Mangrove forest is an important coastal ecosystems for blue carbon. Thus, understanding the carbon dynamic in mangrove forests will help the management the ecosystem with climate changes. many research studies have been quantified the potential C storage in mangrove soil to be about 500 Mg C ha−1. However, mangrove also lost about 43.8 Kg CO2-eq ha-1 yr-1 due to its CO2 and CH4 emissions.
- mangrove
- blue carbon
- greenhouse gas
C1. Storage iIntroduction Mangrove Forests
There are several reasons why mangrove forest ecosystems have high ecosystem C stocks. Coastal ecosystems sequester CO2 from the atmosphere through plant primary production and store it in plant biomass (mostly for woody plants) and soil [1]. Although C accumulation rates vary among coastal wetlands, plant primary production in coastal wetlands in general is comparable to that of terrestrial forests [2]. However, the low decomposition rate of soil C gives coastal wetlands a higher potential to sequester C in sediments [2]. Thus, coastal ecosystems are generally believed to accumulate C up to 100 times faster than terrestrial forest ecosystems [3][4][5][6]. Compared to other coastal ecosystems, mangrove forests are believed to have higher organic C stocks because of their high growth rates [7]. Furthermore, unlike the herbaceous salt marshes, where most organic C stocks are stored in soil, C stocks in mangrove forests are distributed more in plant biomass than soil [8]. Previous research found that most mangrove plant-fixed C is stored in biomass and only 3%–11.7% of it is transferred to and stored in sediment [9].
The soil C stored in mangrove forests can vary widely, but it is generally higher in the tropical regions than the sub-tropical ones [8][10][11][12][13] (Table 1). Different environmental and soil physicochemical factors may explain this difference. Different tidal ranges may create different soil anaerobic conditions among mangrove forests, and thus affect C decomposition rates [13][14]. Moreover, fine soil texture in some mangrove forests may also reduce groundwater drainage and facilitate soil C accumulation [15].
2. CO2 and CH4 Emissions in Mangrove Soils
Although mangrove forests provide high ecosystem C stocks, their wide ranges of anoxic soil conditions also make them a considerable source of greenhouse gases and decrease their net contribution to CO2 reduction (Figure 1). In addition, the presence of sulfate (SO42-) in the saline water can serve as an alternative electron acceptor and help soil microbes yield more energy than methanogens, resulting in CO2 efflux in coastal ecosystems [28][29][30]. As a result, the ecosystem respiration rates in tide-influenced coastal forest wetlands are typically higher than those observed in inland freshwater wetlands [31]. The average CO2 emission from mangrove forests was calculated to be 0.7–3 g C m−2 d−1 [32][33][34][35], which is comparable to CO2 emissions from coastal marshes (0.3–2 g C m−2 d−1) [30][36], but slightly higher than those from inland wetlands (0.8–1.6 g C m−2 d−1) [31] (Table 2).
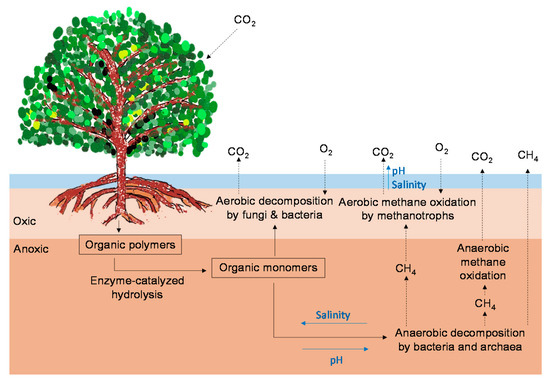
Figure 1. Possible pathways for CO2 and CH4 emissions from mangrove forests (modified from Vepraskas and Craft [37]). The black arrows indicate the C pathways. The blue arrows indicate the direction in which increases in environmental factors (salinity, pH) may affect the C pathways.
