Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Kathrine Holmgaard Bak and Version 2 by Conner Chen.
The volatile composition and odor of meat and meat products is based on the precursors present in the raw meat. These are influenced by various pre-slaughter factors (species, breed, sex, age, feed, muscle type). Furthermore, post-mortem conditions (chiller aging, cooking conditions, curing, fermentation, etc.) determine the development of meat volatile organic compounds (VOCs).
- aroma
- meat
- odor
- dynamic headspace
- GC-O
1. Introduction
Flavor is, obviously, of vital importance for the eating quality of meat. It has been suggested that flavor be defined as a term used to describe the combined influences of taste, odor, the trigeminal system, and touch, with the addition of visual and auditory cues [1]. This is well in line with the ISO definition of flavor, which states that flavor is a “complex combination of the olfactory, gustatory and trigeminal sensations perceived during tasting” [2].
Whereas taste refers to the five basic tastes—sweet, sour, salty, bitter, and umami—odor refers to the aroma elicited by certain volatile compounds [3]. It is well known that raw meat has a bland flavor with little aroma. However, raw meat contains numerous precursors of meat flavor, which result in the formation of volatile odor compounds, especially during cooking [3][4][3,4]. Several hundred or even thousand volatile compounds have been identified in meat from various species [3][4][5][3,4,5]. The odor of meat from different species has many volatile compounds in common [6], though often with quantitative differences between species [4] as well as species-specific odor compounds [7].
The reactions leading to the formation of meat aroma include, for instance, lipid oxidation, Maillard reaction, Strecker degradation, thiamine degradation, and carbohydrate degradation as well the interactions between reaction products, [3] resulting in volatile compounds representative of most classes of organic compounds: hydrocarbons, alcohols, aldehydes, ketones, carboxylic acids, esters, including lactones, ethers, furans, pyridines, pyrazines, pyrroles, oxazoles and oxazolines, thiazoles and thiazolines, thiophenes, and other sulfur- and halogen-containing compounds [8] (as cited by [4]).
When evaluating meat odor, both the qualitative and the quantitative properties must be considered, in addition to the possible synergy between different aroma compounds [4]. It must also be kept in mind that simply because a volatile compound is present in a high concentration does not necessarily mean that it is significant for the odor. The so-called odor activity value (OAV) determines the contribution of a volatile compound to the odor of the food, and is defined as the ratio of the concentration of the volatile compound in the food divided by the odor threshold of the compound in an appropriate matrix (water, air, or even a more complex matrix [9][10][9,10]).
The composition of the food matrix (moisture content, pH, presence of fat and protein, etc.) needs to be given serious consideration when deciding on an appropriate method for analysis, as do the volatile compounds of interest and their potential ranges in the sample [11].
2. Odor Compounds in Meat
2.1. Formation of Odor Compounds
The cooking of meat results in the formation of volatile compounds from water-soluble precursors as well as lipids [5]. The main water-soluble precursors are amino acids, peptides, glycogen, reducing sugars, nucleotides, and thiamine [3][4][5][3,4,5]. The most important reaction pathways are described below, and the main classes of volatile compounds formed during the cooking of meat are shown in Table 1.2.1.1. Maillard Reaction and Strecker Degradation
The Maillard reaction, also known as non-enzymatic browning, is a reaction taking place between a free amino group of an amino acid and the carbonyl group of a reducing sugar [12]. The initial stage of the Maillard reaction is the condensation between the amino group and the reducing sugar to form a glycosylamine, followed by a rearrangement to form either an Amadori compound (if the reducing sugar is an aldose) or a Heyns compound (if the reducing sugar is a ketose) [5][12][13][5,12,13]. The Amadori or Heyns rearrangement products are then dehydrated and degraded via deoxyosones in the intermediate stage [5][13][5,13], generating furanones, furfurals, and dicarbonyl compounds [3][5][3,5]. In the final stage, these compounds react with other reactive compounds such as amines, amino acids, aldehydes, hydrogen sulfide, and ammonia, resulting in the formation of meat flavor compounds belonging to various classes [5]. Related to the Maillard reaction is the Strecker degradation, which may be considered a subset of the Maillard reaction, and is a crucial step for meat flavor generation [14][15]. Strecker aldehydes, which are odor compounds in cooked meat, are formed via the reaction between an amino acid and an α-dicarbonyl compound (e.g., a deoxysone [13]). This reaction generally forms a Strecker aldehyde (which has one less carbon atom than the original amino acid), carbon dioxide, and an α-aminoketone [14][15][15,16]. The α-aminoketones serve as important flavor precursors, leading to the formation of various classes of heterocyclic compounds important for meat flavor [15][16]. Overall, the Maillard reaction and Strecker degradation result in the formation of N-, S-, and O-heterocyclic compounds as well as other sulfur-containing compounds, all of which are important meat flavor compounds [15][16]. The compounds include furans, furanones, methylfuranthiols, imidazoles, pyrazines, pyridines, pyrroles, oxazoles, thiazoles, thiophenes, alkanethiol, alkyl sulfides, and disulfides (see Table 1).Table 1.
