Anand, S.R. Acrosome damage and enzyme leakage of goat spermatozoa during dilution, cooling and freezing. Andrologia 1994, 26, 21–26.
- Nur, Z.; Zik, B.; Ustuner, B.; Sagirkaya, H.; Ozguden, C.G. Effects of different cryoprotective agents on ram sperm morphology and DNA integrity. Theriogenology 2010, 73, 1267–1275.
- Peris-Frau, P.; Martín-Maestro, A.; Iniesta-Cuerda, M.; Sánchez-Ajofrín, I.; Cesari, A.; Garde, J.J.; Villar, M.; Soler, A.J. Cryopreservation of ram sperm alters the dynamic changes associated with in vitro capacitation. Theriogenology 2020, 145, 100–108.
- Da Silva Maia, M.; Bicudo, S.D.; Sicherle, C.C.; Rodello, L.; Gallego, I.C.S. Lipid peroxidation and generation of hydrogen peroxide in frozen-thawed ram semen cryopreserved in extenders with antioxidants. Animal Reprod. Sci. 2010, 122, 118–123.
- Peris, S.I.; Morrier, A.; Dufour, M.; Bailey, J.L. Cryopreservation of ram semen facilitates sperm DNA damage: Relationship between sperm andrological parameters and the sperm chromatin structure Assay. Androl. 2004, 25, 224–233.
- García-Álvarez, O.; Maroto-Morales, A.; Martínez-Pastor, F.; Garde, J.J.; Ramón, M.; Fernández-Santos, M.R.; Esteso, M.C.; Pérez-Guzmán, M.D.; Soler, A. J. Sperm characteristics and in vitro fertilization ability of thawed spermatozoa from black manchega ram: Electroejaculation and postmortem collection. Theriogenology 2009, 72, 160–168.
- M.R. Fernandez-Santos; M.C. Esteso; V. Montoro; Ana J. Soler; J.J. Garde; Cryopreservation of Iberian red deer (Cervus elaphus hispanicus) epididymal spermatozoa: Effects of egg yolk, glycerol and cooling rate. Theriogenology 2006, 66, 1931-1942, 10.1016/j.theriogenology.2006.05.012.
- Yael Gur; Mammalian sperm translate nuclear-encoded proteins by mitochondrial-type ribosomes. Genes & Development 2006, 20, 411-416, 10.1101/gad.367606.
- Baker, M. a.; Nixon, B.; Naumovski, N.; Aitken, R.J. Proteomic insights into the maturation and capacitation of mammalian spermatozoa. Biol. Reprod. Med. 2012, 58, 211–217.
- Oliva, R.; De Mateo, S.; Estanyol, J.M. Sperm cell proteomics. Proteomics 2009, 9, 1004–1017.
- Gayatri Mohanty; Nirlipta Swain; Luna Samanta; Sperm Proteome. Reproductive Sciences 2014, 22, 638-653, 10.1177/1933719114558918.
- Ding-Hui Dai; Izhar Hyder Qazi; Ming-Xia Ran; Kai Liang; Ming Zhang; Ming Zhang; Guangbin Zhou; Christiana Angel; Changjun Zeng; Exploration of miRNA and mRNA Profiles in Fresh and Frozen-Thawed Boar Sperm by Transcriptome and Small RNA Sequencing. International Journal of Molecular Sciences 2019, 20, 802, 10.3390/ijms20040802.
- Janice L. Bailey; J F Bilodeau; N Cormier; Semen cryopreservation in domestic animals: a damaging and capacitating phenomenon.. Journal of Andrology 2000, 21, 1–7.
- Wojtusik, J.; Wang, Y.; Pukazhenthi, B.S. Pretreatment with cholesterol-loaded cyclodextrins prevents loss of motility associated proteins during cryopreservation of addra gazelle (Nanger dama ruficollis) spermatozoa. Cryobiology 2018, 81, 74–80.
- Lessard, C.; Parent, S.; Leclerc, P.; Bailey, J.L.; Sullivan, R. Cryopreservation alters the levels of the bull sperm surface protein P25b. Androl. 2000, 21, 700–707.
- Pini, T.; Rickard, J.P.; Leahy, T.; Crossett, B.; Druart, X.; De Graaf, S.P. Cryopreservation and egg yolk medium alter the proteome of ram spermatozoa. Proteom. 2018, 181, 73–82.
- Peris-Frau, P.; Martín-Maestro, A.; Iniesta-Cuerda, M.; Sánchez-Ajofrín, I.; Mateos-Hernández, L.; Garde, J.J.; Villar, M.; Soler, A.J. Freezing—Thawing procedures remodel the proteome of ram sperm before and after in vitro capacitation. J. Mol. Sci. 2019, 20, 4596.
