Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Masutaka Furue and Version 3 by Dean Liu.
Galactomyces ferment filtrate (GFF) is such a functional ingredient. Its use originated from the empirical observation that the hands of sake brewers who deal with yeast fermentation retain a beautiful and youthful appearance. Consequently, skincare products based on GFF are widely used throughout the world.
- Galactomyces ferment filtrate PiteraTM
- aryl hydrocarbon receptor
- NRF2
1. Introduction
The skin is a vital organ that protects the bodies of terrestrial animals from the effects of dry harsh environments. It also acts as a functional barrier against external mechanical, chemical, and climatological stresses [1][2][1,2]. For example, exposure to ultraviolet (UV) rays and environmental pollutants induces varying degrees of oxidative stress in the skin and the subsequent production of proinflammatory cytokines [3][4][5][6][3,4,5,6]. Low-grade chronic inflammation is a significant risk factor for the type of accelerating aging known as inflammaging [7][8][7,8]. An aged skin appearance and a corresponding histological frailty are aggravated in sun-exposed areas of skin compared with those protected from sunlight [9]. Therefore, the inhibition of oxidative stress by daily applications of suitable antioxidants might be beneficial in retarding skin inflammaging induced by various environmental oxidative stress factors [10][11][10,11].
The barrier function of skin is mainly provided by its outermost epidermal layer, the stratum corneum or cornified layer [1][2][1,2]. The human epidermis is composed of multiple layers of keratinocytes, including basal, spinous, granular, and cornified layers. Keratinocytes proliferate in the basal layer, move up through the spinous and granular layers, and die, but remain functional as corneocytes in the cornified layer, before finally detaching from the skin [1][2][1,2]. Corneocytes are the major components of the cornified layer. However, other biological materials, including the extracellular lamellae of lipids, such as ceramides and cholesterol, and various natural moisturizing factors (NMFs), including free amino acids, pyrrolidone carboxylic acids, lactates, glucose, urea, hyaluronic acid, and electrolytes, are essential for maintaining a healthy skin–water balance [1][2][12][1,2,12]. During the differentiation process from the basal to the cornified layer, keratinocytes sequentially produce epidermal differentiation complex proteins, such as involucrin, loricrin, and filaggrin [13]. The integration of these proteins into cytoskeletal keratin fiber is essential for the proper differentiation of keratinocytes into corneocytes [1][2][12][13][1,2,12,13]. The degradation of filaggrin by proteolytic enzymes, such as caspase-14, in the granular layer is also pivotal in the production of NMFs [1][2][12][13][14][15][16][17][1,2,12,13,14,15,16,17].
The differentiation of keratinocyte is coordinately regulated by various transcription factors, including the aryl hydrocarbon receptor (AHR) [18][19][18,19], OVO-like 1/2 (OVOL1/2) [20][21][22][23][20,21,22,23], MYC [22][23][22,23], NOTCH1 [22][24][22,24], CEBP [25][26][25,26], and PPAR [27][28][27,28]. The expression or activation of these transcription factors is modulated by certain inflammatory cytokines, phytochemicals, and UV-mediated oxidative stress [29][30][31][32][33][34][35][36][37][38][29,30,31,32,33,34,35,36,37,38]. For instance, the expression of filaggrin is downregulated by the interleukins IL-4 and IL-13, which are pathogenic for atopic dermatitis, as well as by IL-17A, which is pathogenic for psoriasis [29]. These might contribute, at least in part, to the dry barrier-impaired skin lesions in atopic dermatitis and psoriasis [39].
In general, dry barrier-impaired skin exhibits a decrease in skin hydration and an increased rate of transepidermal water loss (TEWL) [39]. The topical application of a moisturizer increases skin hydration and decreases TEWL [39][40][39,40]; therefore, skin moisturization is recommended as a basic treatment, especially for atopic dermatitis and senile xerosis [41][42][43][44][41,42,43,44]. It is known that skin moisturization is an important factor in facial skin’s ability to maintain a youthful and healthy appearance [45]. Moreover, antioxidative moisturizers can decrease facial redness and reduce pore dilation [46].