Table 2. Comparison of greenhouse gas effluxes across various salinity ranges. (The absence of data means that the study analyzed did not report these data.)
| Study | Ecosystem | Salinity | CO | 2 | Efflux (mg C m | −2 | h | −1 | ) | CH | 4 | Efflux (mg C m | −2 | h | −1 | ) | N | 2 | O Efflux (mg N m | −2 | h | −1 | ) | Global Warming Potential (GWP) (mg CO | 2-eq | m | −2 | h | −1 | ) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [38] | Mangrove (Taiwan) | 0.14 | ||||||||||||||||||||||||||||
| [39] | Mangrove (China) | 31–74 | ||||||||||||||||||||||||||||
| [40] | Mangrove (India) | 0.018–0.034 | ||||||||||||||||||||||||||||
| [10] | Indonesia | Mangrove | 572 | |||||||||||||||||||||||||||
| [41] | Mangrove (Australia) | −11–128 | Malaysia | Mangrove | 1059 | |||||||||||||||||||||||||
| [8 | ||||||||||||||||||||||||||||||
| [42] | Mangrove (Hong Kong) | 15–21 | 10–1,374 | ] | FL, USA | Mangrove | 307 | |||||||||||||||||||||||
| 0.032–0.534 | ||||||||||||||||||||||||||||||
| [43] | Mangrove (Australia) | 17–25 * | 36.9–59.0 | 0–0.06 | 0–0.05 | 136–245 | [19] | Global | Mangrove | 749 | ||||||||||||||||||||
| [44] | Mangrove (China) | 16–267 | [11] | Australia | Mangrove | 66 | ||||||||||||||||||||||||
| [45] | Mangrove (New Caledonia) | Tidal marsh | 87 | |||||||||||||||||||||||||||
| Seagrass | 24 | |||||||||||||||||||||||||||||
| [12] | Brazil | Mangrove | 341 | |||||||||||||||||||||||||||
| Salt marsh | 257 | |||||||||||||||||||||||||||||
| [20] | MD, USA | Salt marsh ( | S. patens | ) | 24 | |||||||||||||||||||||||||
| Salt marsh ( | S. alterniflora | ) | 22 | |||||||||||||||||||||||||||
| [21] | FL, USA | Salt marsh | 72 |
Aboveground and belowground biomass production in mangrove plants is another major contributor to the ecosystem C stocks in mangrove forests. Unlike herbaceous plants, which have a fast C turnover rate, mangrove plants may be able to fix atmospheric CO2 and store it as biomass for a long period of time (i.e., up to centuries); this would lead to a considerable amount of C stock [22]. Mangrove plants have different degrees of root volumes and aboveground structures that may create a wide range of C storage rates [23][24]. Indeed, field surveys from previous studies in Atlantic coastal mangrove forests showed that aboveground plant biomass comprised 50–250 Mg C ha−1 and the belowground biomass comprised 10–50 Mg C ha−1 [8][12].
The abundant C that mangrove forests provide facilitates the development of soil microbial communities. Studies have shown that the microbial genus Bacteroidetes is abundant in the mangrove rhizosphere, which may be due to the high particulate organic matter in the environment [25][26]. Furthermore, the abundant root systems of mangrove plants may create environmental niches for Proteobacteria, one of the important microbial genera for N and S cycling in mangrove ecosystems [26][27].
| 36–44 | ||||||
| [46] | Mangrove (Colombia) | 2.7–23.4 | 0–23.68 | 0.009–0.375 | ||
| [32] | Mangrove (Philippines) | 16.8–79.3 | 108–151 | 0.06–0.12 | 0–0.084 | 396–604 |
| [47] | Mangrove (China) | 12–14 | −9–140 | 0–4.02 | 0–0.016 | −33–889 |
| [48] | Mangrove (China) | 10–21 | 0–55 | 0.35–23.09 | 0–0.017 | 32–2,326 |
| [33] | Mangrove (Vietnam) | 7–16 | Wet season: 112 Dry season: 25 | |||
| [34] | Mangrove (New Caledonia) | 40.2 | 0.22 | |||
| [35] | Mangrove (Australia) | 28 | ||||
| [49] | Mangrove (Australia) | 9–35 * | 0.04–1.18 | 0.004–0.13 | ||
| [50] | Mangrove (China) | 8.4–14.8 | 0.63–4.12 | |||
| [51] | Mangrove (China) | 12–26 | 11–114 | 0–0.17 | ||
| [52] | Mangrove (Indonesia) | 25–34 | −16.8–46.6 | −0.003–0.007 | −0.17–0.37 | −139–344 |
| [30] | Brackish salt marsh (NC, USA) | 22.5 | −45–88 | −0.17–0.23 | −0.046–0.048 | −202–366 |
| [36] | Tidal freshwater wetland (GA, USA) | 0.4–2.1 | 15–59 | 0.04–0.24 | −0.009–0.012 | 54–244 |
| [53] | Rice paddies (Vietnam) | 0–75 | 0–0.132 | |||
| [54] | Rice paddies (China) | 0–630 | ||||
| [55] | Ponds (Sweden) | 0.75–40.50 |
CH4 efflux in coastal wetlands is considerably lower than in freshwater wetlands, mostly because of the presence of SO42- [30][56]. The CH4 fluxes reported from previous literature show a decreasing trend with increasing salinity (Table 2). Compared to other coastal ecosystems, mangrove forests generally emit 0–23.68 mg C m−2 h−1 of CH4 [32][34][39][48][50][51][57], which is generally higher than in brackish marshes (−0.17–0.23 mg C m−2 h−1) [30], but lower than in tidal freshwater marshes (0.01–10.8 mg C m−2 h−1) [36][58] and freshwater ecosystems such as rice paddies (0–630 mg C m−2 h−1) [53][54][59] or ponds (0.75–40.5 mg C m−2 h−1) [55] (Table 2). In addition, species in mangroves with pneumatophores had significantly lower CH4 emission rates than in mangroves without pneumatophores because pneumatophores increase soil aeration [60]. Moreover, anthropogenic nutrient loading from upland drainage also contributes to the high CH4 emission rates [46][52].