- Tincy Varghese; Bannur C. Divyashree; Sudhir C. Roy; Kajal S. Roy; Press Enter Key For Correspondence Information; Loss of heat shock protein 70 from apical region of buffalo ( Bubalus bubalis ) sperm head after freezing and thawing. Theriogenology 2016, 85, 828-834, 10.1016/j.theriogenology.2015.10.029.
- B. Westfalewicz; M. A. Dietrich; A. Ciereszko; Impact of cryopreservation on bull (Bos taurus) semen proteome1. Journal of Animal Science 2015, 93, 5240-5253, 10.2527/jas.2015-9237.
- Marti, E.; Marti, J.I.; Muiño-Blanco, T.; Cebrián-Pérez, J.A. Effect of the cryopreservation process on the activity and immunolocalization of antioxidant enzymes in ram spermatozoa. Androl. 2008, 29, 459–467.
- Bilodeau, J.-F.; Chatterjee, S.; Sirard, M.A.; Gagnon, C. Levels of antioxidant defenses are decreased in bovine spermatozoa after a cycle of freezing. Hum. Reprod. 2000, 55, 282–288.
- Kumar, A.; Prasad, J.K.; Srivastava, N.; Ghosh, S.K. Strategies to minimize various stress-related freeze–thaw damages during conventional cryopreservation of mammalian spermatozoa. Biobank. 2019, 17, 603–612.
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Reprod. Dev. 2017, 84, 1039–1052.
- Suvro Chatterjee; Eve De Lamirande; Claude Gagnon; Cryopreservation alters membrane sulfhydryl status of bull spermatozoa: Protection by oxidized glutathione. Molecular Reproduction and Development 2001, 60, 498-506, 10.1002/mrd.1115.
- Taylor Pini; Tamara Leahy; Simon P. De Graaf; Sublethal sperm freezing damage: Manifestations and solutions. Theriogenology 2018, 118, 172-181, 10.1016/j.theriogenology.2018.06.006.
- R J Aitken; Free radicals, lipid peroxidation and sperm function. Reproduction, Fertility and Development 1995, 7, 659-668, 10.1071/rd9950659.
- João D. A. Losano; Daniel S.R.Angrimani; A Dalmazzo; Bruno R. Rui; Mm Brito; Cm Mendes; Giulia Kiyomi V. Kawai; C.I. Vannucchi; Meoa Assumpção; V.H Barnabe; et al.M Nichi Effect of mitochondrial uncoupling and glycolysis inhibition on ram sperm functionality. Reproduction in Domestic Animals 2017, 52, 289-297, 10.1111/rda.12901.
- He, Y.; Wang, K.; Zhao, X.; Zhang, Y.; Ma, Y.; Hu, J. Differential proteome association study of freeze-thaw damage in ram sperm. Cryobiology 2016, 72, 60–68.
- Yoon, S.J.; Rahman, M.S.; Kwon, W.S.; Ryu, D.Y.; Park, Y.J.; Pang, M.G. Proteomic identification of cryostress in epididymal spermatozoa. Animal Sci. Biotechnol. 2016, 7, 1–12.
- Kumar, M.; Kumar, K.; Jain, S.; Hassan, T.; Dada, R. Novel insights into the genetic and epigenetic paternal contribution to the human embryo. Clinics 2013, 68, 5–14.
- Lewis, S.E.M.; Aitken, R.J. DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res. 2005, 322, 33–41.
- Yasuhiro Yamauchi; Jeffrey A Shaman; W. Steven Ward; Non-genetic contributions of the sperm nucleus to embryonic development. Asian Journal of Andrology 2010, 13, 31-35, 10.1038/aja.2010.75.
- Verma, A.; Rajput, S.; De, S.; Kumar, R.; Chakravarty, Atish Kumar Datta, T.K. Genome-wide pro fi ling of sperm DNA methylation in relation to buffalo (Bubalus bubalis) bull fertility. Theriogenology 2014, 82, 750–759.
- Ge, S.; Lin, S.; Zhao, Z.; Sun, Q. Epigenetic dynamics and interplay during spermatogenesis and embryogenesis: Implications for male fertility and offspring health. Oncotarget 2017, 8, 53804–53818.
- Collins, L.J.; Schönfeld, B.; Xiaowei, S.C. The epigenetics of non-coding RNA. In Handbook of Epigenetics; Elsevier Inc.: Amsterdam, The Netherlands, 2011; pp. 49–61.
- Xiaoli Chen; Yonggui Wang; Huabin Zhu; Haisheng Hao; Xueming Zhao; Tong Qin; Dong Wang; Comparative transcript profiling of gene expression of fresh and frozen–thawed bull sperm. Theriogenology 2015, 83, 504-511, 10.1016/j.theriogenology.2014.10.015.
