Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Trinidad Perez Palacios and Version 3 by Jessie Wu.
Microencapsulation technology arose in the 1950s from the development of dye capsules to be incorporated into paper. MCurrently, microencapsulation can be defined as a set of technologies aiming to protect sensitive compounds from the external environment and further control their release. For that, the labile compounds, constituting what is known as the core, are entrapped by being surrounded by a shell material (the wall). Microencapsulation has been mainly applied in the pharmaceutical industry, followed in decreasing order by food, cosmetic, textile, biomedical, agricultural and electronic sectors. The recent developments in the microencapsulation of fish oil and natural antioxidant compounds are described.
- fish oil
- microencapsulation
- homogenization
- antioxidant extracts
- wall materials
- quality evaluation
- food enrichment
1. Constituents of Emulsions/Solutions for Microencapsulation
Before microencapsulation, a stable emulsion/solution must be prepared, and its constituents strongly influence the quality of obtained microcapsules. Figure 1 depicts the extent of use of different polymers for microencapsulation of fish oil (Figure 1A) and antioxidants (Figure 1B), with three main different types: saccharides, which were the most frequently used, followed by animal and vegetable proteins. The extent of use of saccharides has been higher for microencapsulated antioxidants in comparison to fish oil microencapsulations (77.8 vs. 52.6%, respectively), while animal proteins have a higher degree of use for fish oil than for antioxidant microencapsulation (34.2% vs. 11.1%), and both types of microcapsules showed a similar extent of use of vegetable proteins (13.16% and 11.11, respectively). Whey protein (isolate (WPI) or concentrate (WPC)) and maltodextrin have been predominantly used in most recent studies on fish oil microcapsules. In the case of antioxidant microcapsules, maltodextrin is the most preferred wall material, but others, as Arabic gum, chitosan, pectin, WPI, inulin and zein, are also remarkable. In addition, distinguishing between plant (including most saccharides and vegetable proteins) and animal-based materials (animal proteins, chitosan, gelatin and collagen), a higher percentage of plant-based materials (in more than 65% of the reviewed papers) in comparison to animal-based ones is noted in both fish oil and antioxidant microencapsulation.
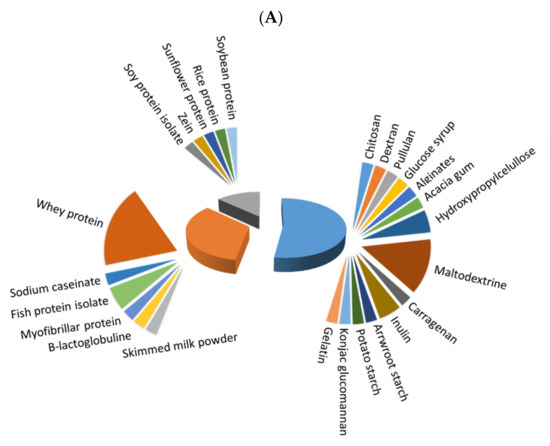
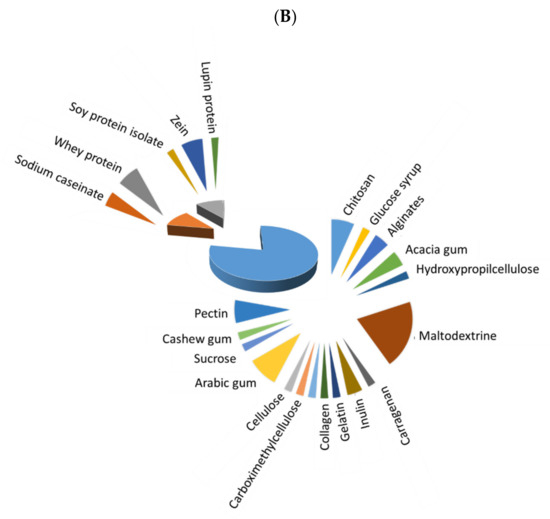
Figure 1. Extent of use of saccharides (blue), vegetable proteins (grey) and animal proteins (orange) as wall material for microencapsulation of fish oil (A) and natural antioxidants (B).