CO2 in mangrove soils is generated by chemoheterotrophs during respiration, but the CH4 fluxes are mainly attributed to methane-producing archaea in soils. However, until now, few studies have focused specifically on identifying the quantity, composition, and environmental niches of methanogenic communities in mangrove soils. The soil total organic C concentrations may stimulate CH4 production and increase the mcrA gene expression (i.e., methanogenic population) in soil [61]. Furthermore, studies on other coastal ecosystems also found that methanogens may be sensitive to soil pH and showed optimum growth at soil pH 6.5–7.5 [62][63].
Along with high SO42- concentrations, CH4 efflux can be reduced by methanotrophs in surface mangrove soils that use CH4 as an energy source and oxidize it into CO2 (Figure 1) [64]. This mechanism can reduce CH4 before it reaches the atmosphere [65][66][67]. However, most previous studies on methanotrophs have been performed in freshwater, not coastal, ecosystems. In fact, mangrove soils may have high CH4 oxidation potentials that are comparable to those of freshwater ecosystems, such as rice paddies and lakes [68][69][70][71][72].
Compared to freshwater ecosystems, mangrove forest soils typically contain more Type I methanotrophic communities [71], which are believed to have higher CH4 oxidation potentials, than Type II methanotrophs, which are typically found in freshwater ecosystems [73][74][75]. Moreover, the Type I methanotrophs Methylosarcina, Methylomonas, and Methylobacter in mangrove forest soils contained the most active CH4-oxidizing genes, despite the fact that the dominant methanotrophs in mangrove soils were uncultured and their genes belong to the deep-sea 5 cluster, which is one of the five major sequence clusters retrieved from marine environments [76]. The presence of NaCl in mangrove soils was proven to be one of the reasons why this environmental niche contains more Type I methanotrophs than Type II ones [77]. As shown in a previous study, Methylobacter is better adapted to various salinity conditions and can be found in water with NaCl concentrations up to 3% [78]. In addition, alkaline environmental conditions may also be an important factor influencing the growth of Type I and Type II methanotrophs [72]. Previous studies revealed that the Type I methanotrophs Methylomonas and Methylobacter are mostly adapted to pH 6.5–7.55, which is generally the pH of saline ecosystems [71][78][79]. This ecological niche provided by the coastal mangrove forests may be one of the key factors resulting in the large Type I methanotrophic populations and low CH4 emissions in this ecosystem.
References
- Alongi, M.D; Impact of global change on nutrient dynamics in mangrove forests. Forests 2018, 9, 596.
- Elizabeth McLeod; Gail L Chmura; Steven Bouillon; Rodney Salm; Mats Björk; Carlos M. Duarte; Catherine E. Lovelock; William H Schlesinger; Brian Silliman; A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO 2. Frontiers in Ecology and the Environment 2011, 9, 552-560, 10.1890/110004.
- Pidgeon, E. Carbon Sequestration by Coastal Marine Habitats: Important Missing Sinks; IUCN: Gland, Switzerland, 2009; pp. 47–51.
- Yo-Jin Shiau; Michael R. Burchell; Ken W. Krauss; Stephen W. Broome; Francois Birgand; Carbon storage potential in a recently created brackish marsh in eastern North Carolina, USA. Ecological Engineering 2019, 127, 579-588, 10.1016/j.ecoleng.2018.09.007.