The main classes of volatile compounds formed during the cooking of meat.
| Volatile Compound Class | Chemical Structure | Formation | General Odor Descriptors [16][17] 1 |
|---|---|---|---|
| Alcohol | 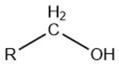 |
Lipid oxidation [5] Carbohydrate fermentation 2 [16][17] |
Saturated alcohols: high threshold. Straight-chain primary alcohols: flavorless. Increase in carbon chain: stronger flavor—greenish, woody, fatty floral. Unsaturated alcohols: mushroom, green leaf, musty. |
| Aldehyde | 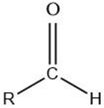 |
Lipid oxidation [5] | C3 and C4: sharp and irritating. C5–C9: green, oily, fatty, tallow. C10–C12: citrus orange peel. Alkyl-branched aldehydes: malty. |
| Carboxylic acid | 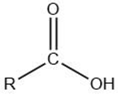 |
Lipid oxidation [5] | Saturated acids: acidic. Unsaturated branched-chain acids: pungent, sour, penetrating. Keto acids: burnt, caramel, sour. |
| Ester | 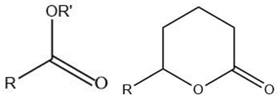 |
Lipid oxidation [5] | Esters from C1–C10 acids: fruity sweet. Esters from long-chain fatty acids: fatty flavor. |
| Ketone | 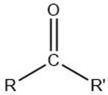 |
Lipid oxidation [5] | Unsaturated ketones: animal fat odors. 2-Alkanones: spicy, fruity, fatty, citrus-like. Lactones: oily, fruity, buttery, fatty. |
| Furan | 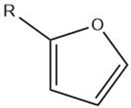 |
Maillard reaction [15][16] Lipid oxidation [5] Carbohydrate degradation [17][14] |
Alkylfurans: grassy, beany odor. |
| Furanone | 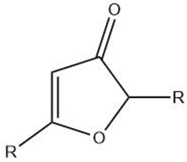 |
Maillard reaction [5] | Fruity, fatty, roasty, roasted almonds, sweet aroma, pungent [11]. |
| Imidazole | 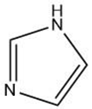 |
Maillard reaction [15][16] | Amine-like [pubchem.org]. |
| Methylfuranthiol | 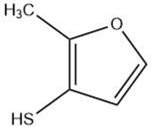 |
Maillard reaction [5] Thiamine degradation [14][15] |
Meaty aroma, roast meat, boiled meat [5][14][5,15]. |
| Oxazole | 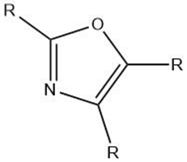 |
Maillard reaction [15][16] Interaction 3 [17][14] |
Green and vegetable-like [15][16]. |
| Pyrazine | 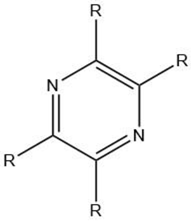 |
Maillard reaction [12] Interaction 3 [17][14] |
Pleasant aroma: nutty roast aroma, earthy, potato-like. |
| Pyridine | 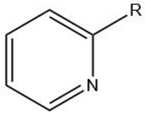 |
Maillard reaction [12] Interaction 3 [17][14] |
Fatty tallow aroma. |
| Pyrrole | 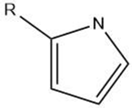 |
Maillard reaction [12] | Burnt earthy odor. |
| Thiazole | 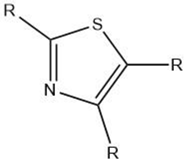 |
Maillard reaction [17][14] Interaction 3 [17][14] |
Green, vegetable-like, nutty, roasted. |
| Thiophene | 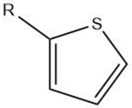 |
Maillard reaction [15][16] Interaction 3 [17][14] Thiamine degradation [12] Nucleotide degradation [17][14] |
Meaty aroma. |
| Alkanethiol | 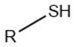 |
Maillard reaction [5] Thiamine degradation [17][14] |
Meat-like, sulfurous, cabbage, onion, garlic-like. Associated with boiled meat. |
| Alkyl sulfide | 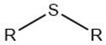 |
Maillard reaction [17][14] Thiamine degradation [17][14] |
|
| Alkyl disulfide | 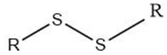 |
Maillard reaction [15][16] Thiamine degradation [17][14] |
1 Odor descriptors from [16][17], unless stated otherwise. 2 Microbial fermentation during the production of dry-cured meat products [16][17]. 3 Interaction signifies the interaction between Maillard reaction products and lipid-derived aldehydes.
