Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Yuangang Liu and Version 2 by Sirius Huang.
Conventional photothermal therapy (PTT) irradiates the tumor tissues by elevating the temperature above 48 °C to exert thermal ablation, killing tumor cells. However, thermal ablation during PTT harmfully damages the surrounding normal tissues, post-treatment inflammatory responses, rapid metastasis due to the short-term mass release of tumor-cellular contents, or other side effects. To circumvent this limitation, mild-temperature photothermal therapy (MTPTT) was introduced to replace PTT as it exerts its activity at a therapeutic temperature of 42–45 °C.
- mild-temperature photothermal therapy
- immunotherapy
- heat shock proteins
- nanoplatforms
- thermal resistance
1. Introduction
Hyperthermia was used to treat breast tumors in Egypt, tracing back to 5000 B.C. Tumor tissue presents increased blood vessels, blood stasis, poor heat dissipation, high resistance, difficult heat dissipation, easy heat accumulation, and rapid temperature increase [1][2][1,2]. Thus hyperthermia is effective for tumor treatment [3]. Photothermal therapy (PTT) is a kind of thermal therapy whereby light energy is converted into heat energy to improve the temperature of lesions to achieve a therapeutic effect [4][5][4,5]. Exogenous photothermal agents (PAs) are not necessary for PTT but can improve the efficiency and efficacy of therapy [6]. PTT is widely applied for the treatment of various types of tumors by promoting apoptosis or necrosis of tumor cells at high temperatures [4][7][8][4,7,8]. PTT relying on the introduction of an exogenous laser can achieve high accuracy, high efficiency, mild toxicity, and non-invasive treatment compared with traditional chemotherapy, radiotherapy, and surgery [5][9][10][5,9,10]. In addition, the laser can be used as a “light-trigger switch” to achieve remote drug control release (light stimulation response) [11][12][11,12]. In contrast, the heat can destroy the lysosome to help the drug-loaded to escape from the lysosome. Nowadays, a division between the concentration of preclinical and clinical PTT research is obvious, with preclinical studies focused on new PAs, whereas clinical studies concentrated on the exploitation of integrated laser devices [6]. The difference may reflect the fact that the effectiveness of PTT can easily be demonstrated in preclinical research, enabling the preparation and application of a wide variety of novel nanomaterials. Nevertheless, PAs hold potential in clinical transformation on account of better selectivity for the target tissue, enabling the utilization of lower-power lasers and simplifying device design. Previous studies have made significant efforts to optimize PAs by modulating the shape, size, and surface chemistry of nanoparticles [7][13][14][7,13,14]. Moreover, the rapid development of nanotechnology has increased advances in PTT through the development of multi-functional nanoparticles [15]. For instance, plasmonic nanoparticles, like gold nanoparticles, and platinum nanoparticles, are chosen as PAs in many reports [16][17][16,17]. In addition, synergistic therapy with PTT improves the therapeutic effect of PTT against tumors [18]. PTT directly kills tumor cells or enhances other therapies by promoting drug delivery, stimulating release, mediating tumor microenvironment (TME), eliciting tumor-specific antigen release, or modulating other biologically related responses [19][20][21][22][23][24][25][19,20,21,22,23,24,25].
However, the clinic application of PTT has been hindered to some extent by several limitations. For instance, it is challenging to completely kill tumor cells using PTT, thus augmenting the risk of tumor recurrence and metastasis owing to limited tissue penetration of the laser (NIR-I widow laser 1~2 cm, NIR-II widow laser > 2 cm) [26]. Therefore, to achieve a high treatment temperature, researchers often increase the laser power or dosage of PAs. However, the American National Standards Institute (ANSI) has established standard tolerance threshold values for the clinically safe use of PTT on the skin [27]. The 808 nm laser power threshold ranges from 330 to 350 mW cm−2 with an exposure time of 10–1000 s. Moreover, PTT inevitably damages normal tissue around the tumor site and leads to in vivo toxicity and side effects [28]. Furthermore, several cell contents and some residual tumor cells caused by thermal ablation may cause a series of side effects, including inflammation, tumor metastasis, harm to normal tissues, and tumor recurrence [29].