- Chmura, G.L.; Anisfeld, S.C.; Cahoon, D.R.; Lynch, J.C. Global carbon sequestration in tidal, saline wetland soils. Glob. Biogeochem. Cycles 2003, 17.
- Kennedy, H.; Bjork, M. Seagrass Meadows; IUCN: Gland, Switzerland, 2009; pp. 23–30.
- Contrib. Mar. Sci.; Geographic variations in salt marsh macrophyte production: A review. Contrib. Mar. Sci. 1976, 20, 47–68.
- L. T. Simpson; T. Z. Osborne; L. J. Duckett; I. C. Feller; Carbon Storages along a Climate Induced Coastal Wetland Gradient. Wetlands 2017, 37, 1023-1035, 10.1007/s13157-017-0937-x.
- Shi-Bo Li; Po-Hung Chen; Jih-Sheng Huang; Mei-Li Hsueh; Li-Yung Hsieh; Chen-Lu Lee; Hsing-Juh Lin; Factors regulating carbon sinks in mangrove ecosystems. Global Change Biology 2018, 24, 4195-4210, 10.1111/gcb.14322.
- Daniel Murdiyarso; Joko Purbopuspito; J. Boone Kauffman; Matthew Warren; Sigit D. Sasmito; Daniel C. Donato; Solichin Manuri; Haruni Krisnawati; Sartji Taberima; Sofyan Kurnianto; The potential of Indonesian mangrove forests for global climate change mitigation. Nature Climate Change 2015, 5, 1089-1092, 10.1038/nclimate2734.
- Carolyn J. Ewers Lewis; Paul E. Carnell; Jonathan Sanderman; Jeff Baldock; Peter I. Macreadie; Variability and Vulnerability of Coastal ‘Blue Carbon’ Stocks: A Case Study from Southeast Australia. Ecosystems 2018, 21, 263-279, 10.1007/s10021-017-0150-z.
- J. Boone Kauffman; Angelo F. Bernardino; Tiago Ferreira; Leila R. Giovannoni; Luiz Eduardo De Oliveira Gomes; Danilo Jefferson Romero; Laís Coutinho Zayas Jimenez; Francisco Ruiz; Carbon stocks of mangroves and salt marshes of the Amazon region, Brazil. Biology Letters 2018, 14, 20180208, 10.1098/rsbl.2018.0208.
- Udo Nehren; Pramaditya Wicaksono; Mapping soil carbon stocks in an oceanic mangrove ecosystem in Karimunjawa Islands, Indonesia. Estuarine, Coastal and Shelf Science 2018, 214, 185-193, 10.1016/j.ecss.2018.09.022.
- Nóbrega, G.N.; Ferreira, T.O.; Siqueira Neto, M.; Queiroz, H.M.; Artur, A.G.; Mendonça, E.D.S.; Silva, E.D.O.; Otero, X.L. Edaphic factors controlling summer (rainy season) greenhouse gas emissions (CO2 and CH4) from semiarid mangrove soils (NE-Brazil). Sci. Total Environ. 2016, 542, 685–693.
- Michael W. I. Schmidt; Margaret S. Torn; Samuel Abiven; Thorsten Dittmar; Georg Guggenberger; Ivan A. Janssens; Markus Kleber; Ingrid Kögel-Knabner; Johannes Lehmann; David Manning; Paolo Nannipieri; Daniel P. Rasse; Steve Weiner; Susan E. Trumbore; Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49-56, 10.1038/nature10386.
- J. Boone Kauffman; R. Flint Hughes; Chris Heider; Carbon pool and biomass dynamics associated with deforestation, land use, and agricultural abandonment in the neotropics.. Ecological Applications 2009, 19, 1211-1222, 10.1890/08-1696.1.
- Daniel C. Donato; J. Boone Kauffman; Daniel Murdiyarso; Sofyan Kurnianto; Melanie Stidham; Markku Kanninen; Mangroves among the most carbon-rich forests in the tropics. Nature Geoscience 2011, 4, 293-297, 10.1038/ngeo1123.
- Benjamin Thompson; Colin P. Clubbe; Jurgenne H. Primavera; David Curnick; Heather Koldewey; Locally assessing the economic viability of blue carbon: A case study from Panay Island, the Philippines. Ecosystem Services 2014, 8, 128-140, 10.1016/j.ecoser.2014.03.004.
- J. Boone Kauffman; Rupesh K. Bhomia; Ecosystem carbon stocks of mangroves across broad environmental gradients in West-Central Africa: Global and regional comparisons. PLOS ONE 2017, 12, e0187749, 10.1371/journal.pone.0187749.
