Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Mariacarla Gonzalez and Version 3 by Rita Xu.
Cervical cancer is one of the most significant global health inequities of our time and is the fourth most common cancer in women worldwide, disproportionally affecting developing countries where the disease burden is 84%. Sometimes referred to as a preventable cancer, it progresses slowly, providing a window of time for routine screening in which pre-cancerous lesions can be identified and treated.
- cervical cancer
- low resource setting
- cervical imaging devices
1. Introduction
Cervical cancer is the fourth most common cancer in women. More than half a million women are diagnosed yearly due to persistent human papillomavirus (HPV) infection, with mortality as high as 311,000 [1]. According to the World Cancer Research Fund, developing countries have 84% of the global disease burden and 80% of the mortality due to a lack of effective screening programs [2]. This causes cervical cancer to be an example of global health inequity since the slow-progressing disease provides time for detecting and treating pre-cancerous lesions. Many women in low and middle-income countries (LMICs) seek clinical care once they experience persistent cancer symptoms. In contrast, cervical cancer screening programs in high-income countries have helped reduce mortality significantly [3]. Several screening techniques have been developed and implemented to aid in low-resource cervical screening.
1.1. Anatomy of The Cervix
The cervix is a cylindrical structure that connects the vaginal canal (ectocervix) to the uterus (endocervix). It is 2–3 cm long, composed mainly of epithelium and stroma. There are two main types of epithelia present in the cervix: columnar and stratified squamous. The columnar epithelium is the lining found in the endocervix and secretes mucus. The stratified squamous epithelium is located in the ectocervix and is a continuation of the vaginal epithelium. The location where these two epithelia meet is called the squamocolumnar junction (SCJ). The location of the SCJ varies depending on continuous cervical remodeling, the main factors being age and hormones (e.g., the SCJ is found in the external os in younger women) [4][5][6,7]. The cervix contains a thick stroma layer under both epithelial types, mainly muscular, elastic, and fibrous tissues. The fibrous stroma occupies three areas with unique orientations surrounding the cervical canal. The inner canal and outer cervix are composed of longitudinally aligned collagen, and in between can be found circumferentially aligned collagen [6][8]. Figure 1 illustrates an anatomical representation of the cervix.
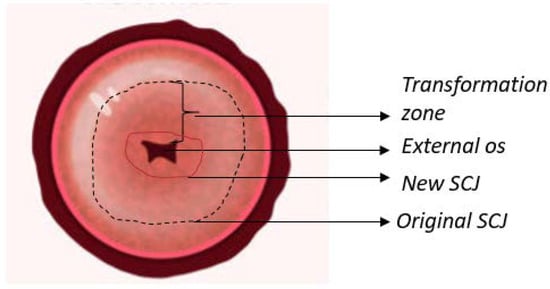
Figure 1. Anatomy of the uterine cervix highlighting the epithelium found on the surface, as well as the transformation zone and squamocolumnar junction (SCJ).
HPV infection is the principal cause of cervical cancer. Types 16 and 18 are responsible for 71% of cases; however, when including HPV types 4, 11, 16, 18, 31, 33, 45, 52, and 58, the percentage of cervical cancer cases due to HPV rises to 90%. HPV is a family of DNA viruses (approximately 15 that can infect the genital tract) that target basal epithelial cells and cause benign and malignant lesions [7][8][9,10]. Common cancers include squamous cells, adenocarcinomas, sarcomas, and small cell neuroendocrine tumors. The immune system clears most infections; if not, the virus proceeds to attack the cells in the cervical SCJ [8][9][4,10,11]. Persistent infection can spread and break through the basal membrane to become an invasive cancer [4].
1.2. Disease Progression
During disease progression, three types of neoplastic states affect the epithelium. Cervical intraepithelial neoplasia (CIN) of first grade or CIN 1, affects one-third of the epithelium and is considered mild and likely caused by a transient HPV infection, which should clear naturally. CIN 2, which affects two-thirds of the epithelium, is a moderate case and combination of self-clearing and pre-cancerous lesions. CIN 3 is considered severe as it affects the whole epithelium; it is a pre-cancer state since the lesions are unlikely to clear up naturally. Using the Bethesda System (classification system used for cytological diagnosis and treatment decisions), CIN 1 is a low-grade squamous intraepithelial lesion (LSIL) and CIN 2 and 3 are high-grade squamous intraepithelial lesions (HSIL). Invasive cervical cancer is a slowly progressing disease, generally taking more than ten years to fully develop from infection [10][11][4,12,13].
Cervical neoplasia is related to changes in both the stroma and epithelial cells [12][13][14][15][14,15,16,17]. Stromal changes stimulate and precede neoplastic progression. Moreover, carcinogenesis results from defective communication between the epithelium and the stroma [13][15][15,17]. The extracellular matrix (ECM) can regulate growth, death, gene expression, and migration, among other processes, all of which regulate physiologic processes such as angiogenesis, tissue morphogenesis, embryonic development, and pathological processes. Furthermore, stroma and tumor cells can exchange growth factors for activating neighboring ECM and aiding the expansion of neoplastic cells [12][14]. The deregulation between the stroma and the epithelium communication promotes carcinogenesis [13][14][15,16]. Neoplastic progression results in changes to the stroma and, therefore, the collagen matrix, which leads to changes in stromal scattering and can be used for optical contrast in the diagnostic measurement of neoplastic tissues [15][17].
