Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Yongchao Li and Version 2 by Camila Xu.
Dizziness is one of the most common reasons for consultation in adult patients. It is an umbrella term used to describe various sensations, including vertigo, disequilibrium, lightheadedness, or presyncope.
- cervicogenic dizziness
- cervical proprioception
- proprioceptors
1. Introduction
Dizziness is one of the most common reasons for consultation in adult patients [1][2][1,2]. It is an umbrella term used to describe various sensations, including vertigo, disequilibrium, lightheadedness, or presyncope (Table 1) [2]. From this perspective, vertigo is just one part of dizziness. However, in the light of the International Bárány Society for NeuroOtology [3], dizziness and vertigo are no longer subordinate but independent allelic symptoms. Dizziness and vertigo may coexist or occur sequentially (Table 2) [3]. In 1955, Ryan and Cope [4] first described dizziness caused by neck disorders as cervical vertigo, also known as cervicogenic vertigo, cervicogenic dizziness or cervical dizziness. HereIn this researchersview, we use cervicogenic dizziness to name this trouble. A recent clinical observation of a large number of cases (1000 cases) found that cervicogenic dizziness accounted for 89% of all dizziness, or vertigo [5]. Cervical spondylosis was one of the common causes of dizziness in the elderly in a community survey [1]. Among patients with cervical vertebral whiplash injuries, the prevalence of dizziness has been variously reported, ranging from 20% to 90% [6]. Nearly half of patients with neck pain have cervicogenic dizziness [1]. However, cervicogenic dizziness is the most controversial among all dizziness because its pathogenesis is unclear, and its diagnosis and treatment are difficult [6][7][8][9][6,7,8,9].
Table 1.
Main categories of dizziness.
| Category | Description |
|---|---|
| Vertigo | A sense of spinning experienced even when someone is perfectly still. |
| Disequilibrium | A loss or lack of equilibrium or stability. |
| Presyncope | Feeling of losing consciousness or blacking out. |
| Lightheadedness | Feeling a little woozy or faint. |
Table 2.
Definition of dizziness and vertigo by the International Bárány Society for NeuroOtology.
| Classification | Description |
|---|---|
| Dizziness | The sensation of disturbed or impaired spatial orientation without a hallucinatory or distorted sense of motion. |
| Vertigo | The sensation of self-motion when no self-motion is occurring or the sensation of distorted self-motion during an otherwise normal head movement. |
Cervicogenic dizziness is considered to have four different pathogenesis, but proprioceptive cervicogenic dizziness is the most common and accepted by most scholars [6]. Unlike other forms of dizziness, cervicogenic dizziness is of interest not only to neurologists but also to physiotherapists, pain physicians, and orthopedic surgeons.
2. Pathophysiology
2.1. Cervical Proprioceptors and Proprioception
The sensorimotor system includes all the afferent, efferent, central integrations, and processing parts [10]. Somatosensory is the afferent component of the sensorimotor system and includes the conscious perception of pressure, temperature, vibration, pain, and proprioception [10][11][10,11]. Proprioception has often been described as theour sixth sensation [12], including kinesthesia, force sensation, and joint position sense [10][11][13][10,11,13]. Proprioceptive information is conducted by specialized nerve endings, called proprioceptors which are situated in the joints, muscles, tendons, and skin [10][12][10,12]. Cervical proprioceptive afferents can be primarily divided into three groups: joint receptors, muscle spindle, and Golgi tendon organs (GTO) [12][14][15][12,14,15], which play a significant role in head-eye coordination and posture maintenance [16]. Proprioceptive information in the neck plays a crucial role in monitoring head orientation and offering a reference for the visual and vestibular receptors [2]. In physiological conditions, muscle spindles play an important role in kinesthesia GTOs conduce to the senses of heaviness and force, while cervical joint receptors may act a secondary role in proprioception [15]. Muscle spindles and GTOs react to the changes in skeletal muscle length and tension, respectively. A high density of muscle spindles has been found in the neck region of humans [16][17][18][16,17,18]. In general, spindle density refers to the number of spindles in wet muscle tissue per gram of muscle sample and is often used to compare the relative abundance of muscle spindles in different muscles of the same species [16][17][16,17]. In a highly cited and classic article, Kulkarni et al. [16] found that the suboccipital small muscles in human fetuses had an abundant spindle density and spindle content but lacked GTOs, making them ideal sensors for detecting the joint position and movements of craniovertebral joints. They also found that the inferior oblique muscle had a spindle density of 242/g, while the trapezius muscle had a spindle density of only 2.2/g [16]. Boyd-Clark et al. [17] found that the spindle density of longus Colli (48.6/g) was significantly higher than that of the multifidus muscle (24.3/g) in human autopsies, and the morphology, distribution, and density of spindle did not change with age. Although spindle density has been widely used in comparative studies, muscle mass has never been shown to be an appropriate reference for spindle number [18]. Banks et