The extensive use of whey protein is related to its excellent surface activity and ability to stabilize oil in water (O/W) emulsions [1][22]. The most common presentations are WPI, WPC and hydrolysates (WPH). In addition, whey protein coproducts, such as the retentate of the final microfiltration step in the production of WPI (Procream) that mainly comprises dairy lipids and aggregated proteins, have been tested for reutilization and valorization as low-cost emulsifiers in microencapsulation [2][23]. Maltodextrin has a high solubility in water and can act as a filler matrix to form stable emulsions. Moreover, it has been reported as good protection from oxidation [3][24]. The use of these polymers is so well established that the current trend is to use them as a control for benchmarking other less-tested materials, alone or in combination, and only a few studies have specifically focused on them. This is the case of some authors [2][23] who blended Procream with intact or hydrolyzed WPC to improve the microencapsulation efficiency and the oxidative stability of fish oil microcapsules or used WPI conjugated with xylooligosaccharides to encapsulate lycopene, thus enhancing the emulsification performance, the antioxidant capacity, and parameters [4][25]. Other researchers [5][26] combined maltodextrin and WPI to microencapsulate fish oil, finding a high oxidative stability of the obtained powders that was attributed to the antioxidant effect of WPI.
Table 1 summarizes the most recent investigations focused on the evaluation of the emulsion constituents for fish oil microencapsulation. Besides the mixtures of whey protein and/or maltodextrin with other wall materials, the evaluation of different polymers of cellulose, inulin, fish protein isolate, chitosan and soy protein isolate has received interest in the most recent studies. García-Moreno et al. [6][27] showed the potential of different carbohydrates (pullulan and dextran or glucose syrup) and WPC mixtures for the nanoencapsulation of fish oil. In the same way, Charles et al. [7][28] demonstrated that arrowroot starch, maltodextrin and WPI combinations successfully encapsulated tuna fish oil, and Damerau et al. [8][29] prepared emulsions of fish oil with WPC and rice proteins as wall materials, obtaining a high stability that was ascribed to the WPC. Jamshidi et al. [9][30] allowed the stabilization of water in oil in water (W1/O/W2) double emulsions containing fish protein hydrolysate within a complex of WPC and inulin to produce fish oil microencapsulates. Özyurtet al. [10][31] also tested the use of fish protein isolate in combination with maltodextrin to encapsulate fish oil, finding better quality characteristics in comparison to the sodium caseinate and maltodextrin mixture. Ogrodowska et al. [11][32] compared different coating materials (maltodextrin, WPC, sunflower and rice proteins and guar gum) for encapsulating fish oil, obtaining the best overall properties when using rice proteins. In addition, the combination of WPC and rice proteins reduced the fishy and rancid odor and flavor of the powder. Chang et al. [12][13][33,34] tested the encapsulation of fish oil using different β-lactoglobulin fibril variants from WPI (β-lactoglobulin fibrils and thiol-modified β-lactoglobulin fibrils), chitosan and maltodextrin. These authors demonstrated that combinations of chitosan (a great emulsion stabilizer) and β-lactoglobulin (with high emulsification properties), which are oppositely charged, improve the microencapsulation efficiency. Encina et al. [14][35] microencapsulated fish oil with hydroxypropylcellulose, due to its solubility in both water and organic solvents, using lecithin as an emulsifier. Hydroxypropylmethylcellulose acetate succinate, which is water-insoluble, was tested by Loughrill er al. [15][36] to obtain compatible fish oil microcapsules with aqueous-based food products. Encina et al. [16][37] synthetized hydroxypropyl-inulin by etherification to increase the solubility in water and organic solvents and obtain a novel encapsulating agent for fish oil. Rios-Mera et al. [17][38] developed fish oil microcapsules with inulin, soy protein isolate and transglutaminase, the enzymatic cross-linking being crucial to improve the retention of fish oil under stress conditions. It is also worth mentioning the use of konjac glucomannan (favorable water solubility and absorption, emulsification and film-forming properties, besides potential health benefits, such as reducing and delaying glucose absorption, inhibiting the synthesis of fatty acids and controlling obesity) [18][39], alginates (which in addition to enhancing satiety, have the ability to form a gel that preserves the bioaccessibility of the core material, controlling the release of essential fatty acids in specific areas of the gastrointestinal tract) [19][40], acacia gum (it has emulsifying and film-forming properties and can act as surface-active substance, and once dried, it constitutes a matrix that prevents contact with oxygen) [20][41], silica (to form a highly organized three-dimensional hybrid matrix nanostructure that enhances bioaccessibility of medium and long chain length triglycerides) [21][42], carrageenan (a water-holding, gelling, stabilizing, thickening and emulsifying agent) [22][43] and zein (a prolamine isolated from maize with hydrophobicity, biocompatibility and film-forming properties) [23][44] as encapsulation materials in recent studies on fish oil microcapsules. Moreover, some recent publications have also been devoted to improving the oxidation stability of fish oil microcapsules by means of including antioxidants together with the wall material or in the core. Thus, Vishnu et al. [24][45] applied vanillic acid grafted chitosan for the microencapsulation of sardine oil, obtaining promising results. Yeşilsu and Özyurt [25][46] evaluated the addition of rosemary, thyme and laurel extracts to a previously formed emulsion of anchovy oil with lactose and sodium caseinate, the highest oxidative stability being found in microcapsules with incorporated rosemary and laurel. In the study of Solomando et al. [26][47], fish oil was mixed with lycopene and microencapsulated by using lecithin and maltodextrin. These authors reported a significant decrease in lipid oxidation markers as the lycopene content increased.
