Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Md. Ferdous Rahman and Version 2 by Dean Liu.
Hydrogen-based energy can play a vital role in this aspect. This energy is green, clean, and renewable. Electrochemical hydrogen devices have been used extensively in nuclear power plants to manage hydrogen-based renewable fuel. Doped zirconate materials are commonly used as an electrolyte in these electrochemical devices. These materials have excellent physical stability and high proton transport numbers, which make them suitable for multiple applications. Doping enhances the physical and electronic properties of zirconate materials and makes them ideal for practical applications.
- perovskite oxide
- proton-conducting oxide
- zirconate
1. Introduction
As a result of the Industrial Revolution and technological advancements, the globe requires alternative energy sources to supply the ever-increasing demand for energy [1][2][3][1,2,3]. In addition, With the rapid depletion of fossil fuel resources and the negative impact of fossil fuel combustion on theour environments [4][5][6][7][4,5,6,7], scientists have turned their attention to other renewable sources, such as electrochemical hydrogen devices based on proton-conducting materials [8][9][10][11][8,9,10,11]. Proton conductors typically have positively charged protonic species, such as H+, H3O+, and NH4+ [12][13][12,13]. Proton-conducting materials provide higher conductivity at lower temperatures with longer lifetimes and less expense than traditional oxide ionic electrolyte conductors [14][15][14,15]. In addition, these conductors lose conductivity at higher temperatures due to reversible or irreversible loss of carriers [16]. These characteristics enable these materials to operate at narrow ranges of temperature.
Proton conductors can be used in various electrochemical energy devices, such as batteries, fuel-cell electrolytes, water electrolyzers’ membrane, hydrogen pumps, hydrogen sensors, and hydrogen gas separation systems [17][18][19][20][17,18,19,20]. Organic polymer, inorganic oxides, and lattice defect oxides are examples of the different types of proton conductors. Compared to the other proton conductors, lattice defect-type oxides, i.e., perovskite-type proton-conducting oxides, are the promising proton conductors due to having the highest proton conductivity and chemical stability within desired temperatures [12][21][22][12,21,22]. A typical chemical formula of a perovskite proton conductor is ABO3 (A = Ba, Ca, Sr, etc.; B = Zr, Ce, Tb, Th, etc.) [23][24][23,24]. In addition, perovskite materials have higher conversion efficiency and are less expensive than other proton conductors [25][26][25,26]. These unique properties of perovskite materials have increased their utility in renewable energy applications, especially in solar cells [27]. Among different types of perovskite proton-conducting materials, zirconate materials are the most widely studied/used due to their high chemical stability and excellent proton conductivity [16][28][29][30][16,28,29,30].
Zirconate materials such as BaZrO3-based materials are considered promising proton-conducting materials and are widely used in chemical and electrical sectors. However, many studies have shown that cerate-based proton conductors such as BaCeO3 have high proton conductivity among perovskite-based materials [12]. The drawback of BaCeO3-based materials is that they are unstable in CO2 and water vapor atmospheres, making them unsuitable for applications [31][32][31,32]. In contrast, BaZrO3-based proton conductors are stable in CO2 and water vapor environments which are attractive properties for electrochemical device application in harsh atmospheres [25]. Moreover, BaZrO3-based materials have better physical properties, including chemical stability and higher mechanical hardness than BaCeO3-based proton-conducting material [33]. Ken Kurosaki et al. reported that BaZrO3 exhibits high thermal conductivity due to the high strength between Zr and O [34]. However, the BaZrO3-based proton conductor’s proton conductivity is lower than the BaCeO3-based proton conductor, which can be improved by doping with trivalent cations such as Gd3+, Y3+, In3+, Yb3+ [35][36][35,36]. Pergolesi et al. have reported that Y3+ doped in BaZrO3 enhances chemical stability, but the poor sinterability increases grain-boundary resistance, which is responsible for reducing proton conductivity [37]. Therefore, the sintering temperature must be increased with decreased grain-boundary resistance to improve electrical properties in zirconate-based proton conductors [38]. Recent research has shown that In-doped zirconate-based perovskite proton conductors exhibit better sintering activities with excellent chemical stability [39]. Consequently, experiments with different doping concentrations and synthesis methods are used to develop high-performing doped BaZrO3 material.
Zirconate materials have low thermal conductivity, low dielectric loss, and very low thermal expansion coefficient [16][40][41][16,40,41], making them more favorable for electrochemical devices than other proton-conducting oxide materials. Furthermore, compared to other proton-conducting materials in hydrogen sensors, zirconate-based hydrogen sensors have been demonstrated to be affordable, portable, and temporally correct due to their high chemical stability, smaller dimensions, and cheapness [16][42][16,42]. Hydrogen can be separated in zirconate-based proton conductors in a controlled way simply by changing the applied current in the electrochemical cell; thus, they can be utilized as hydrogen pumps [16]. Zirconate proton conductors can be used as membrane separators at high temperatures, enabling them to act as a sensitive tritium monitor system [43]. Such a device is helpful in removing inference from radionucleotides and concentrating tritium, since it can operate like an electrochemical hydrogen isotope pump [43]. In addition, tritium release has been reported in zirconate proton-conducting material spheres as far back as 30 years ago, and scientists are making more advancements in that technology [44][45][46][47][48][49][44,45,46,47,48,49].