To circumvent these limitations, mild-temperature photothermal therapy (MTPTT), with a temperature range from 42 °C to 45 °C [3][30][3,30], was introduced to reduce the temperature used, thus alleviating the side effects. In addition, MTPTT does not significantly affect the quality of life of the patient owing to the milder temperature used. However, MTPTT is associated with poor therapeutic effects. Therefore, studies have been exploring methods to achieve better therapeutic efficacy using nanocarriers under MTPTT. Though heat shock protein (HSPs) inhibitors or other compounds can be encapsulated into the nanoplatforms, the antitumor efficacy and safety still need more comprehensive and in-deep studies. Nevertheless, it is a significant integrative treatment and exhibits great potential in future clinical applications [31].
2. The Mechanism of MTPTT
MTPTT effectiveness in cancer treatment does not depend on precise devices or special methods to control the mild temperature but on methods for maintaining treatment efficacy at mild temperature. MTPTT therapeutic effect is attributed to damage to the self-protective mechanism of tumor cells and preventing serious damage from heat stress. Studies report that MTPTT exerts its activity through two self-protective mechanisms, including heat shock reaction and autophagy [32][33][32,33]. In conventional PTT (>48 °C), thermal ablation induces severe and irreversible denaturation of proteins, DNA damage, and denaturation, and destroys the effective defense of the self-protective mechanism. Notably, the self-protective mechanism has a significant effect on the repair of unfolded proteins in MTPTT (<45 °C). Therefore, inhibiting the pathway of the self-protective mechanism is the most effective way to achieve the high efficacy of MTPTT. Studies report that HSR and autophagy are key targets for mediating self-protective mechanisms during MTPTT (Figure 1).
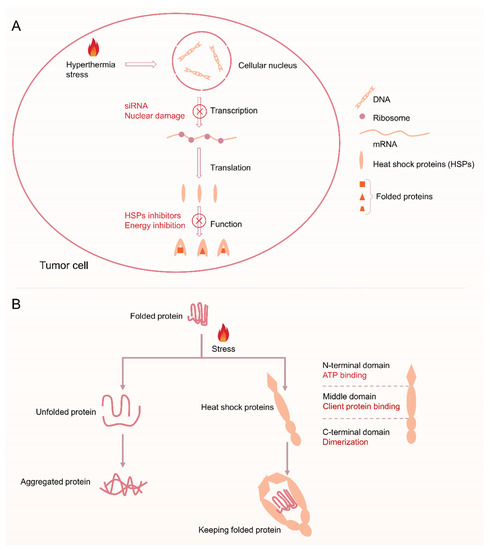
Figure 1. (A) Schematic of the process of heat shock reaction after hyperthermia and the blocking function of heat shock reaction via siRNA, nuclear damage, HSPs inhibitors, and energy inhibition. (B) Illustrating the physiological functions of HSPs: assists protein folding into its native form in MTPTT.
Hyperthermia above 41 °C causes protein denaturation and temporary cell inactivation, which may last for several hours [34][35][34,35]. As a result, upregulation of expression of HSPs is induced by HSR, thus effectively preventing aggregation of other proteins. HSR is a cellular defense mechanism present in all organisms and plays a role in preventing damage from hyperthermia or other adverse stress conditions. HSR limits the therapeutic efficacy of MTPTT through its cytoprotective and antiapoptotic effects [36]. Moreover, HSPs can interact with apoptosis signaling pathway proteins to inhibit the occurrence of apoptosis, thus reducing the therapeutic effect of hyperthermia [37][38][37,38]. In addition, tumor cells overexpress HSPs compared with normal cells, which makes them less sensitive to heat treatment and enables them to remain active at high temperatures [39][40][39,40].