Comparing the obtained results among the different studies might be quite imprecise due to the influence of the encapsulation and analytical procedures. In addition, the quality parameters evaluated are not the same in all reviewed publications. However, in general, the suggested polymers have achieved successful microencapsulation of fish oil with reasonable oxidation stability.
In most recent studies on natural antioxidant microencapsulation (Table 2), the core material is predominantly constituted by an extract from the leaves, seeds or peels of vegetables (green jelly, red chicory, red cabbage, bay, tomato, sea buckthorn, Securigera securidaca, Japanese quince, Moringa stenopetala, Sida rhombifolia), fruits (olive, cocona, camu-camu, cranberry, pomegranate, araza, mulberry, grape, jabuticaba) or plants (green tea) or even from microorganisms (microalgae). The encapsulation of extracts from rice, oat bran and pepper flour, lycopene, curcumin, propolis, resveratrol, thymol and carvacrol has also been recently addressed. As for the wall material, in several works maltodextrin is blended with different polymers, namely hydrolyzed collagen [27][48], sodium caseinate and dried glucose syrup [28][49], Arabic gum [29][30][50,51], peel pectin powders [31][52], cashew gum and Tween 40 [32][53] and inulin [33][54]. In others, maltodextrin is benchmarked against oligofructose [34][14], Arabic gum [35][55] or inulin [33][35][54,55]. In addition, the dextrose equivalent (DE) value of maltodextrin (a measurement of its proportion of reducing sugars) has also been investigated (DE 10-13 vs. DE 17-20) [29][50]. The different maltodextrin blends reached suitable microencapsulation and protection of antioxidant compounds. Moreover, in the comparison studies, maltodextrin was found to be the best polymer for obtaining high-quality microcapsules. Nevertheless, the use of Arabic gum mixed with maltodextrin (1:1 w/w) for encapsulating carotenoids with linseed oil as a carrier resulted in considerable degradation during spray drying and during the gastric phase of simulated digestion [30][51]. The use of zein has also been tested for antioxidant encapsulation. Zein is a corn-derived insoluble protein, only soluble in an aqueous solution of >60% ethanol, capable of encapsulating hydrophobic compounds with low water solubility [36][56]. Thus, it has been successfully used for the encapsulation of an aqueous extract of green jelly leaf [36][56], sea buckthorn leaves [37][57] and thymol [38][58]. Hydroxypropylmethylcellulose [36][56], glycosylated WPI [4][25], alginates [39][59], soy protein isolate [40][60], starch [41][61] gelatin [37][57] and sucrose [42][62] have also been individually investigated as encapsulating materials. In addition, different combinations of these wall materials have been explored to encapsulate antioxidants, i.e., alginate, pectin, WPI and sodium caseinate [39][59]; soy protein isolate with soy soluble polysaccharides and maltodextrin [40][60]; poly(D,L-lactide-co-glycolide), ethylcellulose and polycaprolactone [5][26]; gelatin–acacia gum [43][63]; chitosan–carboxymethylcellulose [43][63]; chitosan, sodium alginate and Arabic gum [44][64]; carrageenan, lupin protein isolate and chitosan [45][65]; and WPI and acacia gum [46][66]. In general, the microencapsulation and protection of the antioxidants have been successfully achieved in these investigations.