- Tracy Elsey-Quirk; Denise M. Seliskar; Christopher K. Sommerfield; John L. Gallagher; Salt Marsh Carbon Pool Distribution in a Mid-Atlantic Lagoon, USA: Sea Level Rise Implications. Wetlands 2011, 31, 87-99, 10.1007/s13157-010-0139-2.
- Kara R. Radabaugh; Ryan P. Moyer; Amanda R. Chappel; Christina E. Powell; Ioana Bociu; Barbara C. Clark; Joseph M. Smoak; Coastal Blue Carbon Assessment of Mangroves, Salt Marshes, and Salt Barrens in Tampa Bay, Florida, USA. Estuaries and Coasts 2018, 41, 1496-1510, 10.1007/s12237-017-0362-7.
- Cheryl L. Doughty; Adam Langley; Wayne S. Walker; Ilka C. Feller; Ronald Schaub; Samantha K. Chapman; Mangrove Range Expansion Rapidly Increases Coastal Wetland Carbon Storage. Estuaries and Coasts 2016, 39, 385-396, 10.1007/s12237-015-9993-8.
- Carlos M. Duarte; Inigo Losada; Iris E. Hendriks; Ines Mazarrasa; Nuria Marbà; The role of coastal plant communities for climate change mitigation and adaptation. Nature Climate Change 2013, 3, 961-968, 10.1038/nclimate1970.
- Rebecca S. Comeaux; Mead A. Allison; Thomas S. Bianchi; Mangrove expansion in the Gulf of Mexico with climate change: Implications for wetland health and resistance to rising sea levels. Estuarine, Coastal and Shelf Science 2012, 96, 81-95, 10.1016/j.ecss.2011.10.003.
- J. Pinhassi; Maria Montserrat Sala; Harry Havskum; Marcus Peters; Oscar Guadayol; Andrea Malits; Cã¯â¿â½Lia Marrasã¯â¿â½; Celia Marrase; Changes in Bacterioplankton Composition under Different Phytoplankton Regimens. Applied and Environmental Microbiology 2004, 70, 6753-6766, 10.1128/aem.70.11.6753-6766.2004.
- Gomes, N.C.M.; Cleary, D.F.R.; Pinto, F.N.; Egas, C.; Almeida, A.; Cunha, A.; Mendonca-Hagler, L.C.S.; Smalla, K. Taking root: Enduring effect of rhizosphere bacterial colonization in mangroves. PLoS ONE 2010, 5.
- Thomas J. Lyimo; Arjan Pol; Mike S. M. Jetten; Huub J. M. Op Den Camp; Diversity of methanogenic archaea in a mangrove sediment and isolation of a newMethanococcoidesstrain. FEMS Microbiology Letters 2009, 291, 247-253, 10.1111/j.1574-6968.2008.01464.x.
- Weston, N.B.; Dixon, R.E.; Joye, S.B. Ramifications of increased salinity in tidal freshwater sediments: Geochemistry and microbial pathways of organic matter mineralization. J. Geophys. Res. Biogeosci. 2006, 111.
- Lisa G. Chambers; K. Ramesh Reddy; Todd Z Osborne; Short-Term Response of Carbon Cycling to Salinity Pulses in a Freshwater Wetland. Soil Science Society of America Journal 2011, 75, 2000-2007, 10.2136/sssaj2011.0026.
- Yo-Jin Shiau; Michael R. Burchell; Ken W. Krauss; Francois Birgand; Stephen W. Broome; Greenhouse Gas Emissions from a Created Brackish Marsh in Eastern North Carolina. Wetlands 2016, 36, 1009-1024, 10.1007/s13157-016-0815-y.
- Weizhi Lu; Jingfeng Xiao; Fang Liu; Yue Zhang; Chang'an Liu; Guanghui Lin; Contrasting ecosystem CO2fluxes of inland and coastal wetlands: a meta-analysis of eddy covariance data. Global Change Biology 2017, 23, 1180-1198, 10.1111/gcb.13424.
- Jose Alan Castillo; Armando Apan; Tek N. Maraseni; Severino G. Salmo; Soil greenhouse gas fluxes in tropical mangrove forests and in land uses on deforested mangrove lands. CATENA 2017, 159, 60-69, 10.1016/j.catena.2017.08.005.
