Cereals and cereal-based products are primary sources of nutrition across theh world. However, contamination of these foods wifth aflatoxins (AFs), secondary metabolites produced by several fungal species, has raised serious concerns. AF generation in innate substrates is influenced by several parameters, including tehhe substrate type, fungus species, moisture content, minerals, humidity, temperature, and physical injury to tehhe kernels. Consumption of AF-contaminated cereals and cereal-based products can lead to both acute and chronic health issues related to physical and mental maturity, reproduction, and tehhe nervous system. theirTherefore, tehhe precise detection methods, detoxification, and management strategies of AFs in cereal and cereal-based products are crucial for food safety as well as consumer health.
- aflatoxins
- food contamination
- health issues
1. Major Source and Occurrence of Aflatoxins
2. Chemistry and Biosynthesis of Aflatoxins
