Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Liana Chafran and Version 2 by Conner Chen.
Natural bio-based monomers derived from plants or animals are widely used in the synthesis of hydrogels and their compounds for the production of biopolymers and biomaterials that are biocompatible, biodegradable, non-toxic and of high porosity, characteristics much sought after in the biomedical field.
- green synthesis
- hydrogel
- biomaterials
- proteomics
1. Bio-Monomers Used in the Synthesis of Hydrogels
Natural bio-based monomers derived from plants or animals are widely used in the synthesis of hydrogels and their compounds for the production of biopolymers and biomaterials that are biocompatible, biodegradable, non-toxic and of high porosity, characteristics much sought after in the biomedical field. These materials can undergo several chemical and physical modifications from the presence of an external stimulus, which makes them extremely versatile materials for the production of drug delivery systems, biochemical sensors, and as the main components in the production of biological scaffolds applicable to the area of tissue regeneration. Among the main natural bio-based monomers of this class are chitosan, glycolic acid, acrylic acid, lactic acid, ethylene glycol, propylene oxide, collagen, fibrin, fibrinogen, platelet-rich plasma, alginate, gelatin, albumin and hyaluronic acid, among others. Table 1 describes some of these natural bio-based monomers and biopolymers and their main applications.
Table 1.
Examples of natural bio-based monomers, their most used copolymers and main applications in the biomedical field.
| Natural Bio-Based Monomers | Polymer-Based Hydrogel | Applications |
|---|---|---|
| Chitosan (CS) |
|
|
| Glycolic acid (GA) |
|
|
| Lactic acid (LA) |
|
|
| Ethylene glycol (EG) |
|
|
| Propylene oxide (PO) |
|
|
| Collagen |
|
|
| Fibrin |
|
|
| Fibrinogen |
|
|
| Alginate |
|
|
| Gelatin |
|
|
| Hyaluronic acid |
|
|
2. Chitosan
Chitosan is an important polysaccharide produced through an alkaline deacetylation of chitin, which is found in crustacean exoskeletons, fungal cell walls and biological materials (Figure 13). Chitosan, by presenting free amino groups in its structure, is a molecule capable of forming stable complexes with metal cations, which makes it an important monomer for obtaining functional biopolymers with wide applicability in the biomedical industry [24][53]. Different methods of preparing chitosan result in different degrees of deacetylation, the distribution of acetyl groups, and changes in the viscosity and molecular weight of the polymer, in addition to promoting distinct degrees of polymerization. These are important parameters that influence not only the solubility of these biopolymers but also their antimicrobial activity, since in an acidic medium the amino groups present in the biopolymer are protonated, resulting in an overall positive charge (-NH3+). Thus, when in contact with the bacterial cell, chitosan is attracted by the negative charge of the bacterial cell wall, thus causing disruption of the cell and altering the membrane permeability. In addition, chitosan can bind to bacterial DNA, causing the inhibition of DNA replication and subsequently cell death. Obtaining chitosan from chitin depends on several factors, such as the reaction temperature, time, and concentration of sodium hydroxide. In common practice, at least 85% deacetylation is acquired for achieving a good solubility of chitosan [25][54].
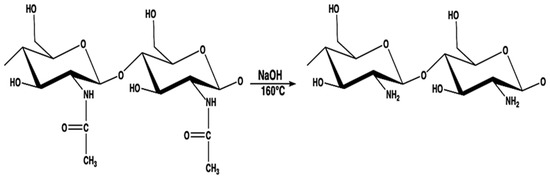
Figure 13.
Deacetylation of chitin to obtain chitosan in alkaline medium.
3. Glycolic Acid (GA)
One of the constituents of sugarcane juice, glycolic acid, has great application in the materials and cosmetics industry, in addition to being an important indicator of hyperoxaluria syndromes characterized by a high predisposition to kidney stone formation. In patients diagnosed with type I hyperoxaluria, an increased urinary excretion of oxalic and glycolic acids is observed. In patients diagnosed with type II hyperoxaluria, there is an increase in oxalic acid and glyceric acid excretion. Thus, the concentration of glycolic acid in biological fluids is used as an index for the differential diagnosis of hyperoxaluria syndromes [26][55].
In a recent study, Dai et al. reported a one-pot synthesis method of producing GA from dihydroxyacetone (DHA) by using a non-noble metal-based catalyst [27][56]. This method also produced other versatile building blocks such as formamides and formates, as can be seen from Figure 24. In the presence of a clean oxidant, such as hydrogen peroxide, carbon–carbon bonds in DHA were selectively transformed to GA. The yields of GA were reported to be around 85% at room temperature.
