Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Dean Liu and Version 2 by Dean Liu.
Galactomyces ferment filtrate (GFF) is such a functional ingredient. Its use originated from the empirical observation that the hands of sake brewers who deal with yeast fermentation retain a beautiful and youthful appearance. Consequently, skincare products based on GFF are widely used throughout the world.
- Galactomyces ferment filtrate PiteraTM
- aryl hydrocarbon receptor
- NRF2
1. Introduction
The skin is a vital organ that protects the bodies of terrestrial animals from the effects of dry harsh environments. It also acts as a functional barrier against external mechanical, chemical, and climatological stresses [1][2]. For example, exposure to ultraviolet (UV) rays and environmental pollutants induces varying degrees of oxidative stress in the skin and the subsequent production of proinflammatory cytokines [3][4][5][6]. Low-grade chronic inflammation is a significant risk factor for the type of accelerating aging known as inflammaging [7][8]. An aged skin appearance and a corresponding histological frailty are aggravated in sun-exposed areas of skin compared with those protected from sunlight [9]. Therefore, the inhibition of oxidative stress by daily applications of suitable antioxidants might be beneficial in retarding skin inflammaging induced by various environmental oxidative stress factors [10][11].
The barrier function of skin is mainly provided by its outermost epidermal layer, the stratum corneum or cornified layer [1][2]. The human epidermis is composed of multiple layers of keratinocytes, including basal, spinous, granular, and cornified layers. Keratinocytes proliferate in the basal layer, move up through the spinous and granular layers, and die, but remain functional as corneocytes in the cornified layer, before finally detaching from the skin [1][2]. Corneocytes are the major components of the cornified layer. However, other biological materials, including the extracellular lamellae of lipids, such as ceramides and cholesterol, and various natural moisturizing factors (NMFs), including free amino acids, pyrrolidone carboxylic acids, lactates, glucose, urea, hyaluronic acid, and electrolytes, are essential for maintaining a healthy skin–water balance [1][2][12]. During the differentiation process from the basal to the cornified layer, keratinocytes sequentially produce epidermal differentiation complex proteins, such as involucrin, loricrin, and filaggrin [13]. The integration of these proteins into cytoskeletal keratin fiber is essential for the proper differentiation of keratinocytes into corneocytes [1][2][12][13]. The degradation of filaggrin by proteolytic enzymes, such as caspase-14, in the granular layer is also pivotal in the production of NMFs [1][2][12][13][14][15][16][17].
The differentiation of keratinocyte is coordinately regulated by various transcription factors, including the aryl hydrocarbon receptor (AHR) [18][19], OVO-like 1/2 (OVOL1/2) [20][21][22][23], MYC [22][23], NOTCH1 [22][24], CEBP [25][26], and PPAR [27][28]. The expression or activation of these transcription factors is modulated by certain inflammatory cytokines, phytochemicals, and UV-mediated oxidative stress [29][30][31][32][33][34][35][36][37][38]. For instance, the expression of filaggrin is downregulated by the interleukins IL-4 and IL-13, which are pathogenic for atopic dermatitis, as well as by IL-17A, which is pathogenic for psoriasis [29]. These might contribute, at least in part, to the dry barrier-impaired skin lesions in atopic dermatitis and psoriasis [39].
In general, dry barrier-impaired skin exhibits a decrease in skin hydration and an increased rate of transepidermal water loss (TEWL) [39]. The topical application of a moisturizer increases skin hydration and decreases TEWL [39][40]; therefore, skin moisturization is recommended as a basic treatment, especially for atopic dermatitis and senile xerosis [41][42][43][44]. It is known that skin moisturization is an important factor in facial skin’s ability to maintain a youthful and healthy appearance [45]. Moreover, antioxidative moisturizers can decrease facial redness and reduce pore dilation [46].