- Hien, H.T.; Marchand, C.; Aimé, J.; Cuc, N.T.K. Seasonal variability of CO2 emissions from sediments in planted mangroves (Northern Viet Nam). Estuar. Coast. Shelf Sci. 2018, 213, 28–39.
- Jacotot, A.; Marchand, C.; Allenbach, M. Tidal variability of CO2 and CH4 emissions from the water column within a Rhizophora mangrove forest (New Caledonia). Sci. Total Environ. 2018, 631–632, 334–340.
- Judith A. Rosentreter; Damien T. Maher; Dirk V. Erler; R. Murray; Bradley D. Eyre; Seasonal and temporal CO2 dynamics in three tropical mangrove creeks – A revision of global mangrove CO2 emissions. Geochimica et Cosmochimica Acta 2018, 222, 729-745, 10.1016/j.gca.2017.11.026.
- Krauss, K.W.; Whitbeck, J.L. Soil greenhouse gas fluxes during wetland forest retreat along the Lower Savannah River, Georgia (USA). Wetlands 2012, 32, 73–81.
- Vepraskas, M.J.; Craft, C.B. Wetland Soils: Genesis, Hydrology, Landscapes, and Classification, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015.
- Tsan-Chang Chang; Shang-Shyng Yang; Methane emission from wetlands in Taiwan. Atmospheric Environment 2003, 37, 4551-4558, 10.1016/s1352-2310(03)00588-0.
- D. M. Alongi; J. Pfitzner; L.A. Trott; F. Tirendi; P. Dixon; D.W. Klumpp; Rapid sediment accumulation and microbial mineralization in forests of the mangrove Kandelia candel in the Jiulongjiang Estuary, China. Estuarine, Coastal and Shelf Science 2005, 63, 605-618, 10.1016/j.ecss.2005.01.004.
- Krithika, K.; Purvaja, R.; Ramesh, R. Fluxes of methane and nitrous oxide from an Indian mangrove. Curr. Sci. 2008, 94, 218–224.
- Catherine E. Lovelock; Soil Respiration and Belowground Carbon Allocation in Mangrove Forests. Ecosystems 2008, 11, 342-354, 10.1007/s10021-008-9125-4.
- Guang C. Chen; Nora Fung Yee Tam; Yong Ye; Spatial and seasonal variations of atmospheric N2O and CO2 fluxes from a subtropical mangrove swamp and their relationships with soil characteristics. Soil Biology and Biochemistry 2012, 48, 175-181, 10.1016/j.soilbio.2012.01.029.
- Stephen Livesley; Sascha M. Andrusiak; Temperate mangrove and salt marsh sediments are a small methane and nitrous oxide source but important carbon store. Estuarine, Coastal and Shelf Science 2012, 97, 19-27, 10.1016/j.ecss.2011.11.002.
- Liang Jin; Changyi Lu; Yong Ye; Gongfu Ye; Soil Respiration in a Subtropical Mangrove Wetland in the Jiulong River Estuary, China. Pedosphere 2013, 23, 678-685, 10.1016/s1002-0160(13)60060-0.
- Leopold, A.; Marchand, C.; Deborde, J.; Chaduteau, C.; Allenbach, M. Influence of mangrove zonation on CO2 fluxes at the sediment-air interface (New Caledonia). Geoderma 2013, 202–203, 62–70.
- Dennis Konnerup; Julian Mauricio Betancourt-Portela; Carlos Villamil; Juan Pablo Parra; Nitrous oxide and methane emissions from the restored mangrove ecosystem of the Ciénaga Grande de Santa Marta, Colombia. Estuarine, Coastal and Shelf Science 2014, 140, 43-51, 10.1016/j.ecss.2014.01.006.
- Guangcheng Chen; Bin Chen; Dan Yu; Nora F Y Tam; Yong Ye; Shunyang Chen; Soil greenhouse gas emissions reduce the contribution of mangrove plants to the atmospheric cooling effect. Environmental Research Letters 2016, 11, 124019, 10.1088/1748-9326/11/12/124019.
- Haitao Wang; Guanshun Liao; Melissa D’Souza; Xiaoqing Yu; Jun Yang; Xiaoru Yang; TianLing Zheng; Temporal and spatial variations of greenhouse gas fluxes from a tidal mangrove wetland in Southeast China. Environmental Science and Pollution Research 2016, 23, 1873-1885, 10.1007/s11356-015-5440-4.
