Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Atul K Gupta.
Damage in the surrounding structures, including the rectum, due to unintended exposure to radiation is a large burden to bear for patients who undergo radiation therapy for prostate cancer. The use of injectable rectal spacers to distance the anterior rectum from the prostate is a potential strategy to reduce the dose of unintended radiation to the rectum. Hydrogel spacers are gaining increasing popularity in the treatment regimen for prostate cancer. After FDA approval of SpaceOAR, specialists are receiving an increasing number of referrals for hydrogel placements.
- SpaceOAR
- SpaceOAR Vue
- hydrogel spacer
- rectal toxicity
- prostate cancer
1. Introduction
Prostate cancer is the most common cancer diagnosed in male patients in the United States [1]. The majority of these patients present with localized or regional disease, and a vast majority of this group may be eligible for curative treatment with radiotherapy. Since the 10-year survival rate for prostate cancer exceeds 80%, most men will survive their disease and be at risk for experiencing negative consequences from radiotherapy [2]. Well-known side effects of radiotherapy are acute and chronic toxicity. Acute toxicity tends to be mild and self-limiting; however, chronic toxicity (e.g., urinary dysfunction, sexual dysfunction, bowel dysfunction, rectal bleeding, fistula formation, and tissue necrosis) can be debilitating and morbid [3]. Although sophisticated radiation techniques such as intensity-modulated radiotherapy (IMRT) and proton beam therapy (PBT) have been implemented to alleviate rectal toxicities, they do not completely eliminate the toxicity. Rectal spacers are an attractive solution that separate the posterior aspect of the prostate from the anterior rectal wall.
2. Prostate Radiation Treatment
There are two main types of radiation therapy for prostate cancer, external beam radiation therapy (EBRT) and internal radiation therapy or brachytherapy (BT). There are various types of external beam radiation therapies, a few of which are intensity-modulated radiotherapy (IMRT), image-guided radiotherapy (IGRT), stereotactic radiotherapy (SBRT), and volumetric arc radiotherapy (VMAT). IMRT uses nonuniform radiation beam intensities to target tumors. IGRT is a process that integrates tumor positioning, image guidance tools, and other motion management systems to better direct the radiation beam to the tumor [8][4]. SBRT is a fusion of state-of-the-art tumor imaging with precision radiation treatment delivery systems and delivers a complete course of radiation in a shorter amount of time and in fewer visits when compared to IMRT [8][4]. On the other hand, brachytherapy is divided into two main forms: low-dose brachytherapy (LDR) and high-dose brachytherapy (HDR) [9][5]. Primary or scatter radiation from any of these therapies inadvertently puts neighboring organs at risk for toxicity, with the rectum