The skin is the largest organ of the human body, comprising 15% of a person’s body weight and with a surface area of about 20 square feet
[1]. It protects the internal structures from any kind of physical, biological, chemical, and mechanical stress from the outer environment. It is also involved in thermoregulation, immune-regulatory observation, water loss prevention, sensation, and vitamin cholecalciferol (D
3) agglutination
[2][3][4][2,3,4]. Any damage or disorder in the healthy structure of skin is described as a wound. Wounds can be classified according to their location, depth, etiology, injury type, and appearance
[5]. Clinically wounds are classified as chronic or acute wounds. Acute wounds spontaneously heal in about 8–12 weeks, whereas chronic wounds require a longer healing time (sometime even some months) due to prolonged inflammation. Age, obesity, injuries, and chronic ailments, such as diabetes, cancer, and other factors, contribute to chronic wounds
[6][7][6,7]. Wound healing is an intricate physiological method consisting of coordinated overlapping phases of hemostasis, inflammation, proliferation, and tissue remodeling
[8]. Hemostasis is the first and foremost reaction within the first few minutes of the injury. At the injured site, the inflammatory cells and platelets begin to adhere and activate fibrin, forming a mesh-like structure that acts as “glue” to bind platelets with each other. This aggregate develops a clot and along with vasoconstriction helps to prevent further bleeding
[9]. The second phase of wound healing is the inflammation that occurs concomitantly with the hemostasis phase. Activation of the complement cascade occurs when a fibrin clot forms, allowing neutrophils to move to the wound site to scavenge bacteria and prepare for healing. The phagocytic cell secretes transforming growth factor β (TGF-β), which initiates a vital signal for the onset of healing at the wound site. The blood monocytes and lymphocytes turn into tissue macrophages, leading to the release of growth factors and cytokines named fibroblast growth factor (FGF), tumor necrosis factor-alpha (TNF-α), and interleukin-1 (IL-1) and, together, these attract endothelial cells, keratinocytes, and fibroblasts to rebuild the damaged blood vessels. Proliferation is the third phase, and during this phase, the contraction of the wound and the replacement of subdermal and dermal tissues take place. Angiogenesis, epithelialization, and collagen production are all initiated by fibroblast cells. TGF-β platelets and macrophages induce collagen production in fibroblasts. In this process, the final phase is remodeling in the healing process; it comprises of new layer formation by fibroblast cells of skin on the wound surface, and the original collagen type III in the injured site is replaced by collagen type I to be cross-linked and realigned along the tension lines
[9][10][9,10]. There are various genetic factors and acquired factors, such as diabetes, which disrupt the wound healing process. The wounds in diabetic patients are characterized by increased inflammation and abnormal cellular infiltration, faulty cytokine production, neuritis, and inadequate neo-angiogenesis. Increasing health care costs, an aging population, biofilm formation, and the continued threat of diabetes and obesity worldwide make chronic wounds a substantial clinical, social, and economic challenge. According to the 2018 retrospective analysis, Medicare beneficiaries identified that approximately 8.2 million people are suffering from wounds. The annual wound care products market is estimated to reach $15–22 billion by 2024. In the United States alone, around 5.7 million patients are suffering from chronic wounds with an annual cost of treatment of about USD 20 billion
[11]. This all leads to the conclusion that there is a huge scope and requirement for research into wound healing. Plants have always been an integrated and most widely studied area in this regard as they promote natural repair mechanisms
[12]. With the advancement in science, interest has shifted from whole plants to active chemical constituents. Curcumin is at the top in the wound healing area. Curcumin is a naturally occurring low-molecular-weight polyphenolic constituent present in the rhizome of
Curcuma longa and
Curcuma aromatic [13]. Curcumin (77%) is the most prominent bioactive constituent of turmeric rhizomes, followed by demethoxycurcumin (17%), bisdemethoxycurcumin (3%), and cyclocurcumin (3%). It has been used for ages as traditional medicine in the treatment of inflammation and healing of impaired wounds. The topical application of curcumin is documented to have an effective role in wound healing mechanisms. Curcumin acts in different stages, such as the inflammatory, maturation and proliferative phases and thus enhances the overall process of wound healing. However, some limiting factors, such as poor bioavailability, low solubility in water, and rapid metabolism hinder curcumin’s therapeutic efficacy. Toxicity at high concentrations in its topical application is another disadvantage of curcumin
[13][14][15][13,14,15]. Hence incorporation of different nano delivery systems to modulate the limiting factors of curcumin is an interesting area to explore to unlock all the possibilities related to this compound in the area of wound healing.