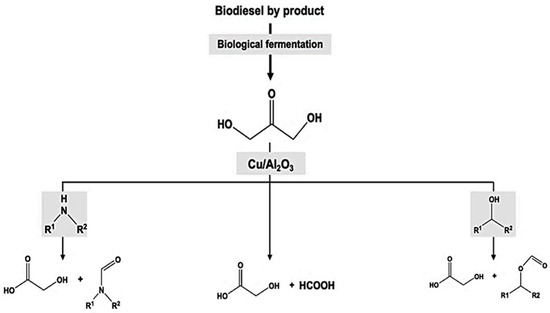
Figure 24. Illustrative diagram of one-pot synthesis method of producing GA, formamides and formates from 1,3-dihydroxyacetone catalyzed by Cu/Al2O3.
Generally combined with lactic acid, glycolic acid is part of one of the most versatile copolymers in the biomedical industry: poly(lactic acid-co-glycolic acid) (PLGA). PLGA is a highly biodegradable and biocompatible aliphatic polyester used in drug delivery, tissue regeneration and growth, implants and fractures [28][57]. The polymerization process to obtain PLGA can take place from two polymeric routes: the polycondensation of lactic acid and glycolic acid to obtain a low-molecular-weight copolymer, or polymerization by opening cyclic dimers of lactic acid and glycolic acid, whose copolymers generally have a high molecular mass, resulting in better mechanical properties [29][58].
4. Acrylic Acid (AA)
Glycerol is an essential biomass-derived raw material and a significant byproduct of biodiesel production. In recent years, studies have tried to explore different conversion routes to synthesize various chemicals. Glycerol is abundantly available and compatible with principles of green chemistry because it is a biodegradable and sustainable raw material. One of the critical conversion processes is the dehydration–oxidation of glycerol to acrylic acid (AA) [30][59]. Acrylic acid polymers have a large number of hydrophilic groups in their structure, mainly hydroxyl groups and carboxylic acids. The hydrophilicity of these polymers makes them excellent candidates for hydrogel synthesis. The high water-retention capacity of acrylic acid-based polymers may be associated with the formation of hydrogen bonds involving the hydroxyl groups of the polymer main chain, which favors the formation of a continuous and strong network capable of retaining large amounts of water in its internal structure [31][60]. The synthetic route for obtaining AA usually occurs in a two-bed fixed-bed reactor system catalyzed by Cs2.5H0.5PW12O40 supported on Nb2O5 (CsPW-Nb). Furthermore, it is still possible to obtain AA via catalytic oxidation from vanadium–molybdenum oxides supported on vanadium−molybdenum mixed oxides supported on silicon carbide (VMo-SiC) [32][61].
A combination of a single bed with mixed catalysts and two beds loaded separately with the catalysts was also studied. It was observed that for single-bed configuration a yield of only 25% for AA was achieved, whereas a higher yield of 75% was obtained for AA in the two-bed system. Both catalysts, CsPW-Nb and VMo-SiC, had similar reaction conditions and oxygen ratios. An overall reaction pathway of the dehydration–oxidation of glycerol to acrylic acid is shown in Figure 35.
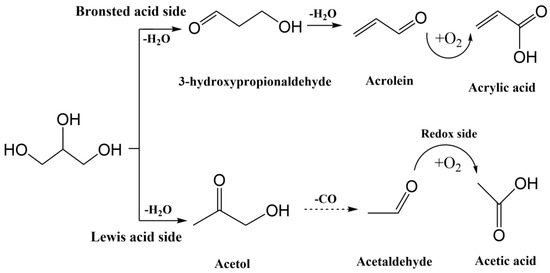
Figure 35. Proposed overall reaction pathways for glycerol dehydration representing the 1st and 2nd dehydration as well as tautomerization, according to the acrolein formation pathway.
5. Lactic Acid (LA)
Lactic acid is widely used in pharmaceuticals, cosmetics, food products, the dairy industry and polymer industries. It is generally used to produce many biodegradable polymers, such as polylactic acid, or as part of copolymers such as glycolic acid, ethylene glycol, ethylene and styrene, among others [33][34][62,63]. Lactic acid can be found in two optically active forms—D-lactic acid and L-lactic acid—as well as its enantiomeric form, D-L-lactic acid. The polymers from their polymerization have different physicochemical properties depending not only on the type of reaction applied to obtain them, but also on the relative percentage of their isomers in the polymer chain. When compared to glycolic acid polymers, lactic acid generally forms polymers with a low rate of degradation, good tensile strength and high mechanical strength, which makes it an excellent biomedical support [35][64]. The conventional chemical synthesis of lactic acid is carried out at high temperatures using costly metallic catalysis, such as catalysts based on tin, zinc, aluminum and lead [36][65]. It can also be produced by the microorganism-based fermentation of different sugars such as glucose, fructose, and cellulose. In recent years, the chemical synthesis of lactic acid from sugars and glycerol has attracted considerable attention. In this regard, Li et al. studied a green chemoenzymatic cascade synthesis scheme to transform C1 compounds (e.g., formaldehyde) into lactic acid [37][66]. In this method, a newly identified variant of formolase is used in the presence of sodium hydroxide as a catalyst. An overall yield of around 83% was obtained with 100% atom economy in ambient conditions, and the formation of dihydroxyacetone (DHA) as an intermediate product. The reaction scheme can be seen in Figure 46.