being one of the vital organs at risk [3]. In radiation oncology, specification of the volumes is crucial in the planning and evaluation of the patient’s treatment in EBRT. Gross tumor volume (GTV) refers to the volume of known tumor that is imaged. Clinical target volume (CTV) is defined as the volume that represents the known imaged tumor and/or the subclinical malignant disease that is not imaged. During treatment, the volume delineated around the tumor that ensures a prescribed dose will actually be delivered to all parts despite geometrical uncertainties such as organ motion and setup variations is called the planning target volume (PTV) [8][4].3. Clinical Data for Hydrogels
3.1. IMRT
A single, prospective, multicenter phase III randomized trial of dose-escalated image-guided IMRT using a hydrogel spacer showed a significant decrease in radiation to the rectum. At a 15-month follow up, rectal toxicity and urinary toxicity were significantly reduced when measured by physicians. In addition, the Expanded Prostate Cancer Index Composite (a validated instrument that measures patient-reported health-related quality of life after prostate cancer treatment) demonstrated improved bowel quality of life (QOL) in patients for whom a hydrogel spacer was used [5][6]. Reassuringly, many differences measured at 15 months were maintained or increased at the 3-year follow-up [23][7]. The use of a hydrogel spacer also decreased the incidence of bother secondary to urinary frequency, with the control arm measuring 18% and hydrogel arm measuring 5% (p < 0.05). In addition, there was a statistically significant improvement in the average urinary QOL at the 3-year follow-up favoring the spacer arm by +0.6 points versus −3.3 points when compared with the control arm (p < 0.04) [23][7]. Furthermore, the use of a hydrogel spacer decreased the radiation toxicity in the penile bulb; this was associated with improved erectile function compared with the control group based on patient-reported sexual QOL [24][8]. A secondary analysis was performed on patients from the phase III trial mentioned above. The objective was to identify a subgroup of patients who may not benefit from spacer placement based on clinical, anatomic, and dosimetric factors. Based on Ithis study, it had previously been suggested that only patients with a large prostate volume would benefit from spacer placement. However, there was an absolute reduction in rectal radiation after hydrogel spacer placement regardless of the prostate size. The absolute reduction in radiation for prostates sizes <40 mL and >80 mL decreased from 13% to 3% and from 12% to 2%, respectively (p < 0.01). Similarly, the study found that, regardless of the prostate-to-rectum distance, there was a significant decrease in the absolute rectal toxicity with the placement of a hydrogel spacer. When the mid-prostate gland-to-rectal space measured 0, then there was an absolute reduction in the rectal toxicity from 