Thus, for both fish oil and antioxidant microencapsulation, the current trend seems to be the comparison and/or combination of more-tested wall materials, mainly maltodextrin and whey proteins, with less-tested ones, most of them being from plants.
Table 1.
Evaluated effect, related to the emulsion constituents, the procedure and the food enrichment, in recent studies for the development of fish oil microcapsules.
Table 2.
Evaluated effect, related to the solution constituents, the procedure and the food enrichment, in recent studies for the development of natural antioxidant microcapsules.
| Effects | Core Material | Microencapsulation Technique | Reference |
|---|---|---|---|
| Constituents of Emulsion/Solution for Microencapsulation | |||
| Use of maltodextrin, inuline and oligofructose | Camu-camu extracts | Spray drying | [34][14] |
| Use of whey protein isolate glycosylated with xylo-oligosaccharides | Lycopene | Freeze drying | [4][25] |
| Use of poly(D,L-lactide-co-glycolide), ethyl cellulose and polycaprolactone | Propolis | Solvent evaporation technique | [5][26] |
| Use of maltodextrin and hydrolyzed collagen | Cocona pulp | Spray drying | [27][48] |
| Use of sodium caseinate, maltodextrin and dried glucose syrup | Resveratrol | Spray drying | [28][49] |
| Use of Arabic gum and maltodextrin (DE 10-13 and 17-20) | Cranberry extract | Spray drying | [29][50] |
| Use of maltodextrin and Arabic gum | Tomato peel extract | Spray drying | [30][51] |
| Use of cashew gum, maltodextrin and Tween 40 | Green tea extract | Spray drying | [32][53] |
| Use of maltrodextrine and/or inulin | Japanese quince juice | Spray drying/freeze drying/vacuum drying | [33][54] |
| Use of maltodextrin or Arabic gum | Araza pulp | Spray drying | [35][55] |
| Use of zein and hydroxypropylmethyl cellulose | Green jelly leaf extract | Spray drying | [36][56] |
| Use of maltodextrin and peel pectin powders | Pomegranate peel extract | Spray drying | [36][56] |
| Use of zein or gelatin | Sea buckthorn leaf extract | Electrohydrodynamic method | [37][57] |
| Use of zein | Thymol | Spray drying | [38][58] |
| Use of alginate alone or in combination with pectin, whey protein isolate and sodium caseinate | Olive leaf extract | Internal gelation | [39][59] |
| Use of soy protein isolate nanocomplexes combined, or not, with soy soluble polysaccharide and/or maltodextrin | Curcumin | Spray drying | [40][60] |
| Use of modified starch (CAPSUL) | Red chicory and red cabbage extract | Spray drying | [41][61] |
| Use of sucrose | Securigera securidaca seed extract | Dried in oven | [42][62] |
| Use of gelatin–acacia gum or chitosan–carboxymethyl cellulose | Black rice extract in combination or not with copigmented anthocyanins | Freeze drying | [43][63] |
| Use of modified chitosan, sodium alginate and Arabic gum | Bay leaf extract | Spray drying | [44][64] |
| Use of carrageenan, lupin protein isolate and chitosan | Astaxanthin oleoresin | Spray drying | [45][65] |
| Use of whey protein isolate and acacia gum | Tomato peel extract | Freeze drying | [46][66] |
| Procedure | |||
| Different maltodextrin:Arabic gum ratio | Red cabbage anthocyanin-rich extract | Double drum dryer | [58][21] |
| Different propolis extract:polymer ratios | Propolis | Solvent evaporation technique | [5][26] |
| Optimization of membrane emulsification process conditions | Resveratrol | Spray drying | [28][49] |
| Optimization of proportion of wall material (maltodextrin or Arabic gum) and drying temperature | Eugenia stipitata pulp | Spray drying | [35][55] |
| Spray drying vs. freeze drying vs. vacuum drying | Japanese quince | Spray drying/freeze drying/vacuum drying | [37][57] |
| Optimization of inlet air temperature, type of emulsion, feeding rate and total solids | Astaxanthin oleoresin | Spray drying | [45][65] |
| Different combinations of total solid content, emulsifier, carrier agent and drying method | Fucoxanthin-rich fraction from microalgae | Spray drying/freeze drying | [51][71] |
| Impact of maltodextine:high methoxyl pectin ratio. Spray drying vs. freeze-dying | Moringa stenopetala leaf extract | Spray drying/freeze drying | [59][78] |
| Different whey protein concentrate:maltodextrin ratios | Oat bran extract | Complex coacervation | [60][79] |