2. Activation of AHR-Filaggrin Axis by GFF
AHR is a ligand-dependent transcription factor that is pivotal in upregulating the expression of filaggrin and other differentiation complex proteins in the epidermis [18][19]. In its steady, nonstimulated condition, AHR resides in the cytoplasm of keratinocytes [47]. Upon stimulation by GFF, activated AHR translocates into the nucleus from the cytoplasm (Figure 1), where it upregulates the expression of filaggrin [47].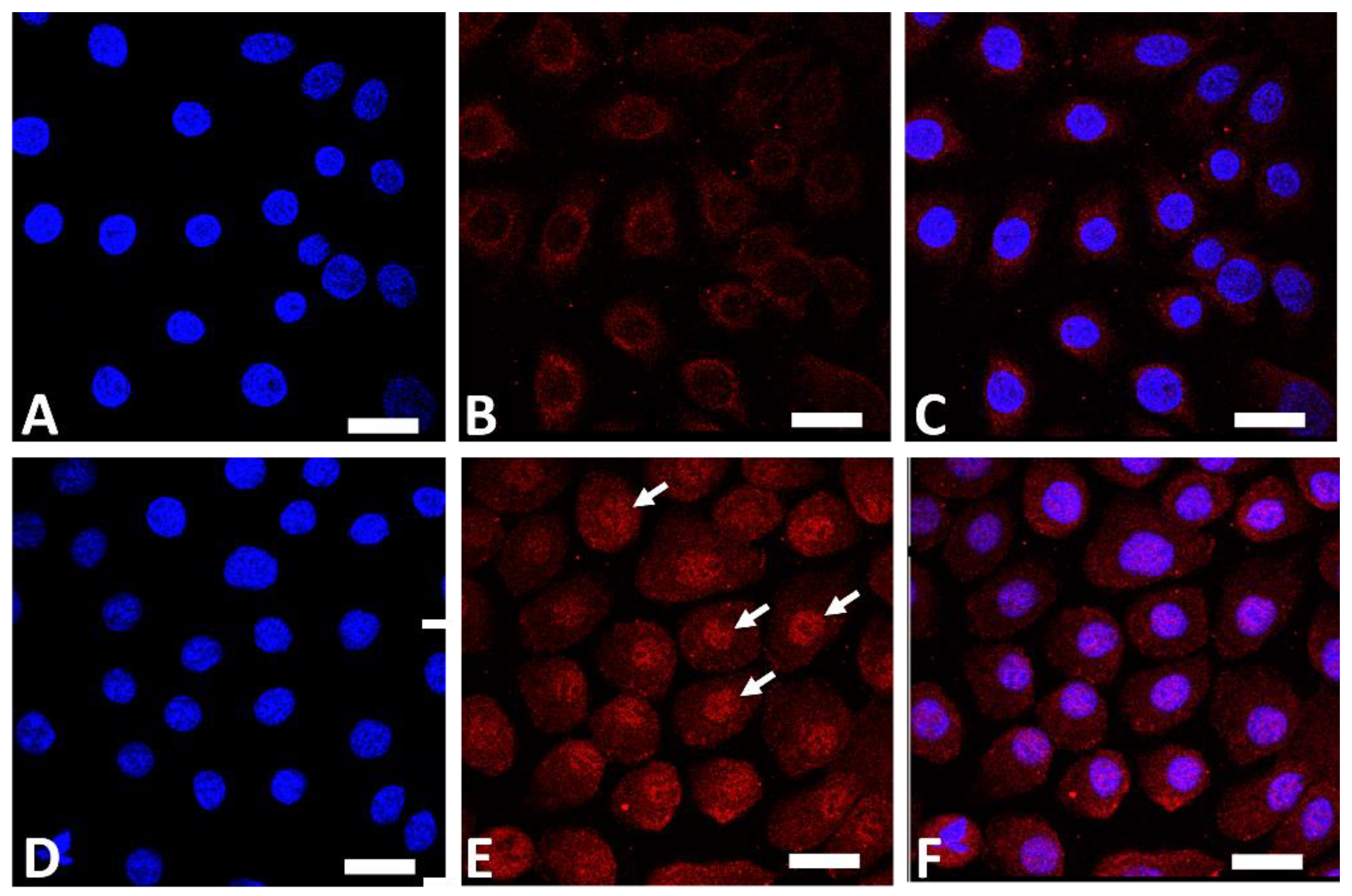
Figure 1. Immunofluorescence staining of AHR (red fluorescence) in human keratinocytes. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). Nonstimulated control keratinocytes ((A); DAPI staining, (B); AHR staining, (C); merged). GFF-treated keratinocytes ((D); DAPI staining, (E); AHR staining, (F); merged). AHR resides mainly in the cytoplasm in nonstimulated keratinocytes (B). GFF induces nuclear translocation of AHR ((E), arrows). AHR: aryl hydrocarbon receptor. GFF: Galactomyces ferment filtrate. Bar; 25 μm.