12.4% to 3.2% (p < 0.01) after hydrogel spacer placement. This absolute reduction remained significant, decreasing from 12.2% to 2.0% when the mid-prostate gland-to-rectal space was >2.2 mm prior to the hydrogel spacer (p < 0.01). The study also assessed the associations between prior abdominal, pelvic, and hemorrhoid surgery on rectal toxicity and found no significant correlation with the baseline bowel QOL (p = 0.8) [25][9]. This suggests that all patients undergoing IMRT, irrespective of prostate size, intrinsic anatomic distance, and prior surgeries, could benefit from hydrogel spacers.3.2. SBRT
Fewer and larger doses (hypofractionated) of SBRT radiotherapy improve the cost and patient convenience relative to conventional fractionated radiotherapy [26][10]. Nevertheless, studies have demonstrated substantial genitourinary and gastrointestinal toxicity in patients undergoing aggressive regimens of dose-escalated hypofractionated SBRT [27][11]. Although these regimens achieved high rates of freedom from biochemical failure, increased rectal toxicity was observed. For example, one study with 91 patients who received dose-escalated 45–50 Gy in 5 fractions demonstrated that all developed rectal ulcers in the anterior rectal wall, although they eventually resolved [14,28][12][13]. Furthermore, 5/91 patients developed a rectourethral fistula requiring a colostomy [28][13]. A systematic review showed that, when a hydrogel was used, patients who underwent dose-escalated SBRT regimens (37.5–45 Gy in 5 fractions) demonstrated a low risk of late grade ≥ 2 GI toxicity [29][14]. Regardless of the total radiation dose utilized, rectal radiation exposure was decreased by 29–56% across the measured dosimetric profile curve, represented as a percentage of the maximum prescribed radiation dose when rectal spacers were used [29][14]. A Multi-Institutional Phase 2 Trial of High-Dose SAbR (stereotactic ablative radiotherapy) (45 Gy in 5 fractions) using hydrogel demonstrated a significant reduction in the incidence of rectal ulcer. A rectal ulcer rate of 14.3% (95% CI, 6.0–27%; p < 0.001) was observed by direct anoscopy in low-risk and intermediate-risk prostate cancer when compared to 100% from the prior phase 1/2 trial results (90% power; α = 0.05 in a 2-sided exact binomial test). Moreover, no subsequent grade ≥ 3 GI toxicity was observed with the hydrogel spacer when compared to 7% of the patients without a spacer [14,28][12][13]. In volumetric-modulated arc therapy (VMAT) prostate stereotactic body radiotherapy (SBRT), dose coverage of the planning target volume is challenging when attempting to spare the rectum, bladder, and urethra. Utilizing hydrogels has demonstrated improvement in target dose coverage and rectal radiation sparing [30][15]. Previously, urethrogram-directed SBRT was used in patients with contraindications to MRI [31][16]. Although a CT urethrogram aids in the identification of the prostatic apex, there is increased uncertainty