- Diane E. Allen; R. C. Dalal; Heinz Rennenberg; Susanne Schmidt; Seasonal variation in nitrous oxide and methane emissions from subtropical estuary and coastal mangrove sediments, Australia. Plant Biology 2011, 13, 126-133, 10.1111/j.1438-8677.2010.00331.x.
- G.C. Chen; Nora Fung Yee Tam; Y.S. Wong; Y. Ye; Effect of wastewater discharge on greenhouse gas fluxes from mangrove soils. Atmospheric Environment 2011, 45, 1110-1115, 10.1016/j.atmosenv.2010.11.034.
- XiaWan Zheng; Jiemin Guo; Weimin Song; Jianxiang Feng; Guanghui Lin; Methane Emission from Mangrove Wetland Soils Is Marginal but Can Be Stimulated Significantly by Anthropogenic Activities. Forests 2018, 9, 738, 10.3390/f9120738.
- Chen, G.C.; Ulumuddin, Y.I.; Pramudji, S.; Chen, S.Y.; Chen, B.; Ye, Y.; Ou, D.Y.; Ma, Z.Y.; Huang, H.; Wang, J.K; et al. Rich soil carbon and nitrogen but low atmospheric greenhouse gas fluxes from North Sulawesi mangrove swamps in Indonesia. Sci. Total Environ. 2014, 487, 91–96.
- Azeem Tariq; Quynh Duong Vu; Lars Stoumann Jensen; Stephane De Tourdonnet; Bjoern Ole Sander; Reiner Wassmann; Trinh Van Mai; Andreas De Neergaard; Mitigating CH 4 and N 2 O emissions from intensive rice production systems in northern Vietnam: Efficiency of drainage patterns in combination with rice residue incorporation. Agriculture, Ecosystems & Environment 2017, 249, 101-111, 10.1016/j.agee.2017.08.011.
- Jingna Liu; Heshui Xu; Ying Jiang; Kai Zhang; Yuegao Hu; Zhao-Hai Zeng; Methane Emissions and Microbial Communities as Influenced by Dual Cropping of Azolla along with Early Rice. Scientific Reports 2017, 7, 40635, 10.1038/srep40635.
- Stadmark, J.; Leonardson, L; Emissions of greenhouse gases from ponds constructed for nitrogen removal. Ecol. Eng. 2005, 25, 542–551.
- Scott D. Bridgham; J. Patrick Megonigal; Jason K. Keller; Norman B. Bliss; Carl Trettin; The carbon balance of North American wetlands. Wetlands 2006, 26, 889-916, 10.1672/0277-5212(2006)26[889:tcbona]2.0.co;2.
- Rita Chauhan; Arindam Datta; A.L. Ramanathan; T.K. Adhya; Factors influencing spatio-temporal variation of methane and nitrous oxide emission from a tropical mangrove of eastern coast of India. Atmospheric Environment 2015, 107, 95-106, 10.1016/j.atmosenv.2015.02.006.
- Karen B. Bartlett; David S. Bartlett; Robert C. Harriss; Daniel I. Sebacher; Methane emissions along a salt marsh salinity gradient. Biodegradation 1987, 4, 183-202, 10.1007/bf02187365.
- Huifeng Liu; Xing Wu; Zongshan Li; Qing Wang; Responses of soil methanogens, methanotrophs, and methane fluxes to land-use conversion and fertilization in a hilly red soil region of southern China. Environmental Science and Pollution Research 2017, 24, 8731-8743, 10.1007/s11356-017-8628-y.
- Yixin He; Wei Guan; Dan Xue; Liangfeng Liu; Changhui Peng; Baowen Liao; Ji Hu; Qiu'an Zhu; Yanzhen Yang; Xu Wang; Guanyi Zhou; Zhongming Wu; Huai Chen; Comparison of methane emissions among invasive and native mangrove species in Dongzhaigang, Hainan Island. Science of The Total Environment 2019, 697, 133945, 10.1016/j.scitotenv.2019.133945.
- Hironori Arai; Ryo Yoshioka; Syunsuke Hanazawa; Vo Quang Minh; Vo Quoc Tuan; Tran Kim Tinh; Truong Quoc Phu; Chandra Shekhar Jha; Suraj Reddy Rodda; Vinay Kumar Dadhwal; Masayoshi Mano; Kazuyuki Inubushi; Function of the methanogenic community in mangrove soils as influenced by the chemical properties of the hydrosphere. Soil Science and Plant Nutrition 2016, 62, 150-163, 10.1080/00380768.2016.1165598.