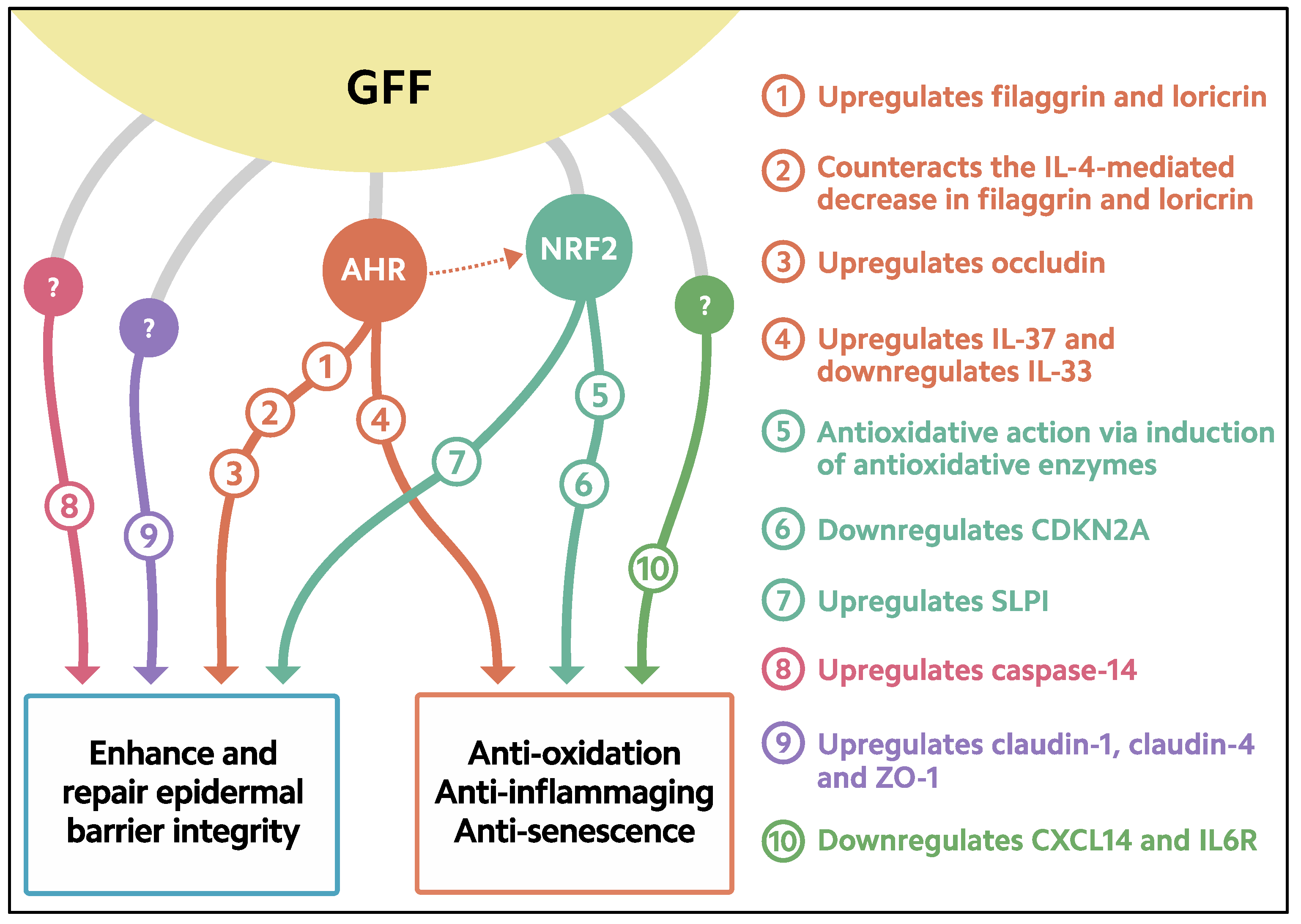
Figure 2. Biological response induced in keratinocytes treated with GFF. GFF: Galactomyces ferment filtrate. AHR: Aryl hydrocarbon receptor. NRF2: Nuclear factor erythroid 2-related factor 2. CDKN2A: cyclin-dependent kinase inhibitor 2A. CXCL14: chemokine (C-X-C motif) ligand 14. IL6R: IL-6 receptor. SLPI: secretory leukocyte peptidase inhibitor.
3. Antioxidative Properties of GFF
The skin is continuously exposed to various oxidative stressors, such as UV radiation, environmental pollutants, and inflammatory cytokines such as tumor necrosis factor-α (TNF-α) [55][61][62][63][64]. These oxidative stressors generate reactive oxygen species (ROS) in skin cells. To ameliorate oxidative damage, excess amounts of ROS require neutralization by an antioxidative system. Nuclear factor erythroid-2-related factor 2 (NRF2) is the antioxidative master transcription factor [10]. Like AHR, nonstimulated NRF2 is mainly located in the cytoplasm of keratinocytes [63][65]. Upon stimulation, the activated NRF2 translocates from the cytoplasm into the nucleus, where it upregulates the transcription of genes for antioxidative enzymes such as glutathione peroxidase 2 (GPX2), NAD(P)H quinone oxidoreductase 1 (NQO1), and heme oxidase 1 (HMOX1), which are responsible for neutralizing excess ROS [63][66][67][68]. For example, GPX2 is known to play a critical role in preventing UVB-mediated carcinogenesis in keratinocyte [67]. In addition to its AHR-stimulating properties, GFF induces nuclear translocation of NRF2 in the cytoplasm (Figure 3) and upregulates the expression of GPX2, NQO1, and HMOX1 [10][65][66][68][69].