| Optimization of maltodextrin:Arabic gum ratio, maltodextrin dextrose equivalent, relative humidity and time during storage | Grape pomace extract | Spray drying | [61][80] |
| Optimization of percentage of solids, maltodextrin:trehalose dehydrate ratio and olive leaf extract:matrix ratio | Olive leaf extract | Freeze drying | [62][81] |
| Maltodextrin concentration | Pomegranate flavedo extract | Lyophilization | [63][82] |
| Amount of sodium alginate and volume of the extract | Araza extracts | Drip extrusion | [64][83] |
| Different homogenization techniques. Optimization of core material:wall material ratio, ultrasonic time, ultrasonic power and ultrasonic temperature | Mulberry polyphenols | Freeze drying | [65][84] |
| Impact of extract concentration | Sida rhombifolia extracts | Spray drying | [66][85] |
| Optimization of dispersion feed rate, drying air inlet temperature and drying air flow rate | Grape peel by-product extract | Spray drying | [67][86] |
| Effect of inlet air temperature | Carvacrol | Spray drying | [68][87] |
| Effect of inlet air temperature | Pepper flour extract | Spray drying | [69][88] |
| Food Enrichment | |||
| Incorporation into dressing | Tomato peel extract | Freeze drying | [46][66] |
| Incorporation into a fruit drink | Pomegranate flavedo extract | Lyophilization | [63][82] |
| Incorporation into cassava starch biscuits | Jabuticaba extracts | Drip extrusion | [64][83] |
| Effects | Microencapsulation Technique | Reference |
|---|---|---|
| Constituents of Emulsion for Microencapsulation | ||
| Use of Procream with intact or hydrolyzed whey protein concentrate | Spray drying | [2][23] |
| Use of octenyl succinic anhydride modified starch, gelatin or whey protein isolate with maltodextrin | Spray drying | [5][26] |
| Use of whey protein concentrate in combination with pullulan and dextran or glucose syrup | Electrospraying | [6][27] |
| Use of arrowroot starch, maltodextrin and whey protein in different combinations | Freeze drying | [7][28] |
| Use of rice and whey protein concentrate | Spray drying | [8][29] |
| Use of fish protein hydrolysate, whey protein concentrate and inulin | Spray drying | [9][30] |
| Use of maltodextrin, thiol-modified β-lactoglobulin fibrils and chitosan | Spray drying | [12][13][33,34] |
| Use of maltodextrin, whey proteins, sunflower proteins, rice proteins, guar gum | Spray drying | [11][32] |
| Use of hydroxylpropylmethylcellulose acetate succinate | Spray drying | [15][36] |
| Use of hydroxypropylcelullose and hydroxypropyl-inulin | Spray drying | [16][37] |
| Use of soy protein isolate, inulin and cross-linked transglutaminase | Complex coacervation + sieving | [17][38] |
| Use of konjac glucomannan, soybean protein isolate and potato starch | Spray drying/freeze drying | [18][39] |
| Use of alginate calcium | Extrusion with calcium chloride | [19][40] |
| Use of skimmed milk powder, acacia gum, and a mixture of acacia gum and grape juice | Spray drying + spray chilling | [20][41] |
| Use of silica | Spray drying | [21][42] |
| Use of myofibrillar protein in the presence, or not, of κ- or λ-carrageenan | Spray drying | [22][43] |
| Use of zein | Electrospraying assisted by pressurized gas | [23][44] |
| Use of vanillic acid grafted chitosan | Spray drying | [24][45] |
| Comparison between fish protein isolate–maltodextrin and sodium caseinate–maltodextrin | Spray drying | [25][46] |
| Use of natural plant extracts (thyme, rosemary, and laurel) in comparison to synthetic antioxidant (BHT) | Spray drying | [25][46] |
| Use of lycopene as antioxidant | Spray drying | [26][47] |
| Comparison between fish protein isolate–maltodextrin and sodium caseinate–maltodextrin | Spray drying | [25][46] |
| Procedure | ||
| Percentage of whey protein concentrate, hydrolyzed whey protein concentrate and Procream | Spray drying | [2][23] |
| Emulsification by means of high-pressure homogenization or rotor–stator | Electrospraying | [6][27] |
| Optimization of emulsion pH and number of homogenization steps | Spray drying | [8][29] |
| Optimization of high-pressure homogenization conditions (pressure and number of cycles) | Spray drying | [9][30] |
| Optimization of fish oil/hydroxypropylcellulose ratio and the air inlet temperature | Spray drying | [14][35] |