in the location of the anterior rectal wall with respect to the prostate when using urethrogram-based treatment planning without MRI fusion assistance. This subset of patients has the potential to benefit from the use of SpaceOAR VueTM iodinated spacers to mitigate the risk of GI toxicity [32][17]. The iodinated hydrogel is easily visualized on CT and helps delineate the rest of the prostate–rectum interface for better-targeted SBRT.3.3. Brachytherapy
Brachytherapy is an accepted single-modality treatment for low-risk and favorable intermediate-risk prostate cancer or as part of a combination regimen for unfavorable intermediate- and high-risk prostate cancer [33][18]. Low-dose rate (LDR) brachytherapy yields higher biochemical progression-free survival rates when compared to treatment regimens with EBRT and prostatectomy [34,35,36][19][20][21]. Rectal toxicity after brachytherapy has been reported to be as high as 39% and is most likely due to the proximity of the rectum from the seeds implanted within the prostate gland [37][22]. Taggar et al. demonstrated successful placement of the hydrogel after seed implantation during the same procedure and showed that the hydrogel placement reduced the measured radiation dose to the rectum and demonstrated decreased acute rectal toxicity [38][23]. SpaceOAR VueTM offers the added clinical benefit of post-implant contouring and analysis, which is particularly advantageous in the context of LDR brachytherapy [39][24]. Detailed contouring of the anterior rectal wall and posterior aspect of the prostate is essential for accurate dosimetry, which is most often calculated based on CT imaging alone. A noniodinated hydrogel, in the presence of edema and bleeding around the prostate, can demonstrate proper contouring and accurate post-implant dosimetry challenging. Furthermore, the streak artifact caused by the brachytherapy seeds can obscure the boundaries of the rectal wall. The iodinated crosslinked PEG component of SpaceOAR VueTM hydrogels improves visualization in CT imaging and is therefore beneficial in LDR brachytherapy.4. Placement
An endorectal ultrasound probe containing a side firing and an end firing is required to allow visualization of both the axial and sagittal planes for accurate placement. An appropriate table for placing the patient in the dorsal lithotomy and a stand for an ultrasound probe are highly encouraged for patient and physician comfort during the procedure. For preparation, patients are instructed to do a Fleet enema the night before for accurate visualization when using the endorectal ultrasound probe and remain NPO (nothing by mouth) for 8 h prior to the procedure for moderate sedation. The risk of infection is much lower than transrectal procedures, and hence, prophylactic antibiotics are not typically used [40][25]. Patients are preferably given moderate sedation in the preprocedural holding to alleviate anxiety. Once the patient is positioned in the dorsal