2. Chemistry and Applications of Curcumin
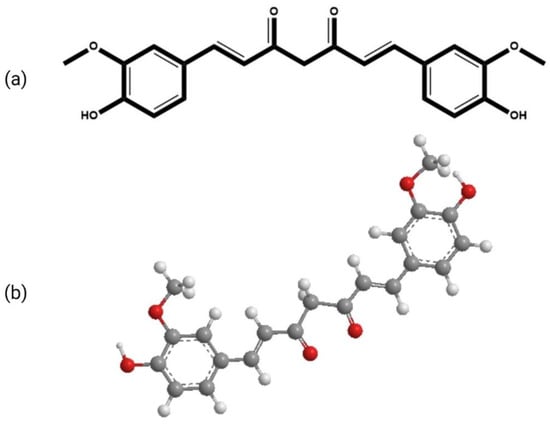
The molecular formula of curcumin is C
21H
20O
6. Its structure is composed of three chemical entities as shown in
Figure 1: two aromatic rings with methoxyl and hydroxyl groups at the ortho position linked by a seven-carbon linker that includes an α, β-unsaturated β-diketone moiety
[16]. Due to the presence of the diketo group, curcumin exhibits keto-enol tautomerism. The keto form predominates in slightly acidic and neutral conditions
[17], whereas the enolic form dominates in alkaline conditions. In solution, it exhibits
cis-trans isomerism. The
trans-form is more stable than the
cis-form due to the placement difference of two phenolic-methoxy groups on the curcumin backbone. The computed dipole moment of curcumin in its ground state is 10.77 D
[18]. The logarithmic value of the octanol/water partition coefficient (
log P) of curcumin is 3.2, thus making it practically insoluble in water but highly soluble in lipids
[16][19][16,19]. Due to its lipophilic nature, it acquires adequate transmembrane permeability. Curcumin can be incorporated into aqueous solvents with the aid of lipids, surfactants, albumins, and biopolymers, etc. For the incorporation of higher concentrations of curcumin, micelles have shown promising results. However, as surfactants can interfere in biological studies, proper controlled experiments must be performed while using these aqueous solutions in biological systems.
Figure 1.
The chemical structure of curcumin (
a
) 2-D (
b
) 3-D.
Curcumin is documented to be effective against a variety of chronic diseases, including Alzheimer’s disease, multiple sclerosis, rheumatoid arthritis, atherosclerosis, and others. It has also shown promising results against cataract formation, liver damage, pulmonary toxicity, fibrosis, and impaired wound healing, inhibits thrombosis and suppresses platelet aggregation
[20]. It is also established as an antineoplastic, antimicrobial, anti-fungal, anti-carcinogenic, anti-infective, anti-mutagenic, anti-inflammatory, anti-proliferative, anti-aging, anti-amyloid, and anti-hypercholesterolemia agent
[21][22][21,22].
It inhibits the activation of free radical-activated transcription factor, nuclear factor kappa B (NFκB)
[15], cytokine production, and other cellular processes essential for cell survival. It also inhibits the signal transducer and activator of transcription (STAT) proteins. Curcumin-led inhibition of NFκB-DNA binding suppresses the pro-inflammatory molecules matrix metalloprotease 9 (MMP-9) and matrix metalloprotease 3 (MMP-3) and also reduces pro-inflammatory cytokines, such as tumor necrosis factor 1 (TNF-1), interleukin 1 (IL-1), and interleukin 8 (IL-8). Curcumin also binds to the COX-2 (prostaglandin-endoperoxide synthase 2) protein, which reduces COX-2 expression, prostaglandin and thromboxane synthesis
[23]. Furthermore, curcumin is a versatile antioxidant molecule that combats free radicals, as it is hypothesized to reduce reactive oxygen species (ROS) production, neutrophil attraction, adhesion, and migration, which leads to the reduction of damage caused by contusion-induced muscle injury
[24]. Curcumin is also documented to lower blood cholesterol and sugar levels and improve insulin sensitivity in diabetic people
[25][26][25,26]. Furthermore, curcumin has also been reported to possess significant antifungal and antibacterial properties against both gram-positive and gram-negative bacteria
[27]. Curcumin-encapsulated PEGylated nanoliposomes showed a potential anti-infective therapeutic effect
[28]. Besides, it also possesses anti-biofilm and antibacterial properties against
Porphyromonas gingivalis [29].