The importance of nucleotides for meat flavor development is also via the Maillard reaction [3]. Ribonucleotides are degraded by enzymatic dephosphorylation post-slaughter, which produces ribose from inosine monophosphate and 5′-ribonucleotides [18]. Ribose then participates in the Maillard reaction and is able to form a number of sulfur-containing volatiles [19].
2.1.2. Lipid Oxidation
Lipid oxidation takes place in three stages—initiation, propagation, and termination. It begins with the formation of lipid hydroperoxides, which are then decomposed into peroxyl and hydroxyl radicals. In the termination step, stable compounds are formed. These are then cleaved or polymerized into the final products [17][14]. Cooking promotes lipid oxidation [18], which results in the formation of several types of lipid oxidation and degradation products, including aliphatic hydrocarbons, aldehydes, ketones, alcohols, carboxylic acids, and esters, including lactones in addition to other aromatic compounds such as alkylfurans [5] (see Table 1)—the specific products depending on the original fatty acid [17][14]. As phospholipids contain a significantly higher proportion of unsaturated fatty acids than triglycerides, it is not surprising that phospholipids are the most important lipid source of meat odor compounds [5], with species-specific odor compounds originating from the lipid fraction of the meat [7]. In general, the odor threshold values for the lipid oxidation-derived volatiles are much higher than those for the N- and S-containing compounds generated from the Maillard reaction, and their importance for cooked meat odor is, therefore, minor. Exceptions are the 6–10 carbon-saturated and -unsaturated aldehydes, which contribute important fatty flavors to cooked meat [5]. The interaction between lipid oxidation products and Maillard reaction products seems also to be important for the development of cooked meat flavor. It has long been speculated that lipid oxidation products inhibit the production of pyrazines [20]. It was later found that phospholipids and their degradation products inhibit the formation of certain heterocyclic compounds, importantly, certain S-heterocyclic compounds [15][16], which help balance the generation of cooked meat odor [15][21][16,21]. The aldehydes formed during lipid oxidation can take part in the Maillard reaction at both initial and later stages to form meat odor compounds. However, most of these lipid oxidation–Maillard reaction interaction-derived compounds have weak odor intensities and high odor thresholds, meaning that their importance for cooked meat odor is likely limited [15][16]. During the storage of meat and meat products, an unwanted degree of lipid oxidation known as warmed-over flavor (WOF) may occur and affect meat quality negatively [22][23][22,23]. WOF arises when previously cooked, refrigerated meat is reheated [24], and is characterized by off-odor and off-flavors described most commonly as “stale”, “cardboard”, “rancid”, and “metallic”, which have been related to hexanal, 2,3-octanedione, and total volatiles [25] as well as to trans-4,5-epoxy-(E)-2-decenal and to the loss of desirable furanones [24]. WOF has been ascribed to oxidation, mainly of phospholipids, during refrigerated storage and is catalyzed by both heme- and non-heme iron released during cooking [3][25][26][3,25,26].2.1.3. Carbohydrate Degradation
Upon heating, carbohydrates are degraded as part of the Maillard reaction as described above. However, they can also be broken down via microbial fermentation in fermented meat products to produce organic acids—most notably, lactic acid—as well as volatile compounds in the form of carboxylic acids and linear alcohols [16][17] (Table 1). Carbohydrate (sugar) degradation can also take place by caramelization, but as this only takes place at rather high temperatures, caramelization is only relevant for meat surface areas during grilling or roasting [3][12][3,12].2.1.4. Thiamine Degradation
Thiamine is a sulfur- and nitrogen-containing vitamin [12][14][12,15]. Several studies using model systems have shown that thiamine is an important precursor of meat odor, with thermal degradation depending on temperature, time, pH, and the matrix composition [3]. The thermal degradation of thiamine into the final meat odor compounds is a complex reaction with various degradation pathways and numerous intermediate products before the production of the final meat odor compounds containing sulfur or nitrogen [12]. These include, for instance, alkanethiols, sulfides, and disulfides [17][14] (Table 1). In cooked ham, thiamine has been proven to play a key role in the formation of known meat odorants 2-methyl-3-furanthiol, 2-methyl-3-methyldithiofuran, and bis(2-methyl-3-furyl)disulfide [27]. The 2-methyl-3-methyldithiofuran from thiamine degradation is also a proven meaty off-odor compound in heat-treated fruit juices [28]. However, adding four times the physiological concentration of thiamine to raw beef resulted in no changes to the flavor of cooked beef, presumably due to a lack of phosphate [29], and similar results have been found for chicken [30]. Hence, some authors deduce that thiamine as a meat flavor precursor seems to be most essential for pork flavor [14][15]. Considering that many of the thiamine degradation products are still found as important meat aroma compounds in other species than pork, as well as in dry-fermented pork products (i.e., no heat treatment), it is not surprising that some of these odor compounds can likewise result from the Maillard reaction or Strecker degradation involving sulfur-containing free amino acids [14][15] or as a result of microbial metabolism [31].2.2. Pre-Slaughter Factors Affecting Meat Volatile and Odor Development
Various pre-slaughter factors such as animal species, breed, sex, age, muscle type, and feed affect the generation of meat flavor precursors and, hence, the generation of volatile and odor compounds [3][32][3,32].2.2.1. Animal Species
The water-soluble precursors are responsible for the majority of the volatile organic compounds (VOCs) in cooked meat, as shown above. However, it is the lipid fraction which is responsible for species-specific flavor. Sensory panel studies carried out in 1968 confirmed previous studies on the volatile compounds of cooked meat from different species [33] (according to [7]). Due to their higher degree of unsaturation, the species-specific flavor has mainly been attributed to phospholipids more so than to triglycerides [5][34][5,34], which also explains the species differences in WOF development (in order of decreasing susceptibility: turkey, chicken, pork, beef, mutton) [35]. However, later research points in the direction that species-specific flavor is the result of a complex interaction between the lipid- and water-soluble compounds [5][21][5,21].2.2.2. Animal Breed
The animal breed has been shown to affect total fat content, intramuscular fat content as well as fatty acid composition [32]. For ruminants such as beef and sheep, species is a significant determinant of fatty acid composition, whereas for monogastric animals such as pigs, feed composition plays a much more important role [36]. An effect of breed on cooked meat flavor was found for beef, e.g. [37]. However, not all studies on ruminants found an effect of breed. For example, [38] found no effect of breed on the flavor of cooked lamb, and [39] only found a minor effect of breed on the flavor of grilled beef, but only two breeds were investigated in each study.2.2.3. Sex of the Animal
The effect of the sex of the animal on meat flavor precursors was reviewed by [32], who found that the amount of subcutaneous and intermuscular fat varies between sexes, with female animals generally having more fat. Differences in cooked meat flavor have been found for beef between heifers and bulls [37], whereas no difference was found for wethers and female lamb [40], nor for male and female foals [41]. The effect of castration on meat flavor is well known for pigs in the form of boar taint, which is mainly an unpleasant odor, but also an unpleasant flavor in meat from entire male pigs, caused primarily by androstenone (5a-androst-16-ene-3-one [42]; urine odor [43]) and skatole (3-methyl indole [42]; fecal/manure odor [3][43][3,43]), but also other volatile compounds [43][44][43,44]. Boar taint can be prevented through surgical castration or immunocastration (vaccination) [43]. Castration also affects the flavor of lamb, with the meat from rams having a more intense flavor than meat from wethers [38].2.2.4. Animal Age
As animal age increases, so does the intensity of the cooked meat flavor, as exemplified by the rather bland flavor of veal compared to beef [3] and the much milder flavor of lamb compared to mutton [45]. The flavor-intensity of beef has been found to increase up to 18 months of age, presumably due to age-related changes in the volatile compound precursors related to the increase in fat content and amount of saturated fat [3].2.2.5. Feed
The fatty acid composition of the meat can easily be manipulated through the feed for monogastric animals such as pigs, while doing the same thing with ruminants is more difficult due to the saturating effect of the rumen [46]. However, it is, nevertheless, possible to some degree [47]. The feeding of pigs and poultry to increase the amount of omega-3 fatty acids in the meat can be problematic due to the development of fishy off-odors and off-flavors, which need to be combatted by a higher content of antioxidants in the feed [46]. Meat from sheep contains species-specific short branched-chain fatty acids, particularly 4-methyloctanoic acid, 4-ethyloctanoic acid, and 4-methylnonanoic acid, which are responsible for the characteristic mutton aroma. The content of these short branched-chain