2. Activation of AHR-Filaggrin Axis by GFF
AHR is a ligand-dependent transcription factor that is pivotal in upregulating the expression of filaggrin and other differentiation complex proteins in the epidermis [18][19][18,19]. In its steady, nonstimulated condition, AHR resides in the cytoplasm of keratinocytes [47][55]. Upon stimulation by GFF, activated AHR translocates into the nucleus from the cytoplasm (Figure 1), where it upregulates the expression of filaggrin [47][55].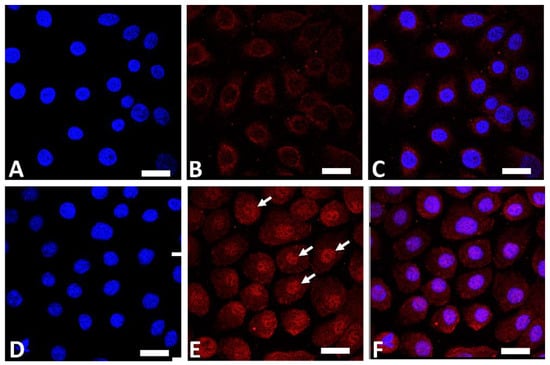
Figure 1. Immunofluorescence staining of AHR (red fluorescence) in human keratinocytes. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). Nonstimulated control keratinocytes ((A); DAPI staining, (B); AHR staining, (C); merged). GFF-treated keratinocytes ((D); DAPI staining, (E); AHR staining, (F); merged). AHR resides mainly in the cytoplasm in nonstimulated keratinocytes (B). GFF induces nuclear translocation of AHR ((E), arrows). AHR: aryl hydrocarbon receptor. GFF: Galactomyces ferment filtrate. Bar; 25 μm.
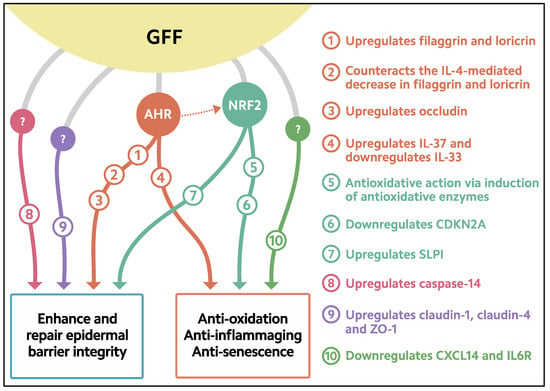
Figure 2. Biological response induced in keratinocytes treated with GFF. GFF: Galactomyces ferment filtrate. AHR: Aryl hydrocarbon receptor. NRF2: Nuclear factor erythroid 2-related factor 2. CDKN2A: cyclin-dependent kinase inhibitor 2A. CXCL14: chemokine (C-X-C motif) ligand 14. IL6R: IL-6 receptor. SLPI: secretory leukocyte peptidase inhibitor.
3. Antioxidative Properties of GFF
The skin is continuously exposed to various oxidative stressors, such as UV radiation, environmental pollutants, and inflammatory cytokines such as tumor necrosis factor-α (TNF-α) [55][61][62][63][64][64,70,71,72,73]. These oxidative stressors generate reactive oxygen species (ROS) in skin cells. To ameliorate oxidative damage, excess amounts of ROS require neutralization by an antioxidative system. Nuclear factor erythroid-2-related factor 2 (NRF2) is the antioxidative master transcription factor [10]. Like AHR, nonstimulated NRF2 is mainly located in the cytoplasm of keratinocytes [63][65][72,74]. Upon stimulation, the activated NRF2 translocates from the cytoplasm into the nucleus, where it upregulates the transcription of genes for antioxidative enzymes such as glutathione peroxidase 2 (GPX2), NAD(P)H quinone oxidoreductase 1 (NQO1), and heme oxidase 1 (HMOX1), which are responsible for neutralizing excess ROS [63][66][67][68][56,72,75,76]. For example, GPX2 is known to play a critical role in preventing UVB-mediated carcinogenesis in keratinocyte [67][75]. In addition to its AHR-stimulating properties, GFF induces nuclear translocation of NRF2 in the cytoplasm (Figure 3) and upregulates the expression of GPX2, NQO1, and HMOX1 [10][65][66][68][69][10,56,74,76,77].
Figure 3. Immunofluorescence staining of NRF2 (green fluorescence) in human keratinocytes. Nonstimulated control keratinocytes ((A); DAPI staining, (B); NRF2 staining, (C); merged). GFF-treated keratinocytes ((D); DAPI staining, (E); NRF2 staining, (F); merged). NRF2 mainly resides in the cytoplasm in nonstimulated keratinocytes (B). GFF induces nuclear translocation of NRF2 ((E), arrows). DAPI: 4′,6-diamidino-2-phenylindole. NRF2: nuclear factor erythroid-2-related factor 2. GFF: Galactomyces ferment filtrate. Bar; 25 μm.

Figure 4. Immunofluorescence staining of ROS (green fluorescence) in human keratinocytes. Nuclei were stained with DAPI. ROS production is minimized in nonstimulated control keratinocytes. IL-13 induces significant ROS production (arrow), which is ameliorated in the presence of GFF. Bar; 25 μm. DAPI: 4′,6-diamidino-2-phenylindole. ROS: reactive oxygen species. GFF: Galactomyces ferment filtrate.