3. Wound Healing Potential of Curcumin: Mechanism of Healing
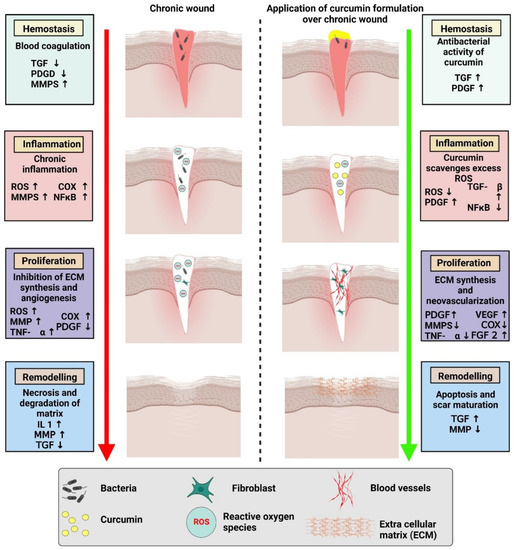
Of late, biomedical applications give strong evidence for the wound healing potential of curcumin as each and every activity plays an important role in wound healing (
Figure 2). Curcumin is a natural polyphenolic antioxidant constituent that has been intensively explored in recent years for its potential as a wound healing agent
[30]. The primary objective of wound healing is to restore tissue integrity and maintain homeostasis
[31]. Curcumin improves the wound contraction rate, thus accelerating the healing of wounds. It is reported to enhance the wound area significantly up to 20%
[32]. Emiroglu et al. have shown that curcumin suppressed the inflammatory response and hastened wound healing
[33]. Heydari et al. discovered that curcumin enhanced collagen deposition and promoted angiogenesis in the chronic wound
[34].
Figure 2.
Comparison of chronic wound healing and accelerated wound healing with the application of a curcumin-based formulation.
3.1. Effects of Curcumin on Inflammation
Inflammation is the critical second phase of the wound healing process, and it is frequently referred to as the first stage in optimal skin regeneration. Controlling inflammation is desirable and can accelerate the wound healing process since tissue injury produces practically a rapid start of acute inflammation. Curcumin, in particular, has been demonstrated to decrease the production of tumor necrosis factor-alpha (TNF-α) and interleukin-1 (IL-1), two essential cytokines generated by monocytes and macrophages that regulate inflammatory responses. Curcumin’s capacity to block the activity of NF-(κ)B (nuclear factor kappa-light-chain-enhancer of activated B cells), a transcription factor involved in the start of inflammatory reactions, is also significant. Curcumin modulates a variety of pathways associated with the activation of NF-(κ)B, which is generally activated by several kinases (AKT, PI3K, IKK)
[35]. For a long time, NF-(κ)B was thought to be oxidant sensitive, emphasizing the link between oxidation and inflammation in wound healing. Other signaling pathways, such as PPAR and myeloid differentiation protein 2-TLR 4 co-receptors, are involved in curcumin’s anti-inflammatory activities (TLR4-MD2)
[36][37][36,37]. Curcumin also reduces angiotensin II-induced inflammatory reactions by inhibiting vascular smooth muscle cell proliferation by increasing PPAR-γ activity. It has also been shown to reduce inflammation by competing with LPS for MD2 binding, effectively inhibiting the TLR4-MD2 signaling complex
[38]. ROS are by-products of aerobic respiration that play a role in intracellular signaling, differentiation, apoptosis, cell development, and immunity. ROS have a role in wound healing because they are necessary for the immune system’s defense against microbes. However, chronic exposure to high levels of ROS causes oxidative stress, which can harm human cells severely. In the wound healing process, oxidative stress is a critical component that limits tissue regeneration. It has been discovered that curcumin has an efficient protective function against oxidative stress through modulating lipoxygenases (LPx), mostly by scavenging free radicals. Curcumin’s capacity to transport electrons or easily give H-atoms from two methoxy phenol groups accounts for its antioxidant properties. It also contains a variety of functional groups, such as b-diketone and a large number of p electrons with high electron-shifting properties. Furthermore, curcumin’s ability to attach to metals is thought to be due to its di-ketone structure, and the phenolic hydroxyl (-OH) groups give ROS scavenging capabilities
[39]. Furthermore, it boosts the generation and functioning of antioxidants and their components, such as glutathione (GSH). Stimulation of cytoprotective signaling pathways, such as the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway, have been identified as linked molecular mechanisms in wound healing that cause oxidative stress and an increase in ROS species. This Nrf2 regulation mechanism is critical for cell protection against oxidative damage. In response to electrophilic and oxidative stress, the reactive cysteine residues of Keap1 change, resulting in diminished E3 ligase activity, continued Nrf2 accumulation in cells, and significant stimulation of a number of cytoprotective genes
[40]. Curcumin works as a Nrf2 activator and thereby decreases oxidative stress by functioning as a direct or indirect antioxidant
[41].