fatty acids is influenced by the diet [32]. Feeding sheep grain-based concentrates as opposed to them being raised on pasture has been found to lead to an increase in the amount of short branched-chain fatty acids [48]. The effect of diet on the composition of aroma compounds in ruminant meat was reviewed by [49]. Additionally, here it was found that grain-based diets lead to a higher content of branched-chain fatty acids, in addition to certain aldehydes (e.g., hexanal [48] and other aldehydes originating from linoleic acid [49]) and lactones (δ-tetradecalactone and δ-hexadecalactone [50] according to [49]) as opposed to meat from grass-fed animals, which had higher contents of various phenols [48][49][48,49], terpenes [49], indoles [48][49][48,49], and sulfur compounds [49]. A higher content of terpenes, among other compounds, in grass-fed sheep was reported by [51]. Another study [39] found that feeding beef a cereal-based concentrate led to an increase in linoleic acid decomposition products among the volatile aroma compounds. On the other hand, feeding beef a grass silage-based diet resulted in a higher amount of α-linolenic acid decomposition products [39]. It has also been found that meat from grass-fed beef has a higher concentration of free amino acids than meat from concentrate-fed animals [52], which potentially leads to the generation of more pyrazines and Strecker aldehydes [3]. The accumulation of plant-derived compounds from the feed into the meat has the potential to influence the volatile composition of meat, as reported, e.g., for pork [43] and sheep [45]. It might be possible to determine the feeding background of an animal based on the analysis of the volatile compounds of the raw meat [53] as well as cooked meat, as reviewed by [32].2.2.6. Muscle Type
Flavor intensity as well as off-flavor intensity varies between muscles, as reviewed by [54]. Although differences in flavor intensity between muscles are evident due to biochemical differences [3], it should be mentioned that the differences in beef flavor between muscles are fairly minor [54].2.3. Post-Mortem Factors Affecting Meat Volatile and Odor Development
Post-mortem factors affecting the development of meat flavor include the slaughter process as well as the subsequent carcass handling, chiller aging, cooking, and storage after cooking [55], as well as processing such as curing and fermentation [3] and preservative technologies such as irradiation [56].2.3.1. Chiller Aging
The slaughter process itself affects the early post-mortem biochemical events, including the pH decline and resultant ultimate pH [55], which is important due to the effect of pH on the Maillard reaction [54], including the increase in pyrazine formation at high pH and inhibition at low pH [57]. Additionally, pH affects the activity of the proteolytic enzymes (calpains and cathepsins) [58], with µ-calpain retaining 24–28% of its maximum activity (pH 7.5, 25 °C) when pH is lowered to 5.5–5.8 post-mortem [59]. During chiller aging, also known as conditioning or maturation, changes in tenderness but also meat flavor may occur [3]. The changes in meat flavor are, to a large degree, caused by increases in free amino acids and peptides [60][61][60,61]. Lipid oxidation [62] and nucleotide breakdown [60][63][60,63] also contribute to the flavor development during chiller aging. Nucleotide breakdown importantly includes the breakdown of inosine 5′-monophosphate to ribose [60], as mentioned earlier. As previously described, these flavor precursors take part in the Maillard reaction and Strecker degradation, and produce pyrazines and Strecker aldehydes [3]. Undesirable changes in the volatile composition during storage may be due to microbial spoilage. Certain alcohols, aldehydes, ketones, fatty acids, esters, and sulfur compounds are all potentially developed in meat during storage as a result of microbial spoilage [64]. The exact development of these undesirable volatile compounds depends on the storage conditions (temperature and packaging atmosphere, i.e., air, modified atmosphere, or vacuum) and on the strains of the microorganisms present [64]. Generally, anaerobic microorganisms produce off-odor compounds, which are more unpleasant than the odors produced by aerobic microorganisms [3]. The development of microbial off-odors during storage is illustrated by [64], where it is shown that, for instance, at the early stages of aerobic storage, fruity and dairy-like odors caused by esters and fatty acids develop, whereas these odors are not found during vacuum storage until much later stages of storage [64]. The main microorganisms responsible for microbial spoilage odor include Pseudomonas fragi, Brochothrix thermosphacta, Enterobacteriaceae, lactic acid bacteria (e.g., Carnobacterium spp., Lactobacillus spp., and Leuconostoc spp.), and Clostridium spp. [3][64][3,64]. For further details regarding which volatile compounds responsible for spoilage odor are produced by which bacteria under specific conditions, the reader is referred to [64].2.3.2. Curing