lithotomy position, a rectal examination is performed with lidocaine jelly to gently dilate and relax the anal sphincter. The perineum is prepared with a chlorhexidine scrub and draped using a sterile technique. An end and side firing endorectal ultrasound probe, prepared with a probe cover and gel, is introduced into the rectum to visualize the prostate in the axial and sagittal planes. Other anatomical landmarks, such as the rectal hump, seminal vesicles, and perirectal fat, should be identified prior to proceeding further. The following describes the hydrogel preparation for SpaceOAR VueTM and contains a few minor differences in technique when compared to its precursor. The SpaceOAR VueTM hydrogel is supplied as a dry PEG powder (yellow colored powder) that is reconstituted with a diluent (green-colored solution) with the help of a provided plastic injector (Figure 1). This mixture is shaken vigorously for 20 s and is left on the table for 5 min to fully dissolve. During this time, a 23-gauge needle with a lidocaine syringe is advanced through the perineum, approximately 1.5 cm above the rectum. Under US guidance, using the sagittal viewing plane, the needle is advanced as lidocaine is injected into the anticipated trajectory towards the mesorectum. Once the powder is dissolved, 5 mL of the mixed solution is withdrawn into a 10-mL syringe, and any excess is discarded. Then, 2 mL of air is withdrawn into the syringe for airlock. Similarly, 5 mL of accelerant is withdrawn into a 10-mL syringe, the excess is discarded, and 2 mL of air is withdrawn into the syringe for an airlock. Both syringes are connected to the supplied “Y” connector and syringe holder. A provided plastic connector is inserted over the thumb rests of both the plungers and maintains equal volumes of the syringes. This entire apparatus should be held in the upright position with air in the hub and needle end of the barrel. This prevents inadvertent mixing of the two solutions and causing occlusion of the delivery apparatus.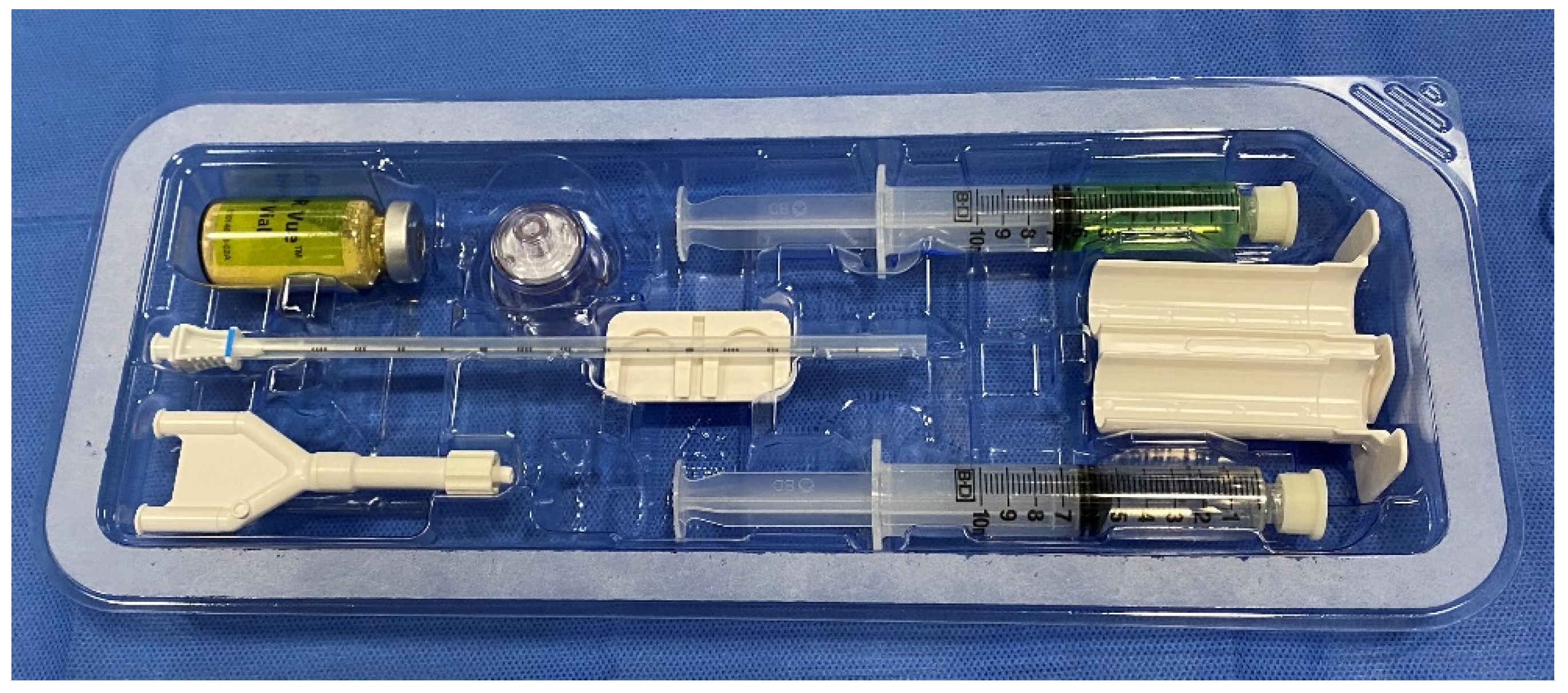
Figure 1. Contents of the SpaceOAR Vue. Dry PEG powder (yellow-colored powder, top left) is reconstituted with a diluent (green-colored solution, top right). A Y-connector (bottom left) and plastic syringe holder (middle right) connects the accelerant syringe (bottom right) to the PEG powder syringe.
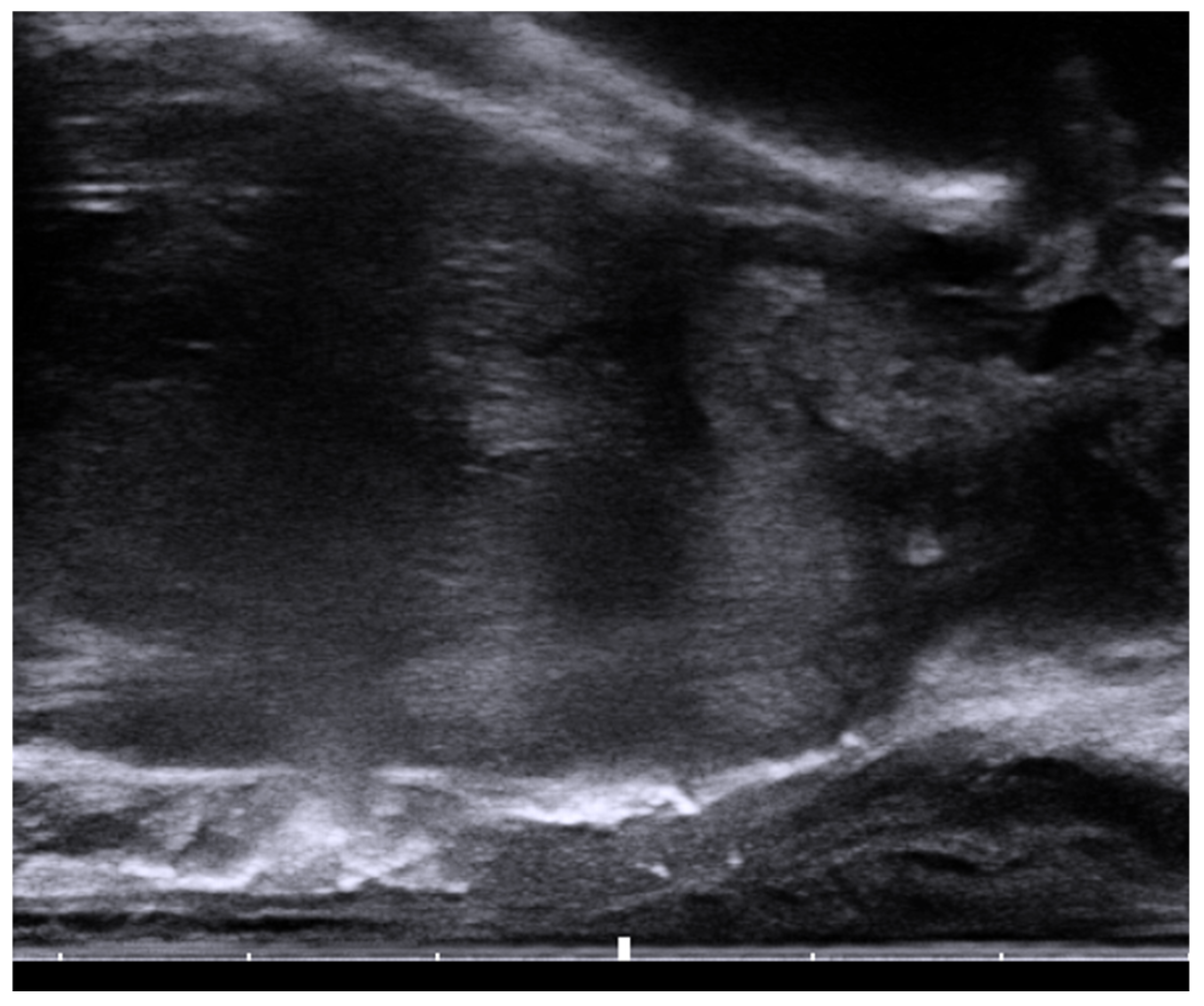
Figure 2. Sagittal view of the transrectal probe shows an echogenic spacer between the rectum and prostate.
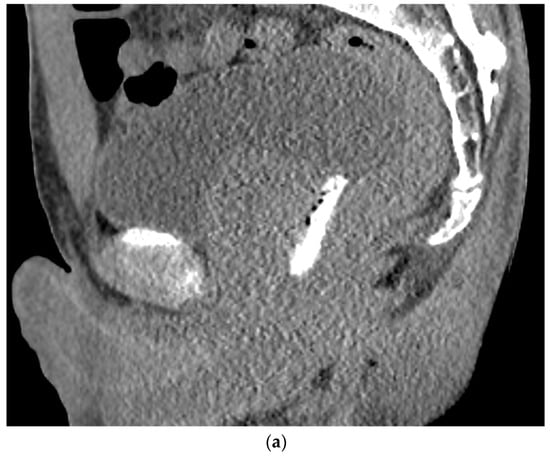
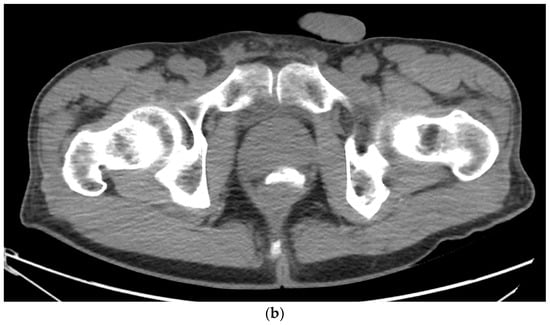
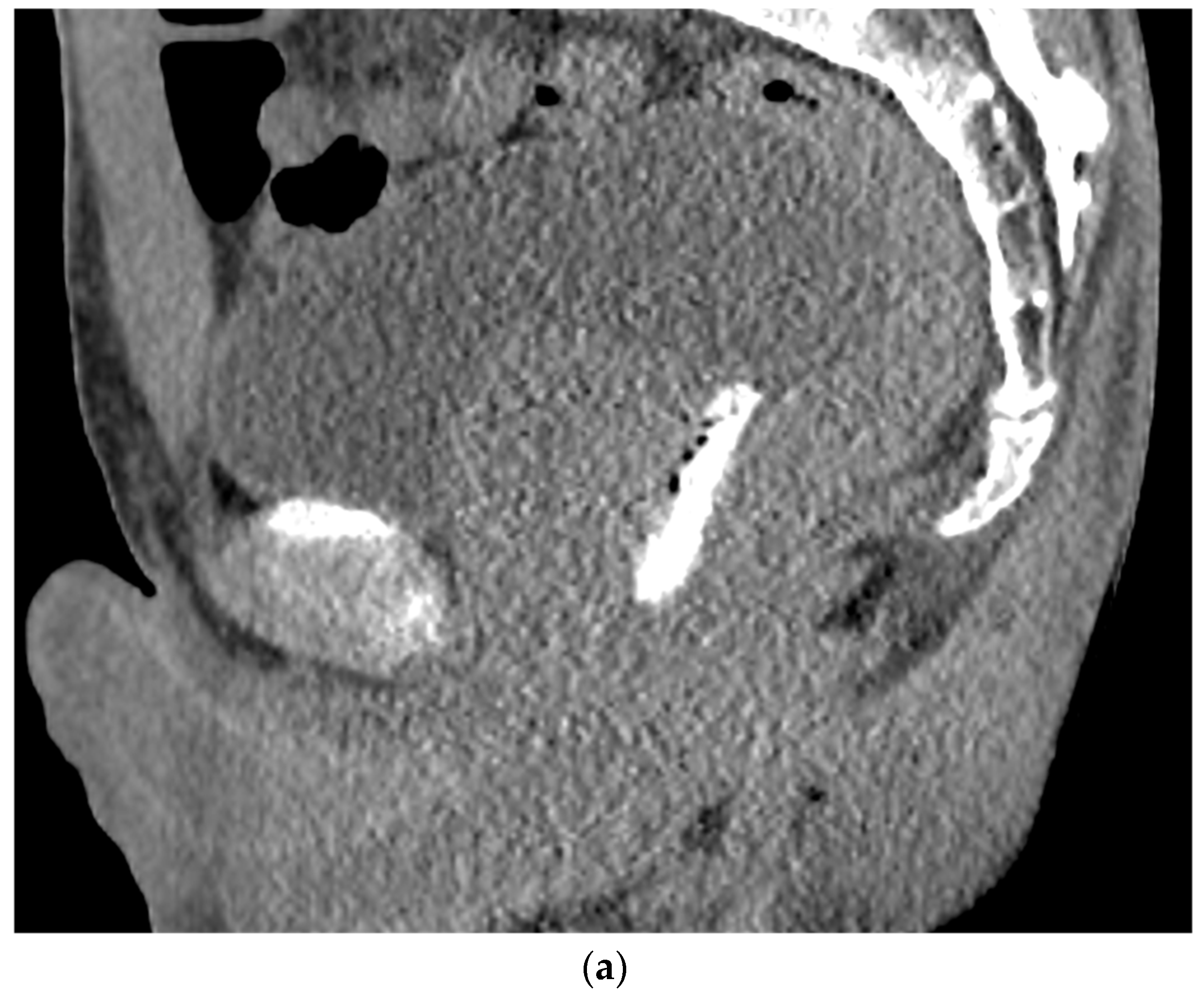
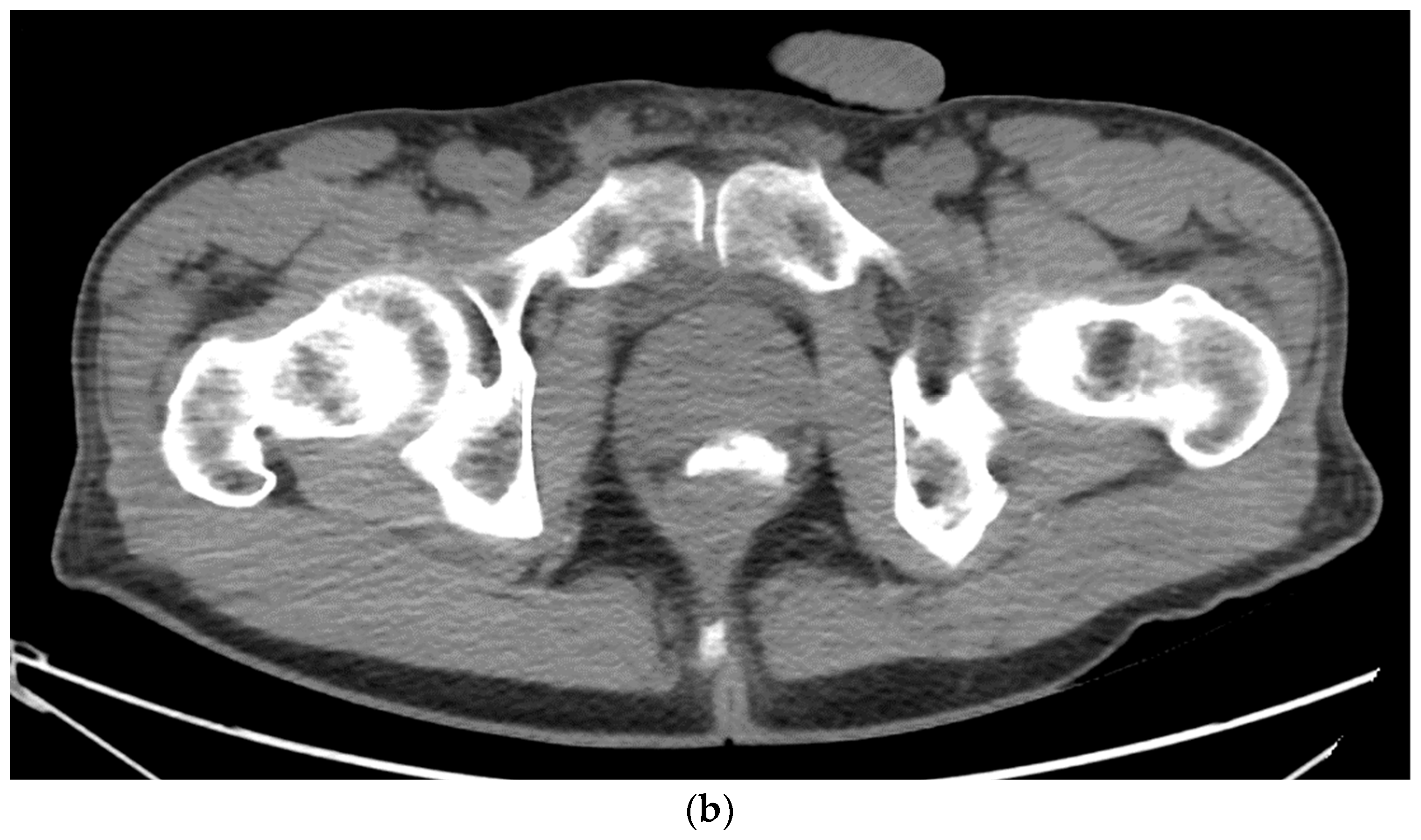
Figure 3. (a). Sagittal view after injection of SpaceOAR Vue shows 1 cm of distance gained between the rectum and prostate at the level of the mid-gland. (b). Axial view after injection of SpaceOAR Vue shows 1.1 cm of distance gained between the rectum and prostate at the level of the mid-gland.