| Direct spray drying vs. production of solid lipid nanoparticle dispersions + spray drying | Spray drying | [15][36] |
| Optimization of fish oil:hydroxypropyl-inulin ratio and the inlet gas temperature | Spray drying | [16][37] |
| Spray drying vs. freeze drying | Spray drying/freeze drying | [18][39] |
| Optimization of oil load | Extrusion with calcium chloride | [19][40] |
| Combination of spray drying and spray chilling | Spray drying + spray chilling | [20][41] |
| Optimization of pH and ratio between myofibrillar protein and κ-carrageenan or λ-carrageenan | Spray drying | [22][43] |
| Optimization of high-pressure homogenization conditions (pressure and number of cycles) | Spray drying | [47][67] |
| Impact of oil load | Spray drying | [48][68] |
| Optimization of soy protein isolate:oil ratio; emulsification by ultra-turrax vs. ultrasonics | Spray drying | [49][69] |
| Vacuum spray-drying technology in comparison to spray drying | Vacuum spray-drying | [50][70] |
| Optimization of temperature, pressure and feed flow rate | Supercritical antisolvent process | [51][71] |
| Food Enrichment | ||
| Incorporation into melted chocolate that covers extruded cereals | Spray drying + spray chilling | [20][41] |
| Incorporation into reconstituted milk | Electrospraying assisted by pressurized gas | [23][44] |
| Incorporation into spreads | Spray drying | [26][47] |
| Incorporation into burgers | Freeze drying | [52][72] |
| Incorporation into cooked and dry-cured sausages | Spray drying | [52][53][54][55][56][57][72,73,74,75,76,77] |
2. Procedure for Microencapsulation
In relation to the microencapsulation procedure, Figure 2 shows some of the different techniques described in recent studies for the production of fish oil and antioxidant microcapsules. Spray drying was the preferred technique in these studies, but the use of freeze drying is also remarkable. Spray drying is a physicomechanical method commonly used for microencapsulation. It is simple, flexible, rapid and easy to scale up; has low operating cost; and allows a large-scale production in continuous mode. Moreover, spray drying has a wide range of equipment availability, and it is considered a clean technology (avoids the utilization of organic solvents). It consists in transforming a liquid solution into powders by means of different steps: atomization of the previously prepared liquid solution (by dissolving or emulsifying the active compounds (core) with the carriers (wall material)) leading to the formation of droplets a few microns in diameter; spraying of the droplets in a heat chamber where they are dehydrated into capsules by applying hot air (160–200 °C) for a few seconds; separation of the capsules from the drying air at a lower temperature (50–80 °C) and recovery of the capsules [70][90]. It is a continuous and single process where the moisture is rapidly evaporated, and this allows keeping a relatively low temperature in the particles. The obtained powder has a very low water activity, ensuring microbiological stability, and it is easy to handle, store and transport. Spray-chilling and spray-cooling techniques work in a similar way to spray drying, but instead of evaporation of the water of the emulsion/solution, a hardening of the surface wall material is achieved. In these processes, the solution is atomized and droplets are cooled instead of evaporated. Thus, the wall material solidifies around the core. Like spray drying, spray chilling and spray cooling are rapid, safe, reproducible, easy to scale up and environmentally friendly and can be operated continuously. However, they also have high process costs and special handling and storage conditions since the obtained material is not as shelf-stable as those produced by spray drying [70][90]. Freeze drying or lyophilization is a drying method that dehydrates the solution of core and wall material mixtures, previously frozen, by sublimation under vacuum and low temperatures. The obtained products present a well-reduced risk of the undesirable effects derived from heating and are easily reconstituted. However, freeze drying is very expensive and time-consuming due to the longer dehydration process, the vacuum technology applied and the low temperatures required [71][4].
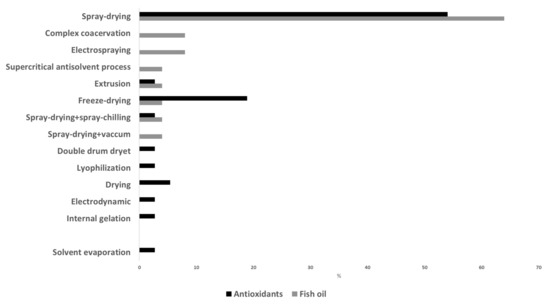
Figure 2.