Figure 3. Immunofluorescence staining of NRF2 (green fluorescence) in human keratinocytes. Nonstimulated control keratinocytes ((A); DAPI staining, (B); NRF2 staining, (C); merged). GFF-treated keratinocytes ((D); DAPI staining, (E); NRF2 staining, (F); merged). NRF2 mainly resides in the cytoplasm in nonstimulated keratinocytes (B). GFF induces nuclear translocation of NRF2 ((E), arrows). DAPI: 4′,6-diamidino-2-phenylindole. NRF2: nuclear factor erythroid-2-related factor 2. GFF: Galactomyces ferment filtrate. Bar; 25 μm.

Figure 4. Immunofluorescence staining of ROS (green fluorescence) in human keratinocytes. Nuclei were stained with DAPI. ROS production is minimized in nonstimulated control keratinocytes. IL-13 induces significant ROS production (arrow), which is ameliorated in the presence of GFF. Bar; 25 μm. DAPI: 4′,6-diamidino-2-phenylindole. ROS: reactive oxygen species. GFF: Galactomyces ferment filtrate.
References
- Rawlings, A.V.; Harding, C.R. Moisturization and skin barrier function. Dermatol. Ther. 2004, 17 (Suppl. S1), 43–48.
- Candi, E.; Schmidt, R.; Melino, G. The cornified envelope: A model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 2005, 6, 328–340.
- Boo, Y.C. Emerging strategies to protect the skin from ultraviolet rays using plant-derived materials. Antioxidants 2020, 9, 637.
- Ryu, Y.S.; Kang, K.A.; Piao, M.J.; Ahn, M.J.; Yi, J.M.; Hyun, Y.M.; Kim, S.H.; Ko, M.K.; Park, C.O.; Hyun, J.W. Particulate matter induces inflammatory cytokine production via activation of NFκB by TLR5-NOX4-ROS signaling in human skin keratinocyte and mouse skin. Redox Biol. 2019, 21, 101080.
- Tanaka, Y.; Uchi, H.; Furue, M. Antioxidant cinnamaldehyde attenuates UVB-induced photoaging. J. Dermatol. Sci. 2019, 96, 151–158.
- Tanaka, Y.; Uchi, H.; Hashimoto-Hachiya, A.; Furue, M. Tryptophan photoproduct FICZ upregulates IL1A, IL1B, and IL6 expression via oxidative stress in keratinocytes. Oxid. Med. Cell Longev. 2018, 2018, 9298052.
- Shive, C.; Pandiyan, P. Inflammation, immune senescence, and dysregulated immune regulation in the elderly. Front. Aging 2022, 3, 840827.
- Heinze-Milne, S.D.; Banga, S.; Howlett, S.E. Frailty and cytokines in preclinical models: Comparisons with humans. Mech. Ageing Dev. 2022, 206, 111706.
- Kimball, A.B.; Alora-Palli, M.B.; Tamura, M.; Mullins, L.A.; Soh, C.; Binder, R.L.; Houston, N.A.; Conley, E.D.; Tung, J.Y.; Annunziata, N.E.; et al. Age-induced and photoinduced changes in gene expression profiles in facial skin of Caucasian females across 6 decades of age. J. Am. Acad. Dermatol. 2018, 78, 29–39.
- Furue, M.; Uchi, H.; Mitoma, C.; Hashimoto-Hachiya, A.; Chiba, T.; Ito, T.; Nakahara, T.; Tsuji, G. Antioxidants for healthy skin: The emerging role of aryl hydrocarbon receptors and nuclear factor-erythroid 2-related factor-2. Nutrients 2017, 9, 223.
- Eassa, H.A.; Eltokhy, M.A.; Fayyaz, H.A.; Khalifa, M.K.A.; Shawky, S.; Helal, N.A.; Eassa, H.A.; Youssef, S.F.; Latz, I.K.; Nounou, M.I. Current topical strategies for skin-aging and inflammaging treatment: Science versus fiction. J. Cosmet. Sci. 2020, 71, 321–350.
- Elias, P.M.; Choi, E.H. Interactions among stratum corneum defensive functions. Exp. Dermatol. 2005, 14, 719–726.
- Kypriotou, M.; Huber, M.; Hohl, D. The human epidermal differentiation complex: Cornified envelope precursors, S100 proteins and the ‘fused genes’ family. Exp. Dermatol. 2012, 21, 643–649.
