Atmospheric aerosols, produced as a consequence of different anthropogenic and natural processes, impart significant control over the global energy budget, climate, and human-environmental health. Their size varies across the nano-micrometer scale. Based on their origin, they may be classified into primary or secondary aerosols. Biomass burning, incomplete combustion of fossil fuels, volcanic eruptions, and traffic-related and wind-driven suspensions contribute to primary aerosol emissions. In contrast, gas-to-particle conversion within the atmosphere leads to secondary particle production. The study of atmospheric aerosols is vital to the field of atmospheric research. The dynamic nature (highly variable concentration composition and size with space and time) of aerosols makes them difficult to investigate. Today, aerosol research involves the application of various spectrometric and spectroscopic techniques. The single-particle analysis of aerosol is yet a challenge. TIn this review, the merits and demerits of various offline and online techniques used for aerosol research are discussed in a nutshell. Mass spectrometric techniques fail in distinguishing certain species.
- aerosols
- global climate
- single-particle analysis
- Raman spectroscopy
- surface-enhanced Raman
1. Introduction
| Offline Techniques | Online Techniques |
|---|---|
| Scanning electron microscopy–energy-dispersive X-ray (SEM-EDX) Micro-Raman spectroscopy (MRS) High-resolution transmission electron microscopy (HRTEM) X-ray photoelectron spectroscopy (XPS) Nano-scale secondary ion mass spectrometry (Nano SIMS) Time-of-flight SIMS (TOF-SIMS) X-ray absorption fine structure spectroscopy (XAFS) X-ray absorption near-edge structure (XANES) spectroscopy Electron energy loss spectrometry (EELS) Proton-induced X-ray emission (PIXE) Single-particle inductively coupled mass spectrometry (SP-ICPMS) Laser microprobe mass spectrometry (LMMS) |
Aerosol time-of-flight mass spectrometer (ATOFMS) Laser-induced breakdown spectroscopy (LIBS) Aerosol mass spectrometry (AMS) |
2. Ambient Aerosol Studies
A large number of studies are available based on the characteristics of ambient aerosols. However, most of them are limited to bulk analysis. To obtain insights into the effect of aerosols on climate, human, and environmental health, it is recommended to carry out a single-particle analysis of ambient aerosols [6]. Due to the dynamic nature of ambient particulates, it is always a challenge to decipher their physical and chemical characteristics. Micro-Raman spectroscopy (MRS) provides the opportunity to rapidly analyze aerosol particles at the microscopic scale. The non-destructive nature of this technique [21] makes it a potential tool for studying ambient aerosols. Aerosols may contain noxious components that are detrimental to human health [22]. The capability of an airborne particle to cause health issues is directly correlated with its size. Particles that have a size of <10 µm are found to cause the most health problems. This is due to their ability to penetrate deeply into the lungs and even into the bloodstream. Thus, size-segregated particle analysis is necessary to identify the role of particulates in causing respiratory and cardiovascular dysfunction. Earlier studies have established