al. [18] revealed that spindle density itself has a nonlinear relationship with muscle mass, so direct linear comparisons of muscle spindle densities across muscle sizes are misleading. They performed an allometric analysis on the number of spindles in mammalian skeletal muscles and suggested the use of residual value as a simple way to measure the relative abundance of muscle spindle components. There was no difference in relative spindle abundance between large and small muscles as measured by residual values [18]. The longus capitis muscle, semispinalis capitis, and obliquus capitis inferior have the highest relative abundance values (7.5, 4.9, and 3.5, respectively) [18]. It is widely accepted that the joint receptors in proprioception act as joint limit detectors, playing a significant role in the sense of position near the limits of joint motion [12][15][19][12,15,19]. However, joint receptors are critical for the control of feedforward muscle activity and muscle stiffness via the gamma muscle spindle system [20]. Slight flexion of the upper cervical joints can lead to significant changes in the discharge rate of muscle spindle afferents in the perivertebral muscles [21]. Thunberg et al. [22] found reflex connections between receptors in the neck facet joints and fusimotoneurones of dorsal cervical muscles and the transient activation of chemically sensitive nerve endings in facet joints to be capable of triggering positive feedback loops that may produce chronic pain and stiffness in the cervical muscles. Therefore, cervical joint receptors are likely to impact postural control and head-eye movement via their influence on the muscle spindle system [20][21][22][20,21,22]. In 1967, Freeman and Wyke [23] classified four kinds of mechanoreceptors in the knee joints of cats. Except as free nerve endings (type IV), three types of proprioceptors are also found in human cervical facet joints [24][25][24,25] and discs [26][27][26,27], including the Ruffini corpuscles (type I), Pacinian corpuscles (type II), and GTOs (type III). However, they are much lower than the amount in the muscles. Although there are a small number of mechanoreceptor endings in the facet capsules and discs, the volume of receptors is relatively large. It is likely that receptive fields are large and that one or two mechanoreceptors may be sufficient to monitor the area of each individual facet capsule or disc [24]. Animal studies have also found that the functional proprioceptors in the facet joint capsule can be activated by low-stretching-level activities [25]. In addition, the intervertebral disc is situated on the central axis of cervical motion, and thus, proprioceptors of cervical discs are in a favorable site to monitor subtle changes in the cervical position or direction of motion. [26][28][26,28].2.2. Central and Reflex Connection for Cervical Proprioceptive Signals
The neck proprioceptor can provide information on the movement and position of the head relative to the trunk but not on the movement of the head in space. However, this sensory information can affect vestibular reflexes, which function to stabilize the posture of the head, eyes, and body and to construct a sense of spatial orientation [29]. The vestibular system provides relevant information about the position of the head relative to space, while the visual system identifies the position of the head relative to the external environment [30]. By combining vestibular signals with neck proprioception information, the motion signals can be coordinately transformed into a body-centered frame of reference [31]. In addition, visual-vestibular and proprioceptive-vestibular interactions are critical for postural control and gaze [31]. The cervical proprioceptive system has unique central and reflex connections with the vestibular and visual systems (Figure 1). It is well recognized that the convergence of cervical proprioceptive afferents with vestibular and visual inputs at different levels of neuroaxis includes the vestibular nuclei, thalamus, and cerebral cortex [10][16][29][32][33][34][10,16,29,32,33,34]. Animal studies suggest that cervical proprioceptive afferents are transmitted to the central cervical nucleus through the dorsal root ganglion and project directly to the vestibular nucleus [35]. Neurons in the central cervical nucleus receive afferent information from the vestibular and cervical proprioceptors and provide integrated data about the head position relative to the trunk and space to the cerebellum and reticular formation [36][37][36,37]. The spinal cord can also indirectly convey input to the vestibular nuclei via the cerebellum and reticular formation [38]. Moreover, the vestibular nuclei can provide direct inputs to the reticular formation and parabrachial nucleus with projections to sympathetic preganglionic neurons of the thoracic spinal cord, which are involved in producing the rapid adjustment of circulation, digestion, and respiration necessary to maintain homeostasis (through the vestibulo-sympathetic reflex pathway) [39][40][39,40]. Reflex activities relating to the cervical, vestibular, and visual systems play a significant role in the coordination of head, neck, and eye movements (Figure 1).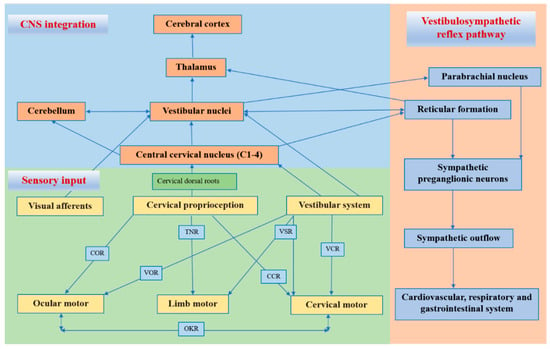
Figure 1. Central and reflex connection for cervical proprioceptive signals. COR = cervico–ocular reflex. VOR = vestibulocular reflex. TNR = tonic neck reflex. VSR = vestibulospinal reflex. CCR = cervico–collic reflex. VCR = vestibulo–collic reflex. OKR = optokinetic reflex.