- Hoober, J.K.; Eggink, L.L. The discovery and function of filaggrin. Int. J. Mol. Sci. 2022, 23, 1455.
- Kim, Y.; Lim, K.M. Skin barrier dysfunction and filaggrin. Arch. Pharm. Res. 2021, 44, 36–48.
- Eckhart, L.; Declercq, W.; Ban, J.; Rendl, M.; Lengauer, B.; Mayer, C.; Lippens, S.; Vandenabeele, P.; Tschachler, E. Terminal differentiation of human keratinocytes and stratum corneum formation is associated with caspase-14 activation. J. Investig. Dermatol. 2000, 115, 1148–1151.
- Hoste, E.; Kemperman, P.; Devos, M.; Denecker, G.; Kezic, S.; Yau, N.; Gilbert, B.; Lippens, S.; De Groote, P.; Roelandt, R.; et al. Caspase-14 is required for filaggrin degradation to natural moisturizing factors in the skin. J. Investig. Dermatol. 2011, 131, 2233–2241.
- Furue, M.; Takahara, M.; Nakahara, T.; Uchi, H. Role of AhR/ARNT system in skin homeostasis. Arch. Dermatol. Res. 2014, 306, 769–779.
- Furue, M.; Tsuji, G.; Mitoma, C.; Nakahara, T.; Chiba, T.; Morino-Koga, S.; Uchi, H. Gene regulation of filaggrin and other skin barrier proteins via aryl hydrocarbon receptor. J. Dermatol. Sci. 2015, 80, 83–88.
- Tsuji, G.; Ito, T.; Chiba, T.; Mitoma, C.; Nakahara, T.; Uchi, H.; Furue, M. The role of the OVOL1-OVOL2 axis in normal and diseased human skin. J. Dermatol. Sci. 2018, 90, 227–231.
- Ito, T.; Tsuji, G.; Ohno, F.; Uchi, H.; Nakahara, T.; Hashimoto-Hachiya, A.; Yoshida, Y.; Yamamoto, O.; Oda, Y.; Furue, M. Activation of the OVOL1-OVOL2 axis in the hair bulb and in pilomatricoma. Am. J. Pathol. 2016, 186, 1036–1043.
- Wells, J.; Lee, B.; Cai, A.Q.; Karapetyan, A.; Lee, W.J.; Rugg, E.; Sinha, S.; Nie, Q.; Dai, X. Ovol2 suppresses cell cycling and terminal differentiation of keratinocytes by directly repressing c-Myc and Notch1. J. Biol. Chem. 2009, 284, 29125–29135.
- Nair, M.; Teng, A.; Bilanchone, V.; Agrawal, A.; Li, B.; Dai, X. Ovol1 regulates the growth arrest of embryonic epidermal progenitor cells and represses c-myc transcription. J. Cell Biol. 2006, 173, 253–264.
- Melnik, B.C. The potential role of impaired Notch signalling in atopic dermatitis. Acta Derm. Venereol. 2015, 95, 5–11.
- Lopez, R.G.; Garcia-Silva, S.; Moore, S.J.; Bereshchenko, O.; Martinez-Cruz, A.B.; Ermakova, O.; Kurz, E.; Paramio, J.M.; Nerlov, C. C/EBPalpha and beta couple interfollicular keratinocyte proliferation arrest to commitment and terminal differentiation. Nat. Cell Biol. 2009, 11, 1181–1190.
- House, J.S.; Zhu, S.; Ranjan, R.; Linder, K.; Smart, R.C. C/EBPalpha and C/EBPbeta are required for Sebocyte differentiation and stratified squamous differentiation in adult mouse skin. PLoS ONE 2010, 5, e9837.
- Yan, Y.; Furumura, M.; Numata, S.; Teye, K.; Karashima, T.; Ohyama, B.; Tanida, N.; Hashimoto, T. Various peroxisome proliferator-activated receptor (PPAR)-γ agonists differently induce differentiation of cultured human keratinocytes. Exp. Dermatol. 2015, 24, 62–65.
- Yang, R.; Chowdhury, S.; Choudhary, V.; Chen, X.; Bollag, W.B. Keratinocyte aquaporin-3 expression induced by histone deacetylase inhibitors is mediated in part by peroxisome proliferator-activated receptors (PPARs). Exp. Dermatol. 2020, 29, 380–386.
- Furue, M. Regulation of filaggrin, loricrin, and involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic implications in atopic dermatitis. Int. J. Mol. Sci. 2020, 21, 5382.