Curing is an old technology used for meat preservation, which also develops desirable cured flavor characteristics in addition to the characteristic cured color [65]. Very simply speaking, curing takes place by the addition of nitrite or nitrate to the meat, the latter then being converted to nitrite by nitrate reductase present in certain microorganisms [65][66][65,66]. Nitrite reacts with the meat pigment myoglobin, ultimately forming nitrosylmyoglobin, which is denatured to the stable nitrosylmyochromogen (stable except for in the presence of both light and oxygen [65]) due to either heat or low pH [66]. Cured meat products can be divided into cooked cured meat products and dry-cured meat products, which have some obvious differences in flavor profiles due to the different processing conditions [3]. It is well known that curing, to a large degree, prevents lipid oxidation [67][68][67,68]. The lack of lipid oxidation products is, at least partially, responsible for cured meat flavor [3]. This is in part due to the strong inhibition of formation of hexanal [3][68][69][3,68,69] and in part due to the general lack of lipid oxidation products, meaning no masking of meaty notes from sulfurous compounds [69]. Cooked, cured meat products, despite seeing a significant reduction in lipid oxidation compared to uncured, cooked meat, still obtain some of their flavor from lipid oxidation products. However, also other reactions related to heat treatment influence the flavor, these being amino acid breakdown, thiamine degradation, and Maillard reaction [69]. Key compounds that have been identified as suppressing the characteristic “cooked ham” odor of cooked, cured ham (as opposed to nitrite-free cooked ham) include 3-methylbutanoic acid (lactic-cheesy odor, origination from amino acid breakdown) and lipid oxidation products 1-octen-3-ol and 1-octen-3-one (mushroom odor), hexanal, octanal, nonanal, 2-nonenal, and 2-decenal (fruity-floral, vegetable, herbaceous, and/or chemical odors) [69]. Dry-cured meat products get their flavor from lipolysis (via lipases), enzymatic oxidation, and proteolysis (via proteolytic enzymes such as calpains and cathepsins) [70] as well as from lipid oxidation [16][18][17,18] and Maillard reaction due to the long ripening time and low water activity [16][17]. Key aroma compounds in dry-cured ham were reviewed by [16][17]. Important aldehydes include hexanal and 3-methylbutanal, while 1-penten-3-ol is an important alcohol. Other important compounds include the nitrogen compounds pyrrole, 2-acetyl-1-pyrroline, dimethylpyrazine, and tetramethylpyrazine, as well as the sulfur compounds dimethyldisulfide, methanethiol, and 2-methyl-3-furanthiol—the latter also known to be produced from the thermal degradation of thiamine [16][17].2.3.3. Fermentation
The addition of starter cultures during the production of fermented meat products results in the formation of certain aroma compounds as a result of the microbial fermentation. Lactic acid bacteria metabolize carbohydrates, resulting in a pH drop due to formation of lactic acid, which makes the taste of fermented meat products rather acidic. Fermentation of carbohydrates as well as lipolysis and proteolysis generate precursors for volatile odor compounds [18][71][18,71]. In addition, chemical reactions in the form of lipid oxidation along with the Maillard reaction and the Strecker degradation contribute to the odor and flavor of fermented meat products. However, the chemical reactions are limited due to processing parameters such as low temperature, low pH, etc. [71]. The important aroma compounds in fermented sausages were reviewed by [71], where it was reported that aldehydes are one of the most important chemical groups with hexanal being the most significant aldehyde, followed by pentanal, octanal, and 2-nonenal (grass, green, herbal odors [16][17]). The most important ketone is 2,3-butandione (buttery odor [16][17]), while carboxylic acids include acetic acid, butanoic acid, and 3-methylbutanoic acid (cheesy notes). Esters in fermented sausages include ethyl butanoate, ethyl 2-methyl butanoate, and ethyl pentanoate (fruity odor and caramel notes). The most important nitrogen compound is reportedly 2-acetyl-pyrroline (nutty, roasted), while low odor threshold-sulfur compounds include diallyl sulfide, diallyl disulfide, dimethyl disulfide, methional, and methionol (garlic, onion, cooked potato, cooked meat). Terpenes (vegetal notes) in fermented sausages originate from the use of spices, while phenolic compounds are formed during the smoking stage [71]. Interestingly, some volatile aroma compounds may have more than one mode of formation. For example, 2-methyl-3-furanthiol is not only produced during the heat degradation of thiamine, but also as a result of the Maillard reaction [31] and microbial metabolism, and has, thus, been detected in fermented meat products [72]. The high free amino acid content, low water activity, low pH, and long drying times are suggested to be responsible for the formation of 2-methyl-3-furanthiol in fermented meat products [31][73][31,73].2.3.4. Irradiation