- Jörg Aurich; Bettina Schreiner; Natascha Ille; Marco Alvarenga; Dragos Scarlet; Cytosine methylation of sperm DNA in horse semen after cryopreservation. Theriogenology 2016, 86, 1347-1352, 10.1016/j.theriogenology.2016.04.077.
- Changjun Zeng; Wenpei Peng; Li Ding; Lian He; Yan Zhang; Donghui Fang; Keyi Tang; A preliminary study on epigenetic changes during boar spermatozoa cryopreservation. Cryobiology 2014, 69, 119-127, 10.1016/j.cryobiol.2014.06.003.
- Chunrong Lv; Guoquan Wu; Qionghua Hong; Guobo Quan; Spermatozoa Cryopreservation: State of Art and Future in Small Ruminants. Biopreservation and Biobanking 2019, 17, 171-182, 10.1089/bio.2018.0113.
- Ugur, M.R.; Abdelrahman, A.S.; Evans, H.C.; Gilmore, A.A.; Hitit, M.; Arifiantini, R.I.; Purwantara, B.; Kaya, A.; Memili, E. Advances in cryopreservation of bull sperm. Vet. Sci. 2019, 6, 268.
- Layek, S.S.; Mohanty, T.K.; Kumaresan, A.; Parks, J.E. Cryopreservation of bull semen: Evolution from egg yolk based to soybean based extenders. Animal Reprod. Sci. 2016, 172, 1–9.
- Barbas, J.P.; Mascarenhas, R.D. Cryopreservation of domestic animal sperm cells. Cell Tissue Bank 2009, 10, 49–62.
- Allai, L.; Benmoula, A.; Marciane da Silva, M.; Nasser, B.; El Amiri, B. Supplementation of ram semen extender to improve seminal quality and fertility rate. Animal Reprod. Sci. 2018, 192, 6–17.
- Purdy, P.H. A review on goat sperm cryopreservation. Small Rumin. Res. 2006, 63, 215–225.
- Sung-Jae Yoon; Saidur Rahman; Woo-Sung Kwon; Yoo-Jin Park; Myung-Geol Pang; Addition of Cryoprotectant Significantly Alters the Epididymal Sperm Proteome. PLoS ONE 2016, 11, e0152690, 10.1371/journal.pone.0152690.
- Fujikawa, T.; Imamura, S.; Tokumaru, M.; Ando, T.; Gen, Y.; Hyon, S.H.; Kubota, C. Cryoprotective effect of antifreeze polyamino-acid (Carboxylated Poly-L-Lysine) on bovine sperm: A technical note. Cryobiology 2018, 82, 159–162.
- Tariq, A.; Ahmad, M.; Iqbal, S.; Riaz, M.I.; Tahir, M.Z.; Ghafoor, A.; Riaz, A. Effect of carboxylated poly L-Lysine as a cryoprotectant on post-thaw quality and in vivo fertility of Nili Ravi buffalo (Bubalus bubalis) bull semen. Theriogenology 2020, 144, 8–15.
- Longobardi, V.; Albero, G.; Canditiis, C. De; Salzano, A.; Natale, A.; Balestrieri, A.; Neglia, G.; Campanile, G.; Gasparrini, B. Cholesterol-loaded cyclodextrins prevent cryocapacitation damages in buffalo (Bubalus bubalis) cryopreserved sperm. Theriogenology 2017, 89, 359–364.
- Mocé, E.; Purdy, P.H.; Graham, J.K. Treating ram sperm with cholesterol-loaded cyclodextrins improves cryosurvival. Animal Reprod. Sci. 2010, 118, 236–247.
- Purdy, P.H.; Graham, J.K. Effect of cholesterol-loaded cyclodextrin on the cryosurvival of bull sperm. Cryobiology 2004, 48, 36–45.
- Leonardo Batissaco; Rubens Paes De Arruda; Maíra Bianchi Rodrigues Alves; Mariana Andrade Torres; Kleber Menegon Lemes; Roberto Romano Prado-Filho; Tamie Guibu De Almeida; André Furugen Cesar De Andrade; E.C.C. Celeghini; Cholesterol-loaded cyclodextrin is efficient in preserving sperm quality of cryopreserved ram semen with low freezability. Reproductive Biology 2020, 20, 14-24, 10.1016/j.repbio.2020.01.002.
- Amidi, F.; Pazhohan, A.; Shabani Nashtaei, M.; Khodarahmian, M.; Nekoonam, S. The role of antioxidants in sperm freezing: A review. Cell Tissue Bank. 2016, 17, 745–756.