- Furue, M. Regulation of skin barrier function via competition between AHR axis versus IL-13/IL-4-JAK-STAT6/STAT3 axis: Pathogenic and therapeutic implications in atopic dermatitis. J. Clin. Med. 2020, 9, 3741.
- Furue, K.; Ito, T.; Tsuji, G.; Ulzii, D.; Vu, Y.H.; Kido-Nakahara, M.; Nakahara, T.; Furue, M. The IL-13-OVOL1-FLG axis in atopic dermatitis. Immunology 2019, 158, 281–286.
- Naher, L.; Kiyoshima, T.; Kobayashi, I.; Wada, H.; Nagata, K.; Fujiwara, H.; Ookuma, Y.F.; Ozeki, S.; Nakamura, S.; Sakai, H. STAT3 signal transduction through interleukin-22 in oral squamous cell carcinoma. Int. J. Oncol. 2012, 41, 1577–1586.
- Swindell, W.R.; Bojanowski, K.; Chaudhuri, R.K. A zingerone analog, acetyl zingerone, bolsters matrisome synthesis, inhibits matrix metallopeptidases, and represses IL-17A target gene expression. J. Investig. Dermatol. 2020, 140, 602–614.
- Chiricozzi, A.; Nograles, K.E.; Johnson-Huang, L.M.; Fuentes-Duculan, J.; Cardinale, I.; Bonifacio, K.M.; Gulati, N.; Mitsui, H.; Guttman-Yassky, E.; Suárez-Fariñas, M.; et al. IL-17 induces an expanded range of downstream genes in reconstituted human epidermis model. PLoS ONE 2014, 9, e90284.
- Di, T.; Zhai, C.; Zhao, J.; Wang, Y.; Chen, Z.; Li, P. Taxifolin inhibits keratinocyte proliferation and ameliorates imiquimod-induced psoriasis-like mouse model via regulating cytoplasmic phospholipase A2 and PPAR-γ pathway. Int. Immunopharmacol. 2021, 99, 107900.
- Lee, J.; Kim, H.J.; Yi, J.Y. A secretome analysis reveals that PPARα is upregulated by fractionated-dose γ-irradiation in three-dimensional keratinocyte cultures. Biochem. Biophys. Res. Commun. 2017, 482, 270–276.
- Furue, M.; Hashimoto-Hachiya, A.; Tsuji, G. Antioxidative phytochemicals accelerate epidermal terminal differentiation via the AHR-OVOL1 pathway: Implications for atopic dermatitis. Acta Derm. Venereol. 2018, 98, 918–923.
- Furue, M.; Uchi, H.; Mitoma, C.; Hashimoto-Hachiya, A.; Tanaka, Y.; Ito, T.; Tsuji, G. Implications of tryptophan photoproduct FICZ in oxidative stress and terminal differentiation of keratinocytes. G. Ital. Dermatol. Venereol. 2019, 154, 37–41.
- Montero-Vilchez, T.; Segura-Fernández-Nogueras, M.V.; Pérez-Rodríguez, I.; Soler-Gongora, M.; Martinez-Lopez, A.; Fernández-González, A.; Molina-Leyva, A.; Arias-Santiago, S. Skin barrier function in psoriasis and atopic dermatitis: Transepidermal water loss and temperature as useful tools to assess disease severity. J. Clin. Med. 2021, 10, 359.
- Maroto-Morales, D.; Montero-Vilchez, T.; Arias-Santiago, S. Study of skin barrier function in psoriasis: The impact of emollients. Life 2021, 11, 651.
- Katoh, N.; Ohya, Y.; Ikeda, M.; Ebihara, T.; Katayama, I.; Saeki, H.; Shimojo, N.; Tanaka, A.; Nakahara, T.; Nagao, M.; et al. Japanese guidelines for atopic dermatitis 2020. Allergol. Int. 2020, 69, 356–369.
- Salvati, L.; Cosmi, L.; Annunziato, F. From emollients to biologicals: Targeting atopic dermatitis. Int. J. Mol. Sci. 2021, 22, 10381.
- Lueangarun, S.; Tragulplaingam, P.; Sugkraroek, S.; Tempark, T. The 24-hr, 28-day, and 7-day post-moisturizing efficacy of ceramides 1, 3, 6-II containing moisturizing cream compared with hydrophilic cream on skin dryness and barrier disruption in senile xerosis treatment. Dermatol. Ther. 2019, 32, e13090.
- Hebert, A.A.; Rippke, F.; Weber, T.M.; Nicol, N.H. Efficacy of nonprescription moisturizers for atopic dermatitis: An updated review of clinical evidence. Am. J. Clin. Dermatol. 2020, 21, 641–655.