1.3. Cervical Testing and Treatment
The standard procedure for cervical cancer diagnosis in the United States includes liquid-based cytology (Pap test) and DNA testing for high-risk HPV. Colposcopy, biopsy, and histological confirmation are performed if abnormal results are obtained. This procedure, however, requires a high level of quality standards, such as trained personnel, medical coverage, and follow-up visits. Therefore, the World Health Organization (WHO) recommends a screen and treat approach, where the primary screening test should be HPV DNA detection every five to ten years after the age of 30 [16][18]. Due to previous recommendations, current screening practices include HPV testing, visual inspection with acetic acid (VIA), and cytology, all followed by treatment. Another commonly used screening option is visual inspection with Lugol’s iodine (VILI), although not explicitly recommended by the WHO. Some of the practices mentioned above cannot be used in the general population; for example, VIA testing is not appropriate for women older than 50 since the transformation zone (where the lesions usually start) moves into the endocervical canal after menopause. The choice of screening techniques depends highly on the local resources, although the latest recommendations by WHO highly recommend the switch from previously mentioned methods to HPV DNA screening due to the objectivity of the test [16][18].
1.3.1. HPV DNA Testing, Cytology, Colposcopy, and Biopsy
Cervical cancer screening in the United States consists of multiple stages. HPV DNA co-testing and cytology (or Pap smear) are the first steps for every cervical cancer diagnosis. A speculum is inserted into the vaginal canal to collect cells from the cervix. The cells are analyzed for abnormality and apparent changes. Cytology results are difficult to score as it has been shown that there is low interobserver agreement. Stoler et al. found only 47.1% agreement in interpreting HSIL for cytology results when comparing the original diagnosis with a quality control group [17][19]. HPV DNA testing determines the presence of high-risk HPV with specificity and accuracy of 55.6% and 75.8%, respectively, and a positive predictive value of 84.8% [18][20].
A second step in the cervical screening is colposcopy when abnormal cells are found (i.e., positive Pap smear). Colposcopy is a visual inspection conducted by trained physicians with a colposcope (a clinical microscope with 3–15 times magnification) that allows for a closer look at the uterine cervix. The accuracy of this procedure is highly dependent on clinicians’ training level and experience. The diagnostic value of the technique has been reported to have high sensitivity (85%). Still, low specificity (69%), meaning the abnormal location can be found, but the severity of the lesion is often inaccurate [19][20][21][22][21,22,23,24].
Furthermore, the interobserver variability for colposcopic data has a kappa value of 0.40 [23][25]. As part of the colposcopy, a biopsy is usually performed where a small portion of the cervix is sampled. Similar to cytology, biopsies have a low interobserver agreement. A study on 2237 cervical histologies showed that the agreement between the original pathologist and the quality control group overlapped only 42.7% of the time for CIN1 cases [17][19].
1.3.2. Visual Inspection
VIA involves applying a 3–5% acetic acid solution to the ectocervix. This application will turn abnormal cells in the epithelium to an opaque white color (referred to as acetowhite), and the tissue is considered VIA positive. These acetowhite lesions are due to the coagulation of proteins in the cells with acetic acid since neoplastic tissue will have a higher protein content than normal tissue. The positive predictive value of VIA is 16.7%, and the negative predictive value of 99%. The specificity and sensitivity are 79.4% and 71.8%, respectively [24][25][26,27]. These results translate to many false positives leading to overdiagnosis and overtreatment.
Another visual inspection technique, VILI, involves applying Lugol’s iodine to the cervical epithelium. This solution reacts with glycogen in normal healthy tissue and turns black upon exposure. In the presence of neoplastic tissue, the glycogen is reduced or absent, and the solution turns the epithelium yellow. The positive predictive value of VILI is 16.8%, and the negative predictive value of 99.7%, resulting in many false positives. The specificity and sensitivity of VILI are 86% and 88%, respectively [25][26][27,28].
Visual inspection for cervical screening suffers from low reproducibility and results in variation depending on the subjectivity of the interpretation of the results [27][29]. It has also been shown that age, parity, menopause, and HPV presence can influence the outcome of visual inspection tests and the level of training of the healthcare providers [28][30]. However, the low cost and real-time results from visual inspection tests make it ideal for the low resource settings and the screen-and-treat approach, especially in areas of high cervical cancer incidence and low medical resources [26][27][29][28,29,31]. To overcome the current screening issues using VIA and VILI, better training of healthcare personnel is needed. Moreover, Raifu et al. recommend specifically better training of personnel on the definition and interpretation of acetowhite lesions of the cervical epithelium in these settings [28][30].