- Fernández-Santos, M.R.; Martínez-Pastor, F.; García-Macías, V.; Esteso, M.C.; Soler, A.J.; Paz, P.; Anel, L.; Garde, J.J. Sperm characteristics and DNA integrity of Iberian red deer (Cervus elaphus hispanicus) epididymal spermatozoa frozen in the presence of enzymatic and nonenzymatic antioxidants. Androl. 2007, 28, 294–305.
- F. Sánchez-Rubio; M.R Fernández-Santos; L. Castro-Vázquez; O. García-Álvarez; A. Maroto-Morales; A.J. Soler; Felipe Martínez-Pastor; J. Julián Garde; Cinnamtannin B-1, a novel antioxidant for sperm in red deer. Animal Reproduction Science 2018, 195, 44-52, 10.1016/j.anireprosci.2018.05.004.
- Khalil, W.A.; El-Harairy, M.A.; Zeidan, A.E.B.; Hassan, M.A.E. Impact of selenium nano-particles in semen extender on bull sperm quality after cryopreservation. Theriogenology 2019, 126, 121–127.
- Hozyen, H.F.; Shamy, A.A.E.; Farghali, A.A. In vitro Supplementation of nano selenium minimizes freeze-thaw induced damage to ram spermatozoa. J. Vet. Sci. 2019, 8, 249–254.
- Shahin, M.A.; Khalil, W.A.; Saadeldin, I.M.; Swelum, A.A.-A.; El-harairy, M.A. Comparison between the effects of adding vitamins, trace elements, and nanoparticles to SHOTOR extender on the cryopreservation of dromedary camel epididymal spermatozoa. Animals 2020, 10, 78.
- Dashtestani, F.; Ghourchian, H.; Najafi, A. Silver-gold-apoferritin nanozyme for suppressing oxidative stress during cryopreservation. Sci. Eng. C 2019, 94, 831–840.
- José A. Cebrián-Pérez; A Casao; M González-Arto; Thais Rose Dos Santos Hamilton; R Perez-Pe; T Muino-Blanco; Melatonin in Sperm Biology: Breaking Paradigms. Reproduction in Domestic Animals 2014, 49, 11-21, 10.1111/rda.12378.
- Iraj Ashrafi; Hamid Kohram; Farhad Farrokhi Ardabili; Antioxidative effects of melatonin on kinetics, microscopic and oxidative parameters of cryopreserved bull spermatozoa. Animal Reproduction Science 2013, 139, 25-30, 10.1016/j.anireprosci.2013.03.016.
- Yi Fang; Chengzhen Zhao; Hai Xiang; Xueli Zhao; Rongzhen Zhong; Melatonin Inhibits Formation of Mitochondrial Permeability Transition Pores and Improves Oxidative Phosphorylation of Frozen-Thawed Ram Sperm. Frontiers in Endocrinology 2020, 10, 896, 10.3389/fendo.2019.00896.
- Pillai, H.; Parmar, M.S.; Shende, A.M.; Thomas, J.; Kartha, H.S.; Sharma, T.; Ghosh, G.S.K.; Kumaresan, A.; Bhure, S.K. Effect of supplementation of recombinant regucalcin in extender on cryopreservation of spermatozoa of water buffalo (Bubalus bubalis). Reprod. Dev. 2017, 84, 1133–1139.
- Ledesma, A.; Zalazar, L.; Buchelly, F.; Ignacio, J.; Brown, P.; Mitch, E.; Hozbor, F.; Cesari, A. Recombinant peptide reverses cryo-capacitation in ram sperm and improves in vitro fertilization. Animal Reprod. Sci. 2019, 207, 61–72.
- Zalazar, L.; Iniesta-Cuerda, M.; Sánchez-Ajofrín, I.; Soler, A.J.; Garde, J.J.; Cesari, A. Recombinant SPINK3 improves ram sperm quality and in vitro fertility after cryopreservation. Theriogenology 2020, 144, 45–55.
- Vanesa Robles; David G. Valcarce; Marta F. Riesco; The Use of Antifreeze Proteins in the Cryopreservation of Gametes and Embryos. Biomolecules 2019, 9, 181, 10.3390/biom9050181.
- Steven R. Payne; J.E. Oliver; G.C. Upreti; Effect of Antifreeze Proteins on the Motility of Ram Spermatozoa. Cryobiology 1994, 31, 180-184, 10.1006/cryo.1994.1021.
- N.S. Prathalingam; W.V. Holt; S.G. Revell; S. Mirczuk; R.A. Fleck; P.F. Watson; Impact of antifreeze proteins and antifreeze glycoproteins on bovine sperm during freeze-thaw. Theriogenology 2006, 66, 1894-1900, 10.1016/j.theriogenology.2006.04.041.