- Miyamoto, K.; Inoue, Y.; Hsueh, K.; Liang, Z.; Yan, X.; Yoshii, T.; Furue, M. Characterization of comprehensive appearances of skin ageing: An 11-year longitudinal study on facial skin ageing in Japanese females at Akita. J. Dermatol. Sci. 2011, 64, 229–236.
- Miyamoto, K.; Dissanayake, B.; Omotezako, T.; Takemura, M.; Tsuji, G.; Furue, M. Daily fluctuation of facial pore area, roughness and redness among young Japanese women; Beneficial effects of Galactomyces ferment filtrate containing antioxidative skin care formula. J. Clin. Med. 2021, 10, 2502.
- Takei, K.; Mitoma, C.; Hashimoto-Hachiya, A.; Takahara, M.; Tsuji, G.; Nakahara, T.; Furue, M. Galactomyces fermentation filtrate prevents T helper 2-mediated reduction of filaggrin in an aryl hydrocarbon receptor-dependent manner. Clin. Exp. Dermatol. 2015, 40, 786–793.
- Howell, M.D.; Kim, B.E.; Gao, P.; Grant, A.V.; Boguniewicz, M.; Debenedetto, A.; Schneider, L.; Beck, L.A.; Barnes, K.C.; Leung, D.Y. Cytokine modulation of atopic dermatitis filaggrin skin expression. J. Allergy Clin. Immunol. 2007, 120, 150–155.
- Kobayashi, J.; Inai, T.; Morita, K.; Moroi, Y.; Urabe, K.; Shibata, Y.; Furue, M. Reciprocal regulation of permeability through a cultured keratinocyte sheet by IFN-gamma and IL-4. Cytokine 2004, 28, 186–189.
- Dębińska, A. New treatments for atopic dermatitis targeting skin barrier repair via the regulation of FLG expression. J. Clin. Med. 2021, 10, 2506.
- Doi, K.; Mitoma, C.; Nakahara, T.; Uchi, H.; Hashimoto-Hachiya, A.; Takahara, M.; Tsuji, G.; Nakahara, T.; Furue, M. Antioxidant Houttuynia cordata extract upregulates filaggrin expression in an aryl hydrocarbon-dependent manner. Fukuoka Igaku Zasshi 2014, 105, 205–213.
- Nakahara, T.; Mitoma, C.; Hashimoto-Hachiya, A.; Takahara, M.; Tsuji, G.; Uchi, H.; Yan, X.; Hachisuka, J.; Chiba, T.; Esaki, H.; et al. Antioxidant Opuntia ficus-indica extract activates AHR-NRF2 signaling and upregulates filaggrin and loricrin expression in human keratinocytes. J. Med. Food 2015, 18, 1143–1149.
- Hirano, A.; Goto, M.; Mitsui, T.; Hashimoto-Hachiya, A.; Tsuji, G.; Furue, M. Antioxidant Artemisia princeps extract enhances the expression of filaggrin and loricrin via the AHR/OVOL1 pathway. Int. J. Mol. Sci. 2017, 18, 1948.
- van den Bogaard, E.H.; Bergboer, J.G.; Vonk-Bergers, M.; van Vlijmen-Willems, I.M.; Hato, S.V.; van der Valk, P.G.; Schröder, J.M.; Joosten, I.; Zeeuwen, P.L.; Schalkwijk, J. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J. Clin. Investig. 2013, 123, 917–927.
- Takei, K.; Mitoma, C.; Hashimoto-Hachiya, A.; Uchi, H.; Takahara, M.; Tsuji, G.; Kido-Nakahara, M.; Nakahara, T.; Furue, M. Antioxidant soybean tar Glyteer rescues T-helper-mediated downregulation of filaggrin expression via aryl hydrocarbon receptor. J. Dermatol. 2015, 42, 171–180.
- Smith, S.H.; Jayawickreme, C.; Rickard, D.J.; Nicodeme, E.; Bui, T.; Simmons, C.; Coquery, C.M.; Neil, J.; Pryor, W.M.; Mayhew, D.; et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J. Investig. Dermatol. 2017, 137, 2110–2119.
- Furue, M.; Nakahara, T. Revival of AHR agonist for the treatment of atopic dermatitis: Tapinarof. Curr. Treat. Options Allergy 2020, 7, 414–421.
- Paller, A.S.; Stein Gold, L.; Soung, J.; Tallman, A.M.; Rubenstein, D.S.; Gooderham, M. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J. Am. Acad. Dermatol. 2021, 84, 632–638.
- Lebwohl, M.G.; Stein Gold, L.; Strober, B.; Papp, K.A.; Armstrong, A.W.; Bagel, J.; Kircik, L.; Ehst, B.; Hong, H.C.; Soung, J.; et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N. Engl. J. Med. 2021, 385, 2219–2229.