The irradiation of fresh meat as a way of controlling spoilage and eliminating foodborne pathogens is approved in the United States by the Food and Drug Administration and the United States Department of Agriculture (mainly used on beef, pork, and poultry) [74], and in the EU by certain member states (mainly used on poultry, offal, frog legs, and animal by-products) [75][76][75,76]. The method is quite efficient. However, irradiated odors described along the lines of “pungent”, “rancid”, “rotten egg”, “sulfur”, “bloody”, etc., may develop due to lipid oxidation and degradation of sulfur-containing amino acids [3][56][3,56]. The most important volatile compounds formed include the hydrocarbons 1-heptene and 1-nonene (influenced mainly by irradiation dose), the aldehydes propanal, pentanal, and hexanal (influenced mainly by packaging atmosphere, i.e., with or without oxygen), and various sulfur-containing compounds such as dimethyltrisulfide and bismethylthiomethane (from the degradation of sulfur-containing amino acids) [56]. In order to prevent the development of off-odors due to irradiation, the use of low temperature, vacuum or inert gas packaging, and the addition of antioxidants are viable options [56].2.3.5. Cooking
The course of the Maillard reaction depends on the nature of the food product in question, i.e., moisture content, pH, reactants involved, and cooking temperature. The rate of the Maillard reaction increases with increasing temperature and decreasing moisture levels, meaning that the formation of the Maillard reaction compounds primarily takes place in the areas of the food that have been dehydrated by the cooking process [77]. According to [21], the cooking method is undoubtedly the most important extrinsic factor influencing the generation of volatile aroma compounds in meat. It has been shown that roasting enhances the formation of sulfur and carbonyl compounds, while stewing produces an entirely different aroma profile with odor compounds belonging to a variety of classes [3]. The cooking method additionally affects the formation of WOF. Microwave heating produces a higher degree of WOF volatiles than pan frying, grilling, or boiling. This has been attributed to the fact that grilling and pan-frying produce Maillard reaction products on the cooked surface, masking the WOF, or perhaps a retardation of the production of the volatiles responsible for WOF [78]. In addition to cooking method, cooking temperature also significantly influences the generation of cooked meat odor volatiles [3]. For example, it has been shown that pan-frying pork at 150 °C leads to domination of lipid-derived volatile compounds, whereas pan-frying at 250 °C leads to formation of predominantly Maillard reaction-derived volatiles [79]. The type of fat used for cooking, not surprisingly, influences the generation of cooked meat aroma. Frying meat in an oil with a high content of polyunsaturated fatty acids such as sunflower oil produces a high content of aliphatic aldehydes and more heterocyclic Maillard reaction compounds than cooking in olive oil, butter, or lard [80]. When frying in olive oil, which contains a higher proportion of monounsaturated fatty acids than sunflower oil, the volatile profile is dominated by lipid-derived volatiles. Frying in butter results in a high amount of high-carbon ketones, while frying in lard results in certain Strecker aldehydes and dimethyl sulfide found only in these samples [80]. The cooked meat aroma and flavor are further influenced by added spices [18] and smoking [3], the latter contributing compounds such as lactones and numerous different phenols to the volatile profiles of meat products [81].2.4. Key Odor Compounds in the Meat of Various Species
Key odor compounds in cooked meat of different species were recently reviewed by [12] for beef, pork, chicken, and lamb. An overview is shown in Table 2. Generalized odor descriptors for compounds from the various chemical classes can be found in [16][17] and are listed in Table 1.Table 2. Types and relative contents of characteristic odor compounds in different thermally processed meats detected by gas chromatography-olfactometry [12] *.
| Classification | Volatile Compound | Relative Content (µg/g) |
Odor Descriptors | |
|---|---|---|---|---|
| Beef | Aldehydes | Hexadecanal | 81.41 | Cardboard [82] |
| [82][83][82,83] | Nonanal | 5.39 | Fat, citrus [82][83][82,83] | |
| Hexanal | 2.08 | Grass, fat [82][83][82,83] | ||
| Benzaldehyde | 0.12 | Almond, burnt sugar [82] | ||
| Alcohols | Z-9-octadecen-1-ol | 0.34 | Fatty, animal 1 | |