2. Proton-Conducting Zirconates
Perovskite proton-conductor oxides, i.e., zirconates and cerate-based materials, are well-known proton conductors for electrochemical device applications due to their excellent physical properties [14][50][14,50]. BaZrO3 is a promising zirconate proton conductor widely used in refractory and electrical sectors. This material has excellent stability in a harsh environment, low proton migration, high melting temperature, high thermal expansion coefficient, excellent structure, and mechanical properties at high temperatures [51][52][51,52]. Furthermore, BaZrO3 does not show any phase transition between low and high temperatures, making it suitable for electrochemical devices, including tritium monitoring systems, tritium recovery systems, hydrogen sensors, and hydrogen pumps [46][53][54][46,53,54]. Although cerate-based proton conductor like BaCeO3, has the highest proton conductivity among other proton-conductor materials, it is unstable in water vapor and CO2 atmosphere, whereas BaZrO3 materials show stability in harsh weather (water vapor and CO2) [16]. Alkaline earth zirconates, such as those found in CaZrO3, BaZrO3, and SrZrO3, are typically more chemically stable and have more mechanical strength than alkaline earth cerate ceramics [55][56][55,56]. Many studies have shown that doping with BaZrO3 can enhance proton conductivity and high chemical stability. The general formula of doping zirconate is AZr1-xDxO3−δ, where trivalent dopant D is used to replace the tetravalent Zr to create oxygen vacancy, which is crucial for proton-conduction perovskite (ABO3) lattice structure [57]. The proton conductivity of the BaZrO3 is greatly affected by the type and amount of the dopant used in the barium zirconate. With increasing Zr materials, the electrolyte sintering temperature is also increased, and as a result, the ionic conductivity is decreased [58]. Moreover, BaZrO3 has high grain-boundary resistance which hinders electrochemical applications. Therefore, to improve the proton conductivity, it is essential to maintain a minimum grain-boundary resistance and high sintering temperature [59][60][59,60]. Studies have shown that Y-doped BaZrO3 (BaZr1−xYxO3−δ) exhibits excellent chemical stability with high proton conductivity [61]. For example, Liu et al. investigated BaZr1−xYxO3−δ electrolyte by partially replacing Zr4+ with neodymium (Nd3+) to enhance the sinterability and conductivity of the electrolyte [62]. The results showed that BaZr0.7 Nd0.1YxO3−δ had higher proton conductivity than BaZr1−xYxO3−δ electrolyte and that Nd3+ doping increased the chemical stability. However, neodymium (Nb) is a rare-earth element and expensive, which is not feasible for commercial application. On the other hand, mixed BaCeO3-BaZrO3 with dopant shows higher chemical stability but enriched Zr, restricting applications due to poor sintering and high grain-boundary resistance [63][64][63,64]. Therefore, further modification is required in zirconate to improve its proton conductivity with suitable stability for electrochemical application.3. Electrochemical Hydrogen Device
Electrochemical devices are an essential scientific innovation enabling the development of an electric vehicle for the future. The principles of electrochemistry have materialized in hydrogen storage [65], hydrogen sensor [66], and hydrogen compressor [67] applications, as well as different chemical sensor applications. The basic electrochemical hydrogen devices have the following components: anode (electrode), electrolyte (proton-conducting solid), and cathode (electrode) (Figure 12) [68]. Electrochemical hydrogen devices use two fundamental principles: electromotive force (EMF) and the hydrogen transport phenomenon of the electrolyte. Recently, electrochemical devices have extensively used proton-conducting zirconates [16]. The small radius of protons enables the ions to fit into the interlayer structure of the cathode.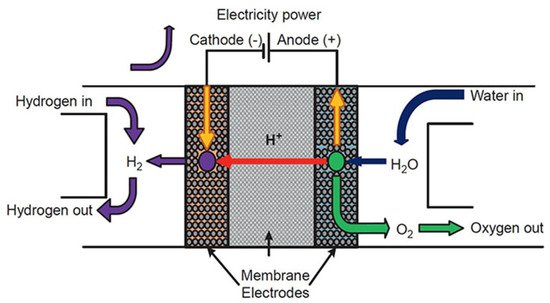
Figure 12. The fundamental design and operation of a proton-exchange membrane (PEM)-based electrochemical hydrogen device. Reprinted with permission from Ref. [68]. Copyright 2019 Elsevier.