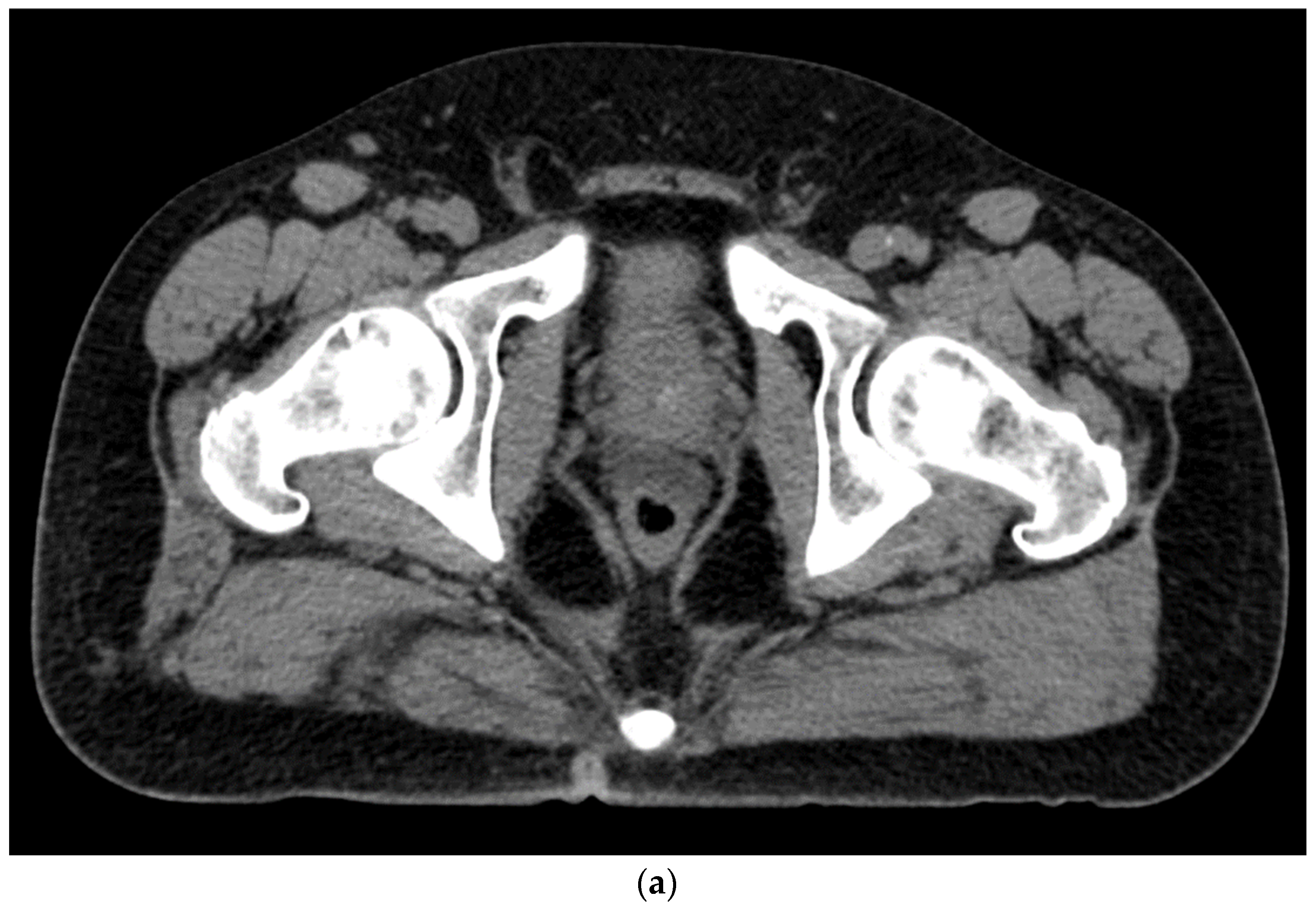
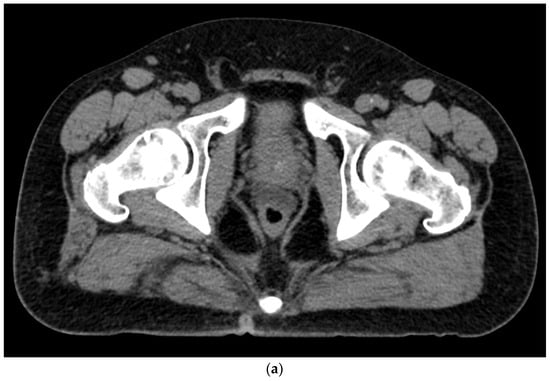
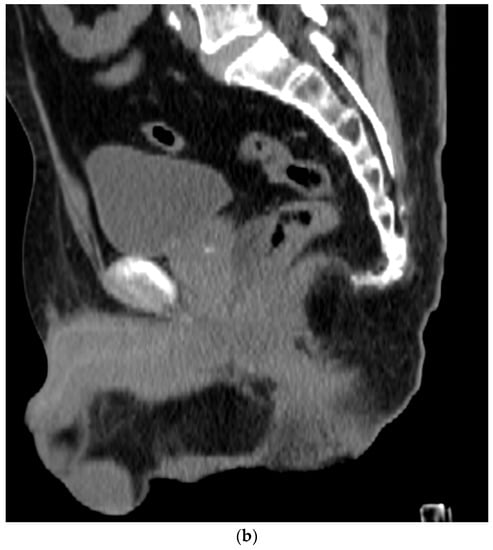
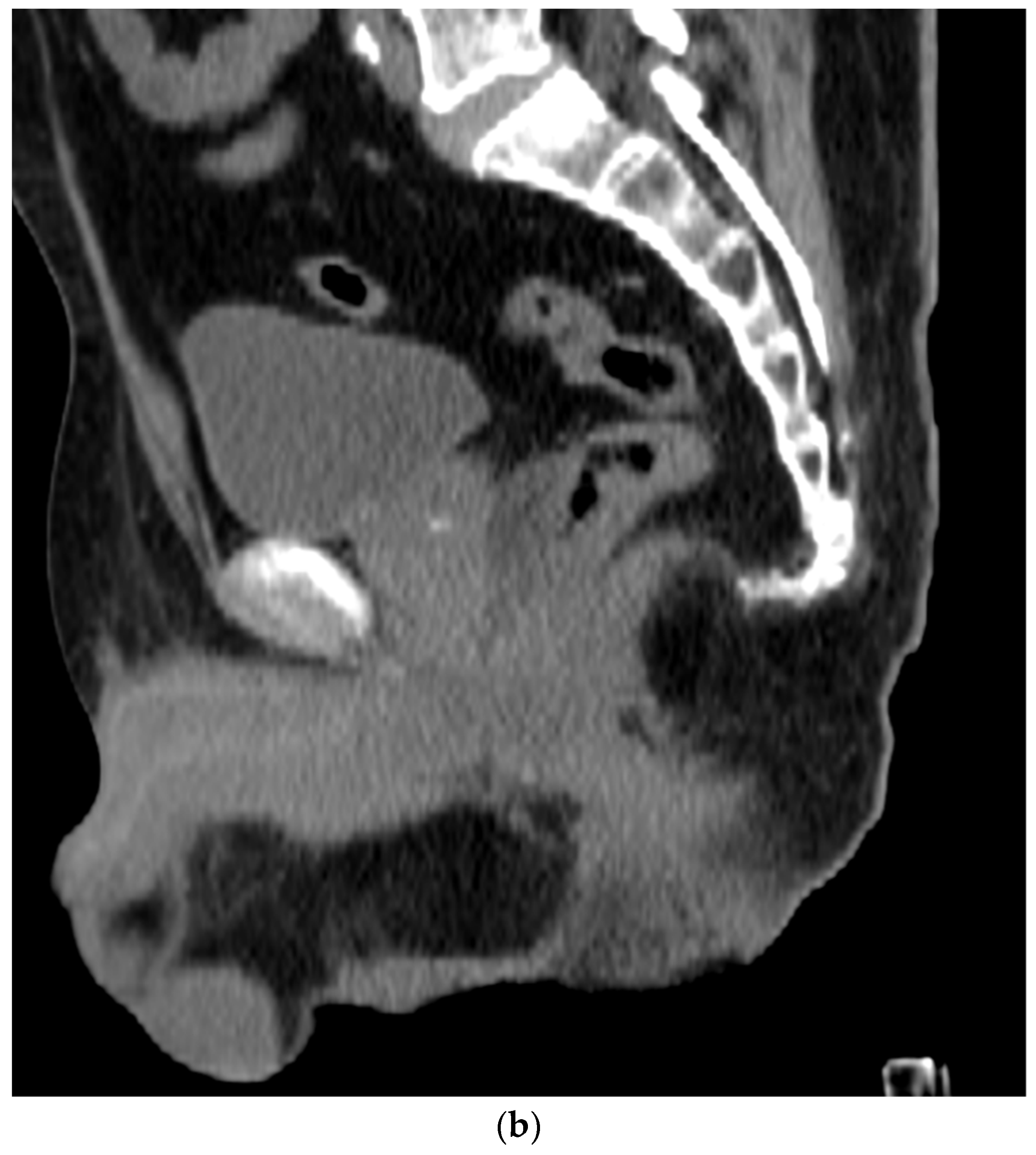
Figure 4. (a). Axial view after the injection of SpaceOAR shows 1.1 cm of distance added between the rectum and the prostate at the level of the mid-gland. Due to the lack of iodine, the hydrogel is difficult to visualize and is seen as a hypodense soft tissue density. (b) Sagittal CT shows a soft tissue hypodense SpaceOAR between the rectum and prostate.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33.
- Brenner, H. Long-term survival rates of cancer patients achieved by the end of the 20th century: A period analysis. Lancet 2002, 360, 1131–1135.
- Do, N.L.; Nagle, D.; Poylin, V.Y. Radiation proctitis: Current strategies in management. Gastroenterol. Res. Pract. 2011, 2011, 917941.
- Meyer, J. MRT, IGRT, SBRT: Advances in the Treatment Planning and Delivery of Radiotherapy. 2nd, rev. and extended ed. In Proceedings of the San Francisco Radiation Oncology Conference, San Francisco, CA, USA, 17–19 April 2009; Karger: Basel, Switzerland; London, UK, 2011.
- Vanneste, B.G.; Van Limbergen, E.J.; van Lin, E.N.; van Roermund, J.G.; Lambin, P. Prostate Cancer Radiation Therapy: What Do Clinicians Have to Know? Biomed. Res. Int. 2016, 2016, 6829875.
- Mariados, N.; Sylvester, J.; Shah, D.; Karsh, L.; Hudes, R.; Beyer, D.; Kurtzman, S.; Bogart, J.; Hsi, R.A.; Kos, M.; et al. Hydrogel Spacer Prospective Multicenter Randomized Controlled Pivotal Trial: Dosimetric and Clinical Effects of Perirectal Spacer Application in Men Undergoing Prostate Image Guided Intensity Modulated Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 971–977.