| 1-octen-3-ol | 0.16 | Mushroom [82][83][82,83] | ||
| Ketones | 3-Hydroxy-2-butanone | 0.70 | Buttery, creamy, fatty, sweet [84] | |
| 2-Octadecanone | 0.55 | Green 1 | ||
| Carboxylic acids | Hexanoic acid | 0.89 | Sweat [82] | |
| 2,4-Hexadienoic acid | 0.21 | Acrid [85] | ||
| Nonanoic acid | 0.03 | Fatty, cheese [86] | ||
| Esters | Ethyl acetate | 50.58 | Pineapple [82] | |
| Ethyl 9-hexadecenoate | 0.18 | Fruity [87] | ||
| Furans | 5-Methyl-2-acetylfuran | 0.71 | Nutty [88] | |
| Tetrahydrofuran | 0.66 | Butter, caramel [89] | ||
| Heterocyclic | 3,5-Diethyl-1,2,4-trithiocyclopentane | 2.85 | Beef aroma [90] | |
| Pork [91][91[92],92] |
Aldehydes | Nonanal | 2.86 | Fatty, floral, wax [93] |
| Benzaldehyde | 2.53 | Bitter almond [93] | ||
| Octanal | 1.97 | Fatty, pungent [93] | ||
| Trans-2-nonenal | 1.47 | Cucumber, farinaceous, greasy, grassy [94][95][94,95] | ||
| Heptanal | 1.25 | Fatty, putty [93] | ||
| Hexanal | 0.95 | Green, grass [93] | ||
| Alcohols | 3-Methyl-1-butanol | 3.10 | Pungent [96] | |
| Hexanol | 1.11 | Woody, cut grass, chemical-winey, fatty, fruity, weak metallic [54] | ||
| 1-Octen-3-ol | 0.83 | Mushroom [93] | ||
| 3-Methyl-3-buten-1-ol | 0.34 | Sweet fruity 1 | ||
| Ketones | 2-Butanone | 0.83 | Chemical, burnt, gas, chocolate [97] | |
| 2-Heptanone | 0.80 | Citrus, grapefruit, floral, fruity, spicy, cinnamon [54][98][54,98] | ||
| Esters | γ-Butyrolactone | 0.96 | Creamy, pleasant, sweet [84] | |
| Ethyl 2-methylbutanoate | 0.35 | Fruity, strawberry-like [99] | ||
| Carboxylic acids | Hexanoic acid | 0.81 | Goaty [11] | |
| Nonanoic acid | 0.25 | Fatty, cheese [86] | ||
| Sulfur compounds | Methional | 1.74 | Cooked potato, roasted [100] | |
| Dimethyl disulfide | 1.24 | Moldy, pungent, rubbery, onion-like [101] |
||
| Pyrazines | 2,5-Dimethyl pyrazine | 0.24 | Nutty, musty, earthy, roasted, cocoa powder [102] | |
| Furans | 2-Pentylfuran | 1.29 | Green bean, butter [54] | |
| Chicken | Aldehydes | P-methoxybenzaldehyde | 20.90 | Anisic 1, hawthorn-like [103] |
| [104][105][106][104,105,106] | Benzaldehyde | 9.88 | Almond, bitter almond, burnt sugar [82][93][82,93] | |
| Nonanal | 0.73 | Fatty, citrus, floral, wax [82][83][93][82,83,93] | ||
| Alcohols | 1-Octen-3-ol | 0.06 | Shiitake mushroom [106] | |
| Ketones | P-methoxypropiophenone | 0.39 | Musty, anisic 1 | |
| Esters | Trans vinyl cinnamate | 0.92 | NR 2 | |
| Furans | 2-Pentylfuran | 0.81 | Green bean, butter [54] | |
| 2-Acetylfuran | 0.21 | Butter, meaty [103] | ||
| Lamb | Aldehydes | Hexanal | 109.23 | Apple, leaf, delicate [107] |
| [107][108][107,108] | Heptanal | 31.32 | Nutty, fruity green [107] | |
| (E)-2-nonenal | 30.09 | Fatty, paper [103] | ||
| Nonanal | 18.25 | Fatty, rancid [107] | ||
| Benzaldehyde | 13.09 | Almond, bitter almond, burnt sugar [82][93][82,93] | ||
| Alcohols | Hexanol | 12.42 | Woody, cut grass, chemical-winey, fatty, fruity, weak metallic [54] | |
| Carboxylic acids | 4-Methylnonanoic acid | 316.73 | Sweet muttony or goaty [109] | |
| 4-Ethyloctanoic acid | 186.22 | Sweet muttony or goaty [109] | ||
| Acetic acid | 5.09 | Pungent, acidic, cheesy, vinegar [84] | ||
| Esters | Ethyl dodecanoate | 6.18 | Fatty [110] | |
| Furans | 2-Methyl-5-(methylthio)furan | 36.09 | Meat, onion [111] | |
| 2-Pentylfuran | 24.21 | Green bean, butter [54] | ||
| Pyrazines | 2,3,5,6-Tetramethylpyrazine | 15.52 | Chocolate-like [112] | |
| Sulfur compounds | Benzyl methyl sulfide | 4.88 | Roasted, muttony, burning [113] |
* Reprinted in adapted form from Food Research International, 151, Sun, Wu, Soladoye, Aluko, Bak, Fu, and Zhang. Maillard reaction of food-derived peptides as a potential route to generate meat flavor compounds: A review, 110823, 2022 with permission from Elsevier. 1 Odor descriptor(s) retrieved from thegoodscentscompany.com. 2 NR Not recorded.
The significance of a volatile compound to the odor of a food is generally determined according to the OAV, which, as previously mentioned, is the ratio of the concentration of the volatile compound in the food divided by the odor threshold of the compound in an appropriate matrix. However, these odor thresholds and OAVs are determined for the individual compound, and it is recognized that the interaction between different volatile compounds results in a loss of aroma properties of the individual compound [16][17]. An additional issue is that the odor threshold is often determined with water as the matrix when the threshold in air might be more relevant [114].
It is well known that volatiles originating from lipid oxidation such as ketones and alcohols have high odor thresholds (mg/L range), aldehydes µg/L to mg/L range, while N- and S-heterocyclic compounds originating from the Maillard reaction and Strecker degradation have odor thresholds in the µg/L range [11]. The odor thresholds for the most important odor-active compounds in cooked meats were reviewed recently [103].