1.3.3. Treatments
The treatments recommended for cervical neoplasia are directed at removing or destroying the transformation zone and abnormal areas found in the cervix. Two main treatment routes include ablation and excision (although there is ongoing research for alternate treatments) [30][32]. Using ablative treatment, the abnormal tissue is destroyed by heating through thermal coagulation or freezing it via cryotherapy. The excisional route removes tissue by large loop excision of the transformation zone (LLETZ) or by cold knife cone (CKC), also known as conization of the cervix [16][18].
1.4. HPV Vaccines
There have been three HPV vaccines available since 2006, although only one is currently used in the United States. Gardasil 9 is a 9-valent vaccine that targets HPV types 6, 11, 16, 18, 31, 33, 45, 52 and 58. The vaccine has an efficacy close to 100% for young adolescents 9–15 years old [31][33]. The vaccine targets infections in anatomical areas other than the cervix (e.g., vulva, penis, anus). Although HPV vaccination has reduced the number of infections in women since its introduction, it does not cover all 15 high-risk HPV types [32][34]. Moreover, it is expensive and difficult to implement in developing countries, leaving screening and treatment of precancerous lesions as the main preventive methods [33][35].
The slow progression of cervical cancer, the anatomic accessibility, and the possible treatment of precancerous lesions make early screening an effective management [10][4,12]. Due to the high costs of traditional cervical screening procedures, several devices have been developed to increase access to cervical testing in the low-resource setting.
2. Cervical Imaging Targeted for Neoplastic Detection
2.1. Callascope
2.1.1. Device
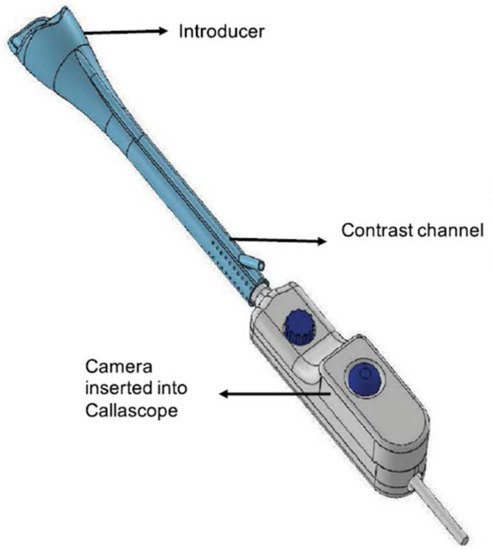
The Callascope is a speculum-free device used for capturing images of the cervix [34][35][36][37][40,41,42,43]. The Callascope was developed at the Department of Biomedical Engineering at Duke University, Durham, NC, USA. The Callascope is designed to create a speculum-free imaging system composed of an introducer and a slender camera. The introducer is a Calla Lily-shaped silicone hollow tube which can be inserted into the vagina (Figure 2) [34][40]. The introducer is approximately 30 mm at the larger proximal end and 12 mm at the distal end [35][41].
The asymmetric tip is designed to allow rotation of the introducer to tilt the cervix into a favorable viewing position. The light source is composed of a ring illuminator with four white LEDs. The camera and housing can be inserted into the introducer to be positioned for imaging the cervix. The camera body is a slim 9 mm diameter tube with a length of approximately 120 mm. The camera is a 2 to 5 Megapixel CMOS sensor with a lens [34][35][40,41]. The camera is fitted with a hydrophobic window at the tip and is positioned in the center of the ring illuminator. The camera is set to a working distance of 25 to 30 mm from the cervix when inserted into the introducer. The Callascope has a field of view of 35 mm. At a working distance of 30 mm and 4× magnification, the smallest resolved feature on a USAF 1951 resolution target was 99.2 µm.
2.1.2. Clinical Testing
Clinic testing of the device has been performed in both the United States and Ghana, looking at two different environments of the Callascope: clinician usage and self-conducted imaging of the cervix [34][40]. Participant eligibility included healthy females 18 years or older. The number of participants in Ghana comprised 25 for clinician testing and 10 for individual usage. In the U.S., 28 participants for clinicians, and 12 were for self-imaging. Participants underwent a pre-exam survey to document demographical information and perceptions using a speculum, Callascope, and clinician vs. self-examination. Post-examination survey was conducted using a modified Universal Pain Assessment tool alongside a written description. Image quality was assessed using one point for visualization of the os and one for each of the four cervical quadrants.
The overall assessment shows a higher preference for the Callascope vs. a standard speculum above 75% in both testing sites (CITE SR 2020). In studies performed by clinicians, the Callascope enabled visualization of the os for 78.6% of U.S. and 80% of Ghana participants. The speculum-based imaging shows the visualization of the os for 96% in the U.S. and 100% in Ghana. Table 1 assesses cervical quadrant visualization for clinician usage [34][40]. Over 60% of participants in both sites found the Callascope easy to insert and use for self-imaging. No patients indicated extreme discomfort, and over 70% of participants stated no or slight pain in the post-examination survey.
Table 1. Callascope cervical quadrant visualization.
| View of at Least Cervical Quadrants | Callascope | |
|---|---|---|
| U.S. | Ghana | |
| [%] | ||
| 2 | 89 | 84 |
| 3 | 72 | 71 |
| 4 | 50 | 44 |