Extent of use of microencapsulation techniques for production of fish oil and antioxidant microcapsules.
Most of the recent studies that focused on the microencapsulation procedure evaluated the influence of different parameters and compared techniques (Figure 3). For example, Carra et al. [67][86] prepared microcapsules of grape extracts by spray drying and investigated the role of dispersion feed rate, drying air inlet temperature and drying air flow rate on several quality parameters, the inlet temperature being the most influential factor. In the study by Sun et al. [68][87], four inlet temperatures (100, 130, 160 and 190 °C) were tested for the encapsulation of carvacrol; these authors found that inlet temperatures between 100 and 130 °C were the most appropriate in terms of antioxidant capacity, dissolution time, hygroscopicity and morphology. de Sá Mendes et al. [69][88] also studied the consequences of inlet temperature (140 to 160 °C) on the physicochemical properties of spray-dried pepper flour extract, concluding that color and morphology were strongly influenced. Brighter, less purple and spherical particles with some shrinkage were obtained with high inlet temperatures. Cui et al. [18][39] evaluated the properties of fish oil microcapsules prepared by either spray drying or freeze drying. In general, these authors found better overall quality in microcapsules prepared with spray drying (higher microencapsulation efficiency, more spherical particles with a compact structure, higher retention rate of core materials). Dadi et al. [59][78] compared the use of spray drying and freeze drying for microencapsulating an extract from Moringa stenopetale leaves. Although the phenolic and flavonoid contents and antioxidant activity were higher in freeze-dried microcapsules, these authors chose the spray-drying technique since the microencapsulation efficiency and storage stability were higher. Similarly, Turkiewicz et al. [33][54] tested freeze drying, spray drying and vacuum drying for the encapsulation of Japanese quince juice. For most of the analyzed parameters in the powders, freeze drying had a positive effect, while vacuum caused deterioration of all parameters. The combination of different microencapsulation technologies has also been tested. Thus, Fadini et al. [20][41] suggested the application of spray-drying and spray-chilling techniques for producing fish oil microcapsules with no fish oil taste. A first shell to cover the fish oil is formed by spray drying; then, spray chilling is applied to create a second shell, using the spray-dried microcapsules as the core and a mixture of vegetable fat and hydrogenated palm oil as the wall material. Solvent and vacuum spray-drying techniques were recently evaluated for the production of fish oil microcapsules in order to avoid the increase in lipid oxidation during spray drying. Solvent spray-drying avoids the preparation of a fish oil-in-water emulsion, and vacuum spray-drying can overcome the high temperature and high oxygen exposure due to the airflow, both of which boost oxidative reactions. Encina et al. [14][35] studied the use of solvent spray-drying with ethanol, methanol and acetone. Although results have shown that solvent spray-drying affects microencapsulation efficiency and stability in different ways, this technique has the potential to be an alternative technology for the encapsulation of fish oil. In the case of vacuum spray-drying, it significantly improved the quality properties of fish oil microcapsules in comparison with spray-dried ones [72][91]. Nevertheless, these authors indicated the need of further studies with these techniques.
Complex coacervation, electrospraying, supercritical antisolvent, extrusion and internal gelation have also been recently applied as encapsulation techniques by some authors. The complex coacervation technique is based on homogenizing two aqueous solutions, one with the bioactive compound mixed with an anionic polymer and the other containing a cationic polymer, to obtain a stable emulsion, which is induced to coacervate and undergo a phase separation by pH and/or temperature modifications. The coacervates are formed by larger particles of higher density at the bottom of the two-phase system. The major limitation of this technique is the high sensitivity to pH and ionic strength of the coacervates, which makes its commercial application difficult [73][13] Rios-Mera et al. [17][38] formed complex coacervates by adding an inulin solution to a previously developed fish oil–soy protein isolate emulsion. TIn this study, the obtained microparticles were sieved and cross-linked with transglutaminase. Bannikova et al. [60][79] added a guar gum solution to a mixture of oat bran extract with maltodextrin and WPC and dried it to produce the microcapsule powder.