a potential link between soot exposure and respiratory and cardiovascular diseases. MRS is a powerful technique for the size-segregated characterization of carbonaceous particulates. A study employed this technique to effectively characterize the different aerosol fractions obtained from an urban atmosphere [23]. The prevalence of soot particles (1323 cm−1 and 1582 cm−1) in finer aerosol fractions, such as PM < 1 and 1–2.5 µm, was noted. Oceans contribute significantly to the total aerosol concentration in the atmosphere [24]. However, limited information exists on the chemical composition of the emitted aerosols. The MRS analysis of the aerosols collected during a long (11,000 km) cruise between Chile and the USA indicated the dominance of long-chained organic compounds [25]. These compounds were identified by their intense doublet peaks occurring at ~2880 cm−1 and ~2850 cm−1 from C–H stretching. Source apportionment studies focused on the long-distance transport of aerosols have effectively used MRS. A study over Kozani, Northern Greece [26] showed the existence of various mineral phases in the collected aerosols, such as calcite (1084, 710, 274, and 149 cm−1), gypsum (1134, 1006, and 413 cm−1), titanium oxide (627, 390, and 148 cm−1), feldspar (510, 474,285, 265, 150, and 109 cm−1), lepidolite (1573, 1347, 728, 382, and 288 cm−1), and smectite (1379, 731, 385, 288, and 141 cm−1). The presence of lepidolite (mica) and smectite (clay) was associated with the long-distance transport of Saharan dust. Acid precipitation is mainly influenced by the availability of acidic components (mainly sulfate and nitrates) in fine atmospheric aerosols. It can occur over less polluted areas due to the long-distance transport of aerosols from industrialized regions. In this respect, MRS was effective in characterizing PM1 samples over a mountain environment subjected to acid precipitation because of the transport of aerosols from industrialized regions [27]. The Raman spectrum could successfully reveal the dominance of sulfate ion (~1000 cm−1) species in the aerosols. Another study used this technique to monitor aerosol samples collected from an urban location [28]. The Raman spectrum indicated the dominance of particles with metal oxides (Fe2O3) coated with carbonaceous species. The study further showed that altering the laser beam intensity can unveil the species distribution in a particle. For example, at a low beam intensity (5%), only G (1361.5 cm−1) and D (1576.5 cm−1) bands of carbonaceous species were noted, whereas at a high beam intensity (50%), the strength of bands (G and D) of carbonaceous species decreased, with a corresponding enhancement in the peaks of Fe2O3. A large volume of studies worldwide have found that various ecosystems face a significant threat from microplastic pollution [29]. Most of these studies are mainly concentrated on marine and freshwater environments. Only a few studies are available indicating the contamination of air with microplastics [30]. The available literature has highlighted the use of the MRS technique to detect microplastics [31][32][33]. Recently, MRS was applied for the analysis of microplastics in air samples collected from a metropolitan area in Hamburg, Germany [34]. The spectra of collected microplastic fragments and fibers revealed polyethylene terephthalate (PET) and polyethylene (PE). Different microplastics can be identified from a pure sample or a mixture from their characteristic peak. The peaks at 1000 cm−1, 1720 cm−1, 1059 cm−1, 695 cm−1, and 402 cm−1 correspond to PS, PET, PE, polyvinyl chloride (PVC), and polypropylene (PP), respectively [35].3. Surface-Enhanced Raman Spectroscopy (SERS)