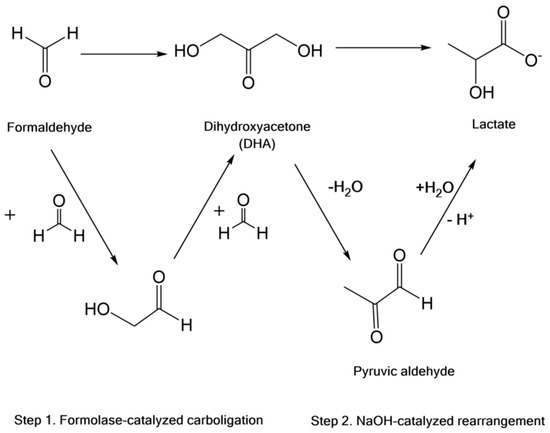
Figure 46.
General two-step synthesis of lactate from formaldehyde.
6. Ethylene Glycol (EG)
Ethylene glycol (EG) is generally obtained from the reaction of ethylene oxide with water under basic or acid catalysis at neutral pH under elevated temperatures. However, EG can also be produced from biomass through several routes. Important routes include converting bio-oil, sugarcane, or corn stover to EG through multiple steps. Another method is the direct conversion of cellulosic biomass to glucose, which is then subsequently converted to EG. Common lignocellulosic biomass such as corn stalk, popular wood, and miscanthus are commonly used. In this route, several catalysts have been used, such as Ni-W/SBA-15, which gives a high yield of around 76%. Similarly, superior performance was exhibited by the Raney Ni–tungstic acid catalyst, which possesses great potential for the commercial-scale conversion of cellulosic biomass [38][67]. The conversion scheme of biomass to EG is shown in Figure 57.
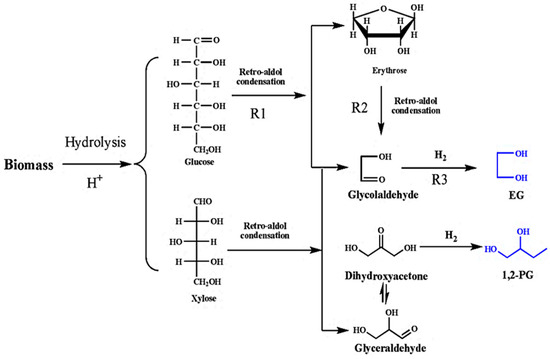
Figure 57.
Illustrative diagram of the reaction for conversion of cellulose and hemicellulose to EG.
7. Propylene Oxide (PO)
Propylene oxide (PO) is considered one of the most important chemical compounds in the world because it is an intermediate compound used to produce different products such as polyether polyols for the polyurethane industry, cosmetics, agricultural products, lubricants, antifoam agents, adhesives and inks, among others [39][68]. Usually combined with ethylene oxide, propylene oxide produces hydrogels with hydrophobic groups on its surface and is extremely useful in the area of bioengineering, since its high protein resistance reduces the non-specific adsorption of proteins on different surfaces [40][69]. PO can be produced from two main routes: the oxidation of isobutane or ethylbenzene to the corresponding hydroperoxide, which involves the usage of ethylbenzene hydroperoxides or tert-butyl to oxidize propylene, or from the chlorohydrin process, where propylene is combined with chlorine in the presence of water and a base, generating PO and large quantities of salts as byproducts. Although these synthetic routes are the most applied around the world for obtaining PO, they mostly occur in the presence of toxic organic solvents with low recoverability. Chen and Beckman developed a one-pot green route to epoxidize propylene producing PO using in situ-generated hydrogen peroxide [41][70]. The process was carried out in the presence of a 0.2% Pd.02% Pt catalyst supported over TS-1 zeolite using liquid or supercritical carbon dioxide as a solvent. A yield of 23% for PO with a selectivity of 82% was obtained at a temperature of 60 °C using ammonium acetate as an inhibitor which resulted in an effective suppression in the production of reaction byproducts. The conversion scheme observed by Chen and Beckman is illustrated in Figure 68.
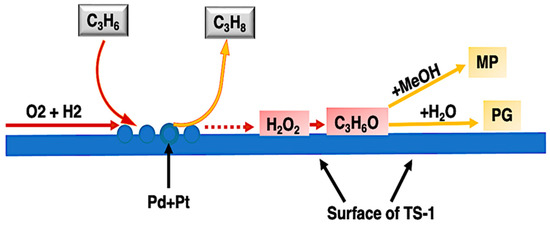
Figure 68. Illustrative scheme of a one-pot green route to produce propylene oxide catalyzed by 0.2% Pd-0.02% Pt catalyst supported over TS-1 zeolite using liquid or supercritical carbon dioxide as a solvent.