Tumor cells mainly regulate the expression of HSPs by activating heat shock transcription factors (HSFs) [41]. Previous studies have explored four HSFs, including HSF1, HSF2, HSF3, and HSF4. Notably, HSF1 is the main transcription factor that mediates HSR. HSF1 is a highly expressed protein in various tumor cells and is related to tumor progression and poor prognosis. The main mechanism of action of HSF1 is by enhancing phosphorylation of its own 326 site serine, thus upregulating expression of HSP70 and HSP27 and ultimately promoting malignant proliferation and apoptosis resistance [42][43][42,43]. Expression levels of HSPs are low, and only 1–2% of the total protein exists under normal physiological conditions [44]. HSF1 is activated and bound to the promoter region of the downstream HSPs gene to promote the expression of HSPs after stimulation by high temperatures, excessive reactive oxygen species (ROS), or inflammation. HSP70 is mainly the first expressed protein as a result of HSR in many HSP families [45][46][45,46]. B-cell lymphoma-2 (Bcl-2) associated athanogene 3 (BAG3) is the chaperone protein of HSP70 and can bind to the ATPase domain of HSP70 through the bag domain to modulate HSP70 function [47][48][47,48]. In addition, the BAG3-HSP70 complex can bind to Bcl-2 and protect it from degradation, thus inhibiting the apoptosis pathway or inhibiting tumor cell apoptosis induced by hyperthermia therapy and chemotherapy [49][50][51][49,50,51].
Therefore, inhibition of HSR can reduce the thermoresistance of tumor cells to increase the effectiveness of sensitizing PTT. Several studies have explored the inhibition of HSR by gene-mediated silencing technology (small interfering RNA or short hairpin RNA, siRNA, or shRN and A), studies are developing heat-sensitive drugs. The efficacy of MTPTT is mainly achieved by blockingHSR, and is mainly through two aspects, including (1) reducing the synthesis of HSPs from HSR [52], and (2) inhibiting the activity of HSPs [53]. The current research mainly focuses on the mechanism of HSPs in improving the efficacy of PTT. The efficacy of PTT can be improved through the following three ways: use of HSPs inhibitors, silencing HSPs gene by siRNA and reducing ATP synthesis. Therefore, it is important to combine HSPs inhibitors (or siRNA, ATP inhibitors) with PAs in the nanosystem, thus improving the sensitivity of tumor cells to heat [54].
Besides, autophagy as a cellular self-protective mechanism rapidly activates cancer cells to maintain energy production and offer recycled materials in response to hyperthermia stress. Autophagy-related tolerance also acts a crucial role in thermal resistance [55]. There are three types of autophagy identified according to different routes in which substrates eventually enter into the lysosomal lumen: microautophagy, chaperone-mediated autophagy, and macroautophagy (Figure 2). Damaged and denatured proteins and organelles are engulfed by autophagosomes, then degraded in the lysosome to provide energy, and macromolecular precursors, and can be recycled to sustain cellular metabolism [56][57][58][56,57,58]. Therefore, intercepting the autophagy pathway can improve the efficacy of MTPTT. Autophagy can be blocked by inhibiting (1) formation of autophagosome (3-methyladenine, wortmannin) [56], (2) fusion of autophagosome and lysosome (hydroxychloroquine, chloroquine, vinblastine) [59], and (3) degradation of autolysosome (pepstatin A) [60]. On the contrary, excessive autophagy does not protect cells but destroys homeostatic functions and induces autophagy-mediated cell death (ACD), known as type II programmed cell death [61]. The excessive autophagy activity far exceeds the degradation capacity of the autolysosome, resulting in the formation of micron vacuoles and degradation blockage [62]. When autophagy fails to stop effectively or is overstimulated, the autophagic activities cannot recycle the cancer cellular components and accelerate ATP depletion, which ultimately leads to cell death and further enhance the therapeutic efficacy of MTPTT. Therein, excessive autophagy is induced via cutting off the inhibition pathway of autophagy or using autophagy inducers, including carbamazepine, C2-ceramide, rapamycin, and xestospongin B/C [63].
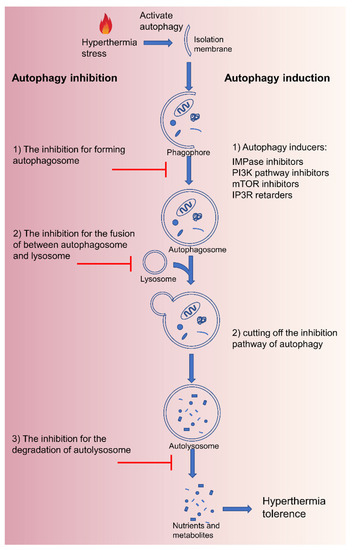
Figure 2.
Schematic of the process of macroautophagy after hyperthermia and the various strategies to inhibit or induce autophagy.