- Bissonnette, R.; Stein Gold, L.; Rubenstein, D.S.; Tallman, A.M.; Armstrong, A. Tapinarof in the treatment of psoriasis: A review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J. Am. Acad. Dermatol. 2021, 84, 1059–1067.
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative stress in the skin: Impact and related protection. Int. J. Cosmet. Sci. 2021, 43, 495–509.
- Tsuji, G.; Takahara, M.; Uchi, H.; Takeuchi, S.; Mitoma, C.; Moroi, Y.; Furue, M. An environmental contaminant, benzo(a)pyrene, induces oxidative stress-mediated interleukin-8 production in human keratinocytes via the aryl hydrocarbon receptor signaling pathway. J. Dermatol. Sci. 2011, 62, 42–49.
- Takei, K.; Hashimoto-Hachiya, A.; Takahara, M.; Tsuji, G.; Nakahara, T.; Furue, M. Cynaropicrin attenuates UVB-induced oxidative stress via the AhR-Nrf2-Nqo1 pathway. Toxicol. Lett. 2015, 234, 74–80.
- Bak, D.H.; Lee, E.; Lee, B.C.; Choi, M.J.; Kwon, T.R.; Hong, J.; Mun, S.K.; Lee, K.; Kim, S.; Na, J.; et al. Therapeutic potential of topically administered γ-AlOOH on 2,4-dinitrochlorobenzene-induced atopic dermatitis-like lesions in Balb/c mice. Exp. Dermatol. 2019, 28, 169–176.
- Fuyuno, Y.; Uchi, H.; Yasumatsu, M.; Morino-Koga, S.; Tanaka, Y.; Mitoma, C.; Furue, M. Perillaldehyde inhibits AHR signaling and activates NRF2 antioxidant pathway in human keratinocytes. Oxid. Med. Cell Longev. 2018, 2018, 9524657.
- Hashimoto-Hachiya, A.; Tsuji, G.; Furue, M. Antioxidants cinnamaldehyde and Galactomyces fermentation filtrate downregulate senescence marker CDKN2A/p16INK4A via NRF2 activation in keratinocytes. J. Dermatol. Sci. 2019, 96, 53–56.
- Walshe, J.; Serewko-Auret, M.M.; Teakle, N.; Cameron, S.; Minto, K.; Smith, L.; Burcham, P.C.; Russell, T.; Strutton, G.; Griffin, A.; et al. Inactivation of glutathione peroxidase activity contributes to UV-induced squamous cell carcinoma formation. Cancer Res. 2007, 67, 4751–4758.
- Takei, K.; Takahara, M.; Hachiya, A.; Inoue, K.; Yan, X.; Tsuji, G.; Nakahara, T.; Furue, M. Galactomyces ferment filtrate (Pitera) inhibits UVB-induced ROS production by Ahr/NRrf2/Nqo1 signaling, Aesthet. Dermatol. 2014, 24, 342–350. (In Japanese)
- Cooper, J.K.W.; Koshoffer, A.; Kadekaro, A.L.; Hakozaki, T.; Boissy, R.E. Galactomyces ferment filtrate suppresses reactive oxygen species generation and promotes cellular redox balance in human melanocytes via Nrf2-ARE pathway. J. Clin. Cosmet. Dermatol. 2019, 3, 1.
- Mitamura, Y.; Murai, M.; Mitoma, C.; Furue, M. NRF2 activation inhibits both TGF-β1- and IL-13-mediated periostin expression in fibroblasts: Benefit of cinnamaldehyde for antifibrotic treatment. Oxid. Med. Cell Longev. 2018, 2018, 2475047.
- Schäfer, M.; Farwanah, H.; Willrodt, A.H.; Huebner, A.J.; Sandhoff, K.; Roop, D.; Hohl, D.; Bloch, W.; Werner, S. Nrf2 links epidermal barrier function with antioxidant defense. EMBO Mol. Med. 2012, 4, 364–379.
- Skeate, J.G.; Porras, T.B.; Woodham, A.W.; Jang, J.K.; Taylor, J.R.; Brand, H.E.; Kelly, T.J.; Jung, J.U.; Da Silva, D.M.; Yuan, W.; et al. Herpes simplex virus downregulation of secretory leukocyte protease inhibitor enhances human papillomavirus type 16 infection. J. Gen. Virol. 2016, 97, 422–434.
- Nakajima, A.; Sakae, N.; Yan, X.; Hakozaki, T.; Zhao, W.; Laughlin, T.; Furue, M. Transcriptomic analysis of human keratinocytes treated with Galactomyces ferment filtrate, a beneficial cosmetic ingredient. J. Clin. Med. 2022, 11, 4645.
More