Extrusion techniques involve forcing a liquid mixture (core and wall materials) through an orifice to form droplets at the discharge point of the nozzle. After the droplets are formed, capsules are immediately solidified by physical or chemical processes. In coextrusion, core and wall materials are injected separately in a double fluid nozzle [74][8]. Apart from coextrusion, there are other different types of extrusion techniques, melt injection, hot-melt extrusion and electrostatic. There are some recent works that achieved the microencapsulation of fish oil and natural antioxidants by means of the extrusion technique. Bannikova et al. [19][40] applied this technique to microencapsulate fish oil by preparing an emulsion with alginate that was dripped into calcium chloride followed by sieving, with the obtained microcapsules being stable during the gastric stage and released at the intestinal phase. Similarly, de Cássia Sousa Mendes et al. [64][83] dripped a mixture of sodium alginate with an extract of jaboticaba peel and seeds in a calcium chloride solution and obtained microcapsules with high encapsulation efficiency and antioxidant potential.
Two main electrodynamic techniques are used to encapsulate bioactive compounds, electrospraying and electrospinning. Both involve the application of an electrical field to charge the polymer solution, which is subsequently induced to free charges and forms nanofibers (electrospinning) or is atomized into droplets, giving charged nanodroplets (electrospraying). Despite being novel techniques, both processes are slow, difficult to scale up and have low yield and efficiency, which limit their commercial exploitation for foods [71][4]. Busolo et al. [23][44] and García-Moreno et al. [6][27] applied electrospraying to microencapsulate fish oil by using whey protein carbohydrates and zein as wall materials, respectively, with stable microcapsules being attained; Lyu et al. [37][57] tested this technology for encapsulating polyphenols and flavonoids from the leaves of sea buckthorn, resulting in successful protection from degradation and bioaccessibility in the intestine.
The use of supercritical fluid processes to encapsulate bioactive compounds is gaining attention. This technique is based on the peculiar properties of supercritical fluid, which is an intermediate between liquid and gas and can be easily changed with modifications in its pressure and temperature. Depending on the role that the supercritical fluid plays in microcapsule formation, this technique can be classified as solvent (the target compound and the wall material are dissolved in a supercritical fluid at high pressure; once the supercritical fluid is expanded, pressure is reduced and precipitation of the particles occurs), antisolvent (the target compound and the wall material are dissolved in a liquid solvent and atomized together with the supercritical fluid, which decreases the solubility of the solute in the mixture, with the consequent formation of nano- or microparticles) or solute (the supercritical fluid is dissolved into a suspension of the target compound and wall material, followed by a rapid depressurization of the solution through a nozzle and the final formation of solid particles or liquid droplets) [71][4]. By submitting the fluids to high pressure, this technique allows applying low temperatures, small solvent volume and sample quantity, and short extraction times. In addition, this method eliminates or reduces the solvents due to the high solubility of the solvent in the supercritical fluid, which is easily removed from the final product by depressurization. However, due to the high pressure variations applied, this technique implies a high cost. Based on their findings on particle size, moisture content, surfaces, microencapsulation efficiency, stability against oxidation and other microcapsule quality parameters, Karim et al. [75][92] pointed out the capability of the supercritical antisolvent process in encapsulating fish oil for industrial application using hydroxypropyl methyl cellulose as a wall material.
The ionotropic gelation method is based on the interaction between ionic polymers and ions with opposite charges that can be positioned externally or incorporated within the polymer solution in an inactive form, giving rise to external and internal ionotropic gelation methods, respectively. In both cases, hydrogel microbeads are produced. Flamminii et al. [39][59] achieved the microencapsulation of olive leaf extract through internal gelation by using alginate alone or mixed with pectin, whey proteins or sodium caseinate. The combination of alginate with pectin achieved the highest microencapsulation efficiency and retention of phenolic compounds.
Moreover, in some recent studies for antioxidant microencapsulation, simple solvent evaporation has been used, as reported by Paulo et al. [5][26], who microencapsulated propolis extract after evaporating the solvents of a double emulsion in a fume hood at room temperature. Behnamnik et al. [42][62] encapsulated Securigera securidaca seed extract using sucrose and heating to 132 °C to co-crystalize the product, which was subsequently dried in an oven. In the work of Sakulnarmrat et al. [58][21], cabbage anthocyanin-rich extract was encapsulated by mixtures of maltodextrin and Arabic gum using a double drum dryer. In these studies, the obtained microcapsules showed high antioxidant properties.