Traditionally, the most preferred methods for the chemical characterization of aerosols involve the use of mass spectrometric techniques. This is due to their capability in detecting species present at trace (ng/L) concentrations [36]. These techniques are, however, costly and time-consuming. Because of the dynamic nature of the aerosols, it is always better to analyze samples rapidly after collection. The mass spectrometric technique often falls short for the rapid analysis of samples, as it requires pre-sample processing [36]. These steps may alter the properties of the aerosols. This highlights the necessity of a sophisticated technique that is fast and does not require pre-sample processing. With surface-enhanced Raman spectroscopy (SERS), it is now possible to detect species at the atto-gram to femtogram level in particles [18]. In the SERS technique, the signals emanating from the analytes are amplified to a great extent by the localized surface plasmon resonance (LSPR) produced by the nanoparticle framework in the substrate used for the analysis (Figure 1) [36].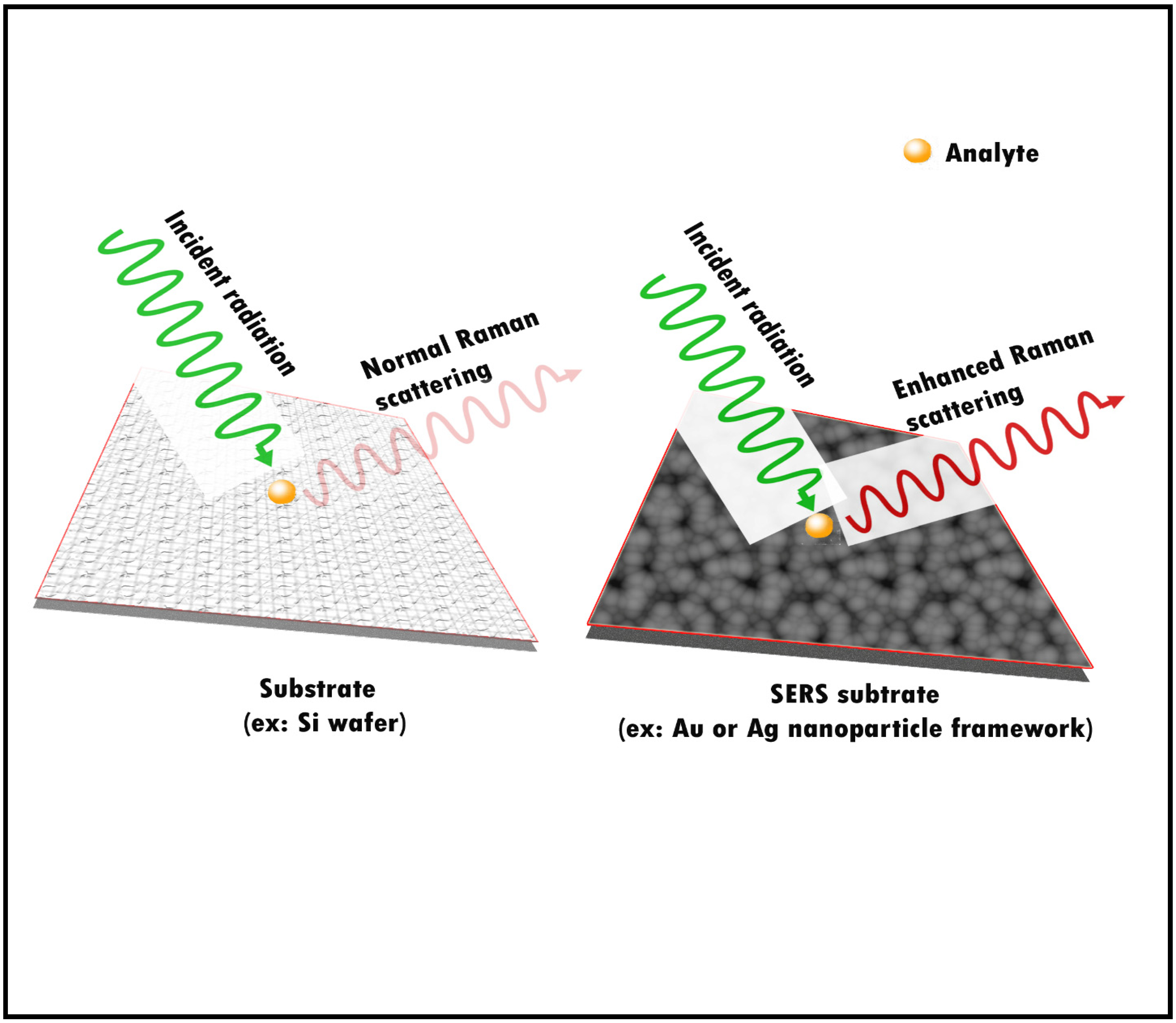
References
- Pöschl, U. Atmospheric Aerosols: Composition, Transformation, Climate and Health Effects. Angew. Chem. Int. Ed. 2005, 44, 7520–7540.
- Williams, J.; de Reus, M.; Krejci, R.; Fischer, H.; Ström, J. Application of the variability-size relationship to atmospheric aerosol studies: Estimating aerosol lifetimes and ages. Atmos. Chem. Phys. 2002, 2, 133–145.
- McMurry, P.H. A review of atmospheric aerosol measurements. Atmos. Environ. 2000, 34, 1959–1999.
- Finlayson-Pitts, B.; Pitts, J. Chemistry of Upper and Lower Atmosphere; Academic Press: Cambridge, MA, USA, 2000.
- Pratt, K.A.; Prather, K.A. Mass spectrometry of atmospheric aerosols—Recent developments and applications. Part II: On-line mass spectrometry techniques. Mass Spectrom. Rev. 2012, 31, 17–48.
- Elmes, M.; Gasparon, M. Sampling and single particle analysis for the chemical characterisation of fine atmospheric particulates: A review. J. Environ. Manage. 2017, 202, 137–150.
- Laskin, A.; Cowin, J.P. Automated Single-Particle SEM/EDX Analysis of Submicrometer Particles down to 0.1 μm. Anal. Chem. 2001, 73, 1023–1029.
- Mäkelä, J.M.; Hoffmann, T.; Holzke, C.; Väkevä, M.; Suni, T.; Mattila, T.; Aalto, P.P.; Tapper, U.; Kauppinen, E.I.; O’Dowd, C.D. Biogenic iodine emissions and identification of end-products in coastal ultrafine particles during nucleation bursts. J. Geophys. Res. Atmos. 2002, 107, PAR 14-11–PAR 14-14.
- Ma, C.-J.; Kasahara, M.; Höller, R.; Kamiya, T. Characteristics of single particles sampled in Japan during the Asian dust–storm period. Atmos. Environ. 2001, 35, 2707–2714.
- Angyal, A.; Kertész, Z.; Szikszai, Z.; Szoboszlai, Z. Study of Cl-containing urban aerosol particles by ion beam analytical methods. Nucl. Instrum. Methods Phys. Res. B 2010, 268, 2211–2215.