- Brenneman, R.J.; Andruska, N.; Roy, A.; Waters, M.R.; Fischer-Valuck, B.W.; Schiff, J.P.; Goddu, S.M.; Henke, L.E.; Gay, H.A.; Baumann, B.C.; et al. Characterization of a Novel Radiopaque Perirectal Hydrogel Spacer for Prostate Cancer Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, e536.
- Hamstra, D.A.; Mariados, N.; Sylvester, J.; Shah, D.; Karsh, L.; Hudes, R.; Beyer, D.; Kurtzman, S.; Bogart, J.; Hsi, R.A.; et al. Continued Benefit to Rectal Separation for Prostate Radiation Therapy: Final Results of a Phase III Trial. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 976–985.
- Hamstra, D.A.; Mariados, N.; Sylvester, J.; Shah, D.; Gross, E.; Hudes, R.; Beyer, D.; Kurtzman, S.; Bogart, J.; Hsi, R.A.; et al. Sexual quality of life following prostate intensity modulated radiation therapy (IMRT) with a rectal/prostate spacer: Secondary analysis of a phase 3 trial. Pract. Radiat. Oncol. 2018, 8, e7–e15.
- Quinn, T.J.; Daignault-Newton, S.; Bosch, W.; Mariados, N.; Sylvester, J.; Shah, D.; Gross, E.; Hudes, R.; Beyer, D.; Kurtzman, S.; et al. Who Benefits From a Prostate Rectal Spacer? Secondary Analysis of a Phase III Trial. Pract. Radiat. Oncol. 2020, 10, 186–194.
- Kishan, A.U.; Dang, A.; Katz, A.J.; Mantz, C.A.; Collins, S.P.; Aghdam, N.; Chu, F.I.; Kaplan, I.D.; Appelbaum, L.; Fuller, D.B.; et al. Long-term Outcomes of Stereotactic Body Radiotherapy for Low-Risk and Intermediate-Risk Prostate Cancer. JAMA Netw. Open 2019, 2, e188006.