- Sowers, K.R.; Baron, S.F.; Ferry, J.G; Methanosarcina acetivorans sp. nov., an acetotrophic methane-producing bacterium isolated from marine sediments. Applied and Environmental Microbiology 1984, 47, 971–978.
- Lyimo, T.J.; Pol, A.; Op den Camp, H.J.; Harhangi, H.R.; Vogels, G.D; Methanosarcina semesiae sp. nov., a dimethylsulfide-utilizing methanogen from mangrove sediment. Int. J. Syst. Evol. Microbiol. 2000, 50, 171–178.
- Coyne, M. Soil Microbiology: An Exploratory Approach; Delmar Publishers: New York, NY, USA, 1999.
- Peter Roslev; Gary M. King; Regulation of methane oxidation in a freshwater wetland by water table changes and anoxia. FEMS Microbiology Ecology 1996, 19, 105-115, 10.1111/j.1574-6941.1996.tb00203.x.
- Jean Le Mer; Pierre Roger; Production, oxidation, emission and consumption of methane by soils: A review. European Journal of Soil Biology 2001, 37, 25-50, 10.1016/s1164-5563(01)01067-6.
- Megonigal, J.P.; Schlesinger, W.H. Methane-limited methanotrophy in tidal freshwater swamps. Glob. Biogeochem. Cycles 2002, 16.
- Cai, Y.; Zheng, Y.; Bodelier, P.L.E.; Conrad, R.; Jia, Z. Conventional methanotrophs are responsible for atmospheric methane oxidation in paddy soils. Nat. Commun. 2016, 7.
- Pranitha S. Pandit; Dilip R. Ranade; Prashant K. Dhakephalkar; Monali Rahalkar; A pmoA-based study reveals dominance of yet uncultured Type I methanotrophs in rhizospheres of an organically fertilized rice field in India.. 3 Biotech 2016, 6, 135, 10.1007/s13205-016-0453-3.
- Kirsten Oswald; Jon S Graf; Sten Littmann; Daniela Tienken; Andreas Brand; Bernhard Wehrli; Mads Albertsen; Holger Daims; Michael Wagner; Marcel Mm Kuypers; Carsten J Schubert; Jana Milucka; Crenothrix are major methane consumers in stratified lakes. The ISME Journal 2017, 11, 2124-2140, 10.1038/ismej.2017.77.
- Shiau, Y.J.; Cai, Y.F.; Lin, Y.T.; Jia, Z.; Chiu, C.Y. Community structure of active aerobic methanotrophs in Red Mangrove (Kandelia obovata) soils under different frequency of tides. Microb. Ecol. 2017.
- Yo-Jin Shiau; Yuanfeng Cai; Zhongjun Jia; Chi-Ling Chen; Chih-Yu Chiu; Phylogenetically distinct methanotrophs modulate methane oxidation in rice paddies across Taiwan. Soil Biology and Biochemistry 2018, 124, 59-69, 10.1016/j.soilbio.2018.05.025.
- He, R.; Wooller, M.J.; Pohlman, J.W.; Catranis, C.; Quensen, J.; Tiedje, J.M.; Leigh, M.B; Identification of functionally active aerobic methanotrophs in sediments from an arctic lake using stable isotope probing. Environ. Microbiol. 2012, 14, 1403–1419.
- Dumont, M.G.; Luke, C.; Deng, Y.; Frenzel, P. Classification of pmoA amplicon pyrosequences using BLAST and the lowest common ancestor method in MEGAN. Front. Microbiol. 2014, 5.
- Zheng, Y.; Jia, Z. The application of biomarker genes for DNA/RNA-stable isotope probing of active methanotrophs responsible for aerobic methane oxidation in six paddy soils. Acta Pedol. Sin. 2016, 53, 490–501.
- Claudia Lüke; Peter Frenzel; Potential of pmoA Amplicon Pyrosequencing for Methanotroph Diversity Studies ▿†. Applied and Environmental Microbiology 2011, 77, 6305, 10.1128/AEM.05355-11.
- Adrian Ho; Yongliang Mo; Hyo Jung Lee; Leopold Sauheitl; Zhongjun Jia; Marcus A. Horn; Effect of salt stress on aerobic methane oxidation and associated methanotrophs; a microcosm study of a natural community from a non-saline environment. Soil Biology and Biochemistry 2018, 125, 210-214, 10.1016/j.soilbio.2018.07.013.
- Bowman, J.P. Methylobacter. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015.
- Bowman, J.P. Methylomonas. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015.