3.2. Effects of Curcumin on the Proliferative Phase of Wound Healing
Granulation tissue formation and collagen deposition (the production of the extracellular protein matrix), fibroblast proliferation, epithelialization, and apoptosis of undesired cells are all part of the proliferative phase of wound healing
[42]. Various studies have examined the impact of curcumin on these processes, as well as the time for wound closure in curcumin-treated animals compared to controls. According to Panchtantram et al., curcumin promoted collagen production and enhanced cellular proliferation at the wound site, as seen by increased DNA, total protein, and type III collagen content in wound tissues
[43]. According to a histological investigation by Gopinath et al., curcumin-increased cytokine production causes fibroblasts to migrate to wound sites, resulting in enhanced fibroblast and collagen proliferation
[44]. A comparison research employing a skin incision model showed that curcumin supported complete epithelial repair, enhanced angiogenesis during the proliferative phase of wound healing, and faster wound closure
[45].
3.3. Effects of Curcumin on Fibroblast Proliferation
The migration of fibroblasts into the wound area is necessary for the formation/remodeling of granulation tissue, as well as the creation and deposition of collagen
[46]. As a result, fibroblasts in the wound environment are undoubtedly the most significant mediator in guaranteeing quick and aesthetically pleasing wound closure. Fibroblasts naturally develop into myofibroblasts during the creation of granulation tissue. Curcumin treatment has been demonstrated to cause fibroblast infiltration into wound sites in various research findings
[47][48][47,48]. Ravanfar et al. observed that curcumin dramatically sped up cellular proliferation and shortened the inflammatory phase, showed considerably greater fibroblast distribution/one mm
2 of wound area, and quick re-epithelialization
[48].
3.4. Effects of Curcumin on Granulation Tissue Formation
After four days of a skin injury, granulation tissue, or new stroma, starts to form, where small capillaries develop, and fibroblasts infiltrate, facilitating the creation of an extracellular matrix. Curcumin improves granulation tissue formation, which further facilitates re-epithelialization by providing a stable foundation for epithelial cells to migrate and heal the wound gap. A study done by Aslam et al. has shown that granulation tissue was present in the curcumin-treated group, along with mild collagen deposition, neovascularization, and moderate inflammatory cells (D–F). Furthermore, they have also shown that the group that received treatment with curcumin + ZnO nanoconjugates displayed traits that were close to normal, including enlarged collagen bundles in the dermis, severe fibrosis, angiogenesis, and an altered collagen matrix (G–I)
[49].
3.5. Effect of Curcumin on Collagen Deposition
The extracellular matrix must be reorganized and remodeled in order for wounds to heal entirely. The extracellular matrix, which includes granulation tissue and collagen, gives support to cells and comprises of various proteins and polysaccharides. Collagen is the most abundant protein in the extracellular matrix of the skin, which accounts for 70–80% of its mass. The creation of scar tissue, which is largely made up of collagenous fibers, is the end outcome of wound healing. As a result, sufficient collagen production and deposition in a wound site would be ideal for improving wound repair. Curcumin has been reported to enhance collagen and extracellular matrix synthesis and thereby accelerate the process of wound healing
[50][51][50,51]. It has been observed that curcumin tends to accelerate wound healing as it can start synthesis and release of collagen on the third day after its application on a wound as shown by Mahmoud et al., 2022
[52]. Qian et al. showed that curcumin can aggregate wound exudate to cause a cascade release of curcumin, which speeds up the healing process while promoting collagen deposition and vascularization
[53].