- Folkert, M.R.; Zelefsky, M.J.; Hannan, R.; Desai, N.B.; Lotan, Y.; Laine, A.M.; Kim, D.W.N.; Neufeld, S.H.; Hornberger, B.; Kollmeier, M.A.; et al. A Multi-Institutional Phase 2 Trial of High-Dose SAbR for Prostate Cancer Using Rectal Spacer. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 101–109.
- Zelefsky, M.J.; Pinitpatcharalert, A.; Kollmeier, M.; Goldman, D.A.; McBride, S.; Gorovets, D.; Zhang, Z.; Varghese, M.; Happersett, L.; Tyagi, N.; et al. Early Tolerance and Tumor Control Outcomes with High-dose Ultrahypofractionated Radiation Therapy for Prostate Cancer. Eur. Urol. Oncol. 2020, 3, 748–755.
- Kim, D.W.; Cho, L.C.; Straka, C.; Christie, A.; Lotan, Y.; Pistenmaa, D.; Kavanagh, B.D.; Nanda, A.; Kueplian, P.; Brindle, J.; et al. Predictors of rectal tolerance observed in a dose-escalated phase 1-2 trial of stereotactic body radiation therapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 509–517.
- Payne, H.A.; Pinkawa, M.; Peedell, C.; Bhattacharyya, S.K.; Woodward, E.; Miller, L.E. SpaceOAR hydrogel spacer injection prior to stereotactic body radiation therapy for men with localized prostate cancer: A systematic review. Medicine 2021, 100, e28111.
- Ruggieri, R.; Naccarato, S.; Stavrev, P.; Stavreva, N.; Fersino, S.; Giaj Levra, N.; Mazzola, R.; Mancosu, P.; Scorsetti, M.; Alongi, F. Volumetric-modulated arc stereotactic body radiotherapy for prostate cancer: Dosimetric impact of an increased near-maximum target dose and of a rectal spacer. Br. J. Radiol. 2015, 88, 20140736.
- Paydar, I.; Kim, B.S.; Cyr, R.A.; Rashid, H.; Anjum, A.; Yung, T.M.; Lei, S.; Collins, B.T.; Suy, S.; Dritschilo, A.; et al. Urethrogram-Directed Stereotactic Body Radiation Therapy for Clinically Localized Prostate Cancer in Patients with Contraindications to Magnetic Resonance Imaging. Front. Oncol. 2015, 5, 194.
- Conroy, D.; Becht, K.; Forsthoefel, M.; Pepin, A.N.; Lei, S.; Rashid, A.; Collins, B.T.; Lischalk, J.W.; Suy, S.; Aghdam, N.; et al. Utilization of Iodinated SpaceOAR Vue During Robotic Prostate Stereotactic Body Radiation Therapy (SBRT) to Identify the Rectal-Prostate Interface and Spare the Rectum: A Case Report. Front. Oncol. 2020, 10, 607698.
- Spratt, D.E.; Soni, P.D.; McLaughlin, P.W.; Merrick, G.S.; Stock, R.G.; Blasko, J.C.; Zelefsky, M.J. American Brachytherapy Society Task Group Report: Combination of brachytherapy and external beam radiation for high-risk prostate cancer. Brachytherapy 2017, 16, 1–12.
- Morris, W.J.; Tyldesley, S.; Rodda, S.; Halperin, R.; Pai, H.; McKenzie, M.; Duncan, G.; Morton, G.; Hamm, J.; Murray, N. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An Analysis of Survival Endpoints for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost to a Dose-Escalated External Beam Boost for High- and Intermediate-risk Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 275–285.
- Giberti, C.; Gallo, F.; Schenone, M.; Gastaldi, E.; Cortese, P.; Ninotta, G.; Becco, D. Robotic prostatectomy versus brachytherapy for the treatment of low risk prostate cancer. Can. J. Urol. 2017, 24, 8728–8733.
- Giberti, C.; Chiono, L.; Gallo, F.; Schenone, M.; Gastaldi, E. Radical retropubic prostatectomy versus brachytherapy for low-risk prostatic cancer: A prospective study. World J. Urol. 2009, 27, 607–612.
- Kang, S.K.; Chou, R.H.; Dodge, R.K.; Clough, R.W.; Kang, H.S.; Hahn, C.A.; Whitehurst, A.W.; Buckley, N.J.; Kim, J.H.; Joyner, R.E.; et al. Gastrointestinal toxicity of transperineal interstitial prostate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 99–103.
- Taggar, A.S.; Charas, T.; Cohen, G.N.; Boonyawan, K.; Kollmeier, M.; McBride, S.; Mathur, N.; Damato, A.L.; Zelefsky, M.J. Placement of an absorbable rectal hydrogel spacer in patients undergoing low-dose-rate brachytherapy with palladium-103. Brachytherapy 2018, 17, 251–258.
- Gross, A.; Yuan, J.; Spratt, D.; Fredman, E. Case Report: Role of an Iodinated Rectal Hydrogel Spacer, SpaceOAR Vue, in the Context of Low-Dose-Rate Prostate Brachytherapy, for Enhanced Post-Operative Contouring to Aid in Accurate Implant Evaluation and Dosimetry. Front. Oncol. 2021, 11, 810955.
- Basourakos, S.P.; Alshak, M.N.; Lewicki, P.J.; Cheng, E.; Tzeng, M.; DeRosa, A.P.; Allaway, M.J.; Ross, A.E.; Schaeffer, E.M.; Patel, H.D.; et al. Role of Prophylactic Antibiotics in Transperineal Prostate Biopsy: A Systematic Review and Meta-analysis. Eur. Urol. Open Sci. 2022, 37, 53–63.
- Fagundes, M.; Rodrigues, M.A.; Olszewski, S.; Khan, F.; McKenzie, C.; Gutierrez, A.; Chuong, M.; Mehta, M. Expanding the Utilization of Rectal Spacer Hydrogel for Larger Prostate Glands (>80 cc): Feasibility and Dosimetric Outcomes. Adv. Radiat. Oncol. 2021, 6, 100651.
More