- Cheng, W.; Weng, L.-T.; Li, Y.; Lau, A.; Chan, C.K.; Chan, C.-M. Surface Chemical Composition of Size-Fractionated Urban Walkway Aerosols Determined by X-Ray Photoelectron Spectroscopy. Aerosol Sci. Technol. 2013, 47, 1118–1124.
- Davidson, R.A.; Anderson, D.S.; Van Winkle, L.S.; Pinkerton, K.E.; Guo, T. Evolution of Silver Nanoparticles in the Rat Lung Investigated by X-ray Absorption Spectroscopy. J. Phys. Chem. A 2015, 119, 281–289.
- Sobanska, S.; Falgayrac, G.; Rimetz-Planchon, J.; Perdrix, E.; Brémard, C.; Barbillat, J. Resolving the internal structure of individual atmospheric aerosol particle by the combination of Atomic Force Microscopy, ESEM–EDX, Raman and ToF–SIMS imaging. Microchem. J. 2014, 114, 89–98.
- Riemer, N.; West, M. Quantifying aerosol mixing state with entropy and diversity measures. Atmos. Chem. Phys. 2013, 13, 11423–11439.
- Lee, S.-H.; Allen, H.C. Analytical Measurements of Atmospheric Urban Aerosol. Anal. Chem. 2012, 84, 1196–1201.
- Zangmeister, C.D.; Pemberton, J.E. Raman Spectroscopy and Atomic Force Microscopy of the Reaction of Sulfuric Acid with Sodium Chloride. J. Am. Chem. Soc. 2000, 122, 12289–12296.
- Craig, R.L.; Bondy, A.L.; Ault, A.P. Computer-controlled Raman microspectroscopy (CC-Raman): A method for the rapid characterization of individual atmospheric aerosol particles. Aerosol Sci. Technol. 2017, 51, 1099–1112.
- Craig, R.; Bondy, A.; Ault, A. Surface Enhanced Raman Spectroscopy Enables Observations of Previously Undetectable Secondary Organic Aerosol Components at the Individual Particle Level. Anal. Chem. 2015, 87, 7510–7514.
- Rindelaub, J.D.; Craig, R.L.; Nandy, L.; Bondy, A.L.; Dutcher, C.S.; Shepson, P.B.; Ault, A.P. Direct Measurement of pH in Individual Particles via Raman Microspectroscopy and Variation in Acidity with Relative Humidity. J. Phys. Chem. A 2016, 120, 911–917.
- Tirella, P.N.; Craig, R.L.; Tubbs, D.B.; Olson, N.E.; Lei, Z.; Ault, A.P. Extending surface enhanced Raman spectroscopy (SERS) of atmospheric aerosol particles to the accumulation mode (150–800 nm). Environ. Sci. Process Impacts 2018, 20, 1570–1580.
- Darimont, G.; Gilbert, B.; Cloots, R. Non-destructive evaluation of crystallinity and chemical composition by Raman spectroscopy in hydroxyapatite-coated implants. Mater. Lett. 2004, 58, 71–73.
- Heidam, N.Z.; Christensen, J.; Wåhlin, P.; Skov, H. Arctic atmospheric contaminants in NE Greenland: Levels, variations, origins, transport, transformations and trends 1990–2001. Sci. Total Environ. 2004, 331, 5–28.
- Batonneau, Y.; Sobanska, S.; Laureyns, J.; Bremard, C. Confocal Microprobe Raman Imaging of Urban Tropospheric Aerosol Particles. Environ. Sci. Technol. 2006, 40, 1300–1306.
- Prospero, J. Mineral and sea salt aerosol concentrations in various ocean regions. J. Geophys. Res. Oceans 1979, 84, 725–731.
- Deng, C.; Brooks, S.D.; Vidaurre, G.; Thornton, D.C.O. Using Raman Microspectroscopy to Determine Chemical Composition and Mixing State of Airborne Marine Aerosols over the Pacific Ocean. Aerosol Sci. Technol. 2013, 48, 193–206.
- Iordanidis, A.; Garcia-Guinea, J.; Garas, S.; Asvesta, A.; Triantafyllou, A.G. Application of μRaman Microscopy to the Identification of Individual Airborne Particles: Preliminary Results from Kozani’s Area, Northern Greece. Part. Sci. Technol. 2014, 32, 355–359.
- Vineyard, M.F.; LaBrake, S.M.; Ali, S.F.; Nadareski, B.J.; Safiq, A.D.; Smith, J.W.; Yoskowitz, J.T. Characterization of atmospheric aerosols in the Adirondack Mountains using PIXE, SEM/EDX, and Micro-Raman spectroscopies. Nucl. Instrum. Methods Phys. Res. B 2015, 350, 77–80.
- Gonzalez, L.T.; Longoria-Rodriguez, F.E.; Sanchez-Dominguez, M.; Leyva-Porras, C.; Acuna-Askar, K.; Kharissov, B.I.; Arizpe-Zapata, A.; Alfaro-Barbosa, J.M. Seasonal variation and chemical composition of particulate matter: A study by XPS, ICP-AES and sequential microanalysis using Raman with SEM/EDS. J. Environ. Sci. 2018, 74, 32–49.
- Zhang, S.; Wang, J.; Liu, X.; Qu, F.; Wang, X.; Wang, X.; Li, Y.; Sun, Y. Microplastics in the environment: A review of analytical methods, distribution, and biological effects. Trends Anal. Chem. 2019, 111, 62–72.
- Enyoh, C.E.; Verla, A.W.; Verla, E.N.; Ibe, F.C.; Amaobi, C.E. Airborne microplastics: A review study on method for analysis, occurrence, movement and risks. Environ. Monit. Assess. 2019, 191, 668.
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.; Ribeiro-Claro, P.J. Identification of microplastics using Raman spectroscopy: Latest developments and future prospects. Water Res. 2018, 142, 426–440.
- Schymanski, D.; Goldbeck, C.; Humpf, H.-U.; Fürst, P. Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res. 2018, 129, 154–162.
- Kniggendorf, A.-K.; Wetzel, C.; Roth, B. Microplastics detection in streaming tap water with Raman spectroscopy. Sensors 2019, 19, 1839.
- Klein, M.; Fischer, E.K. Microplastic abundance in atmospheric deposition within the Metropolitan area of Hamburg, Germany. Sci. Total Environ. 2019, 685, 96–103.
- Sobhani, Z.; Al Amin, M.; Naidu, R.; Megharaj, M.; Fang, C. Identification and visualisation of microplastics by Raman mapping. Anal. Chim. Acta 2019, 1077, 191–199.
- Ong, T.T.X.; Blanch, E.W.; Jones, O.A.H. Surface Enhanced Raman Spectroscopy in environmental analysis, monitoring and assessment. Sci. Total Environ. 2020, 720, 137601.
- Fu, Y.; Kuppe, C.; Valev, V.K.; Fu, H.; Zhang, L.; Chen, J. Surface-Enhanced Raman Spectroscopy: A Facile and Rapid Method for the Chemical Component Study of Individual Atmospheric Aerosol. Environ. Sci. Technol. 2017, 51, 6260–6267.
- Hu, J.; Duan, F.; He, K.; Ma, Y.; Dong, S.; Liu, X. Characteristics and mixing state of S-rich particles in haze episodes in Beijing. Front Environ. Sci. Eng. 2016, 10, 12.
- Eom, H.-J.; Gupta, D.; Li, X.; Jung, H.-J.; Kim, H.; Ro, C.-U. Influence of collecting substrates on the characterization of hygroscopic properties of inorganic aerosol particles. Anal. Chem. 2014, 86, 2648–2656.
- He, K.; Yang, F.; Ma, Y.; Zhang, Q.; Yao, X.; Chan, C.K.; Cadle, S.; Chan, T.; Mulawa, P. The characteristics of PM2.5 in Beijing, China. Atmos. Environ. 2001, 35, 4959–4970.
- Sun, Z.; Duan, F.; He, K.; Du, J.; Yang, L.; Li, H.; Ma, T.; Yang, S. Physicochemical analysis of individual atmospheric fine particles based on effective surface-enhanced Raman spectroscopy. J. Environ. Sci. 2019, 75, 388–395.
- Liu, C.; Li, J.; Zhang, Y.; Wang, L.; Deng, J.; Gao, Y.; Yu, L.; Zhang, J.; Sun, H. Widespread distribution of PET and PC microplastics in dust in urban China and their estimated human exposure. Environ. Int. 2019, 128, 116–124.
- Zhang, M.; Liu, Y.; Jia, P.; Feng, Y.; Fu, S.; Yang, J.; Xiong, L.; Su, F.; Wu, Y.; Huang, Y. Ag Nanoparticle-Decorated Mesoporous Silica as a Dual-Mode Raman Sensing Platform for Detection of Volatile Organic Compounds. ACS Appl. Nano Mater. 2021, 4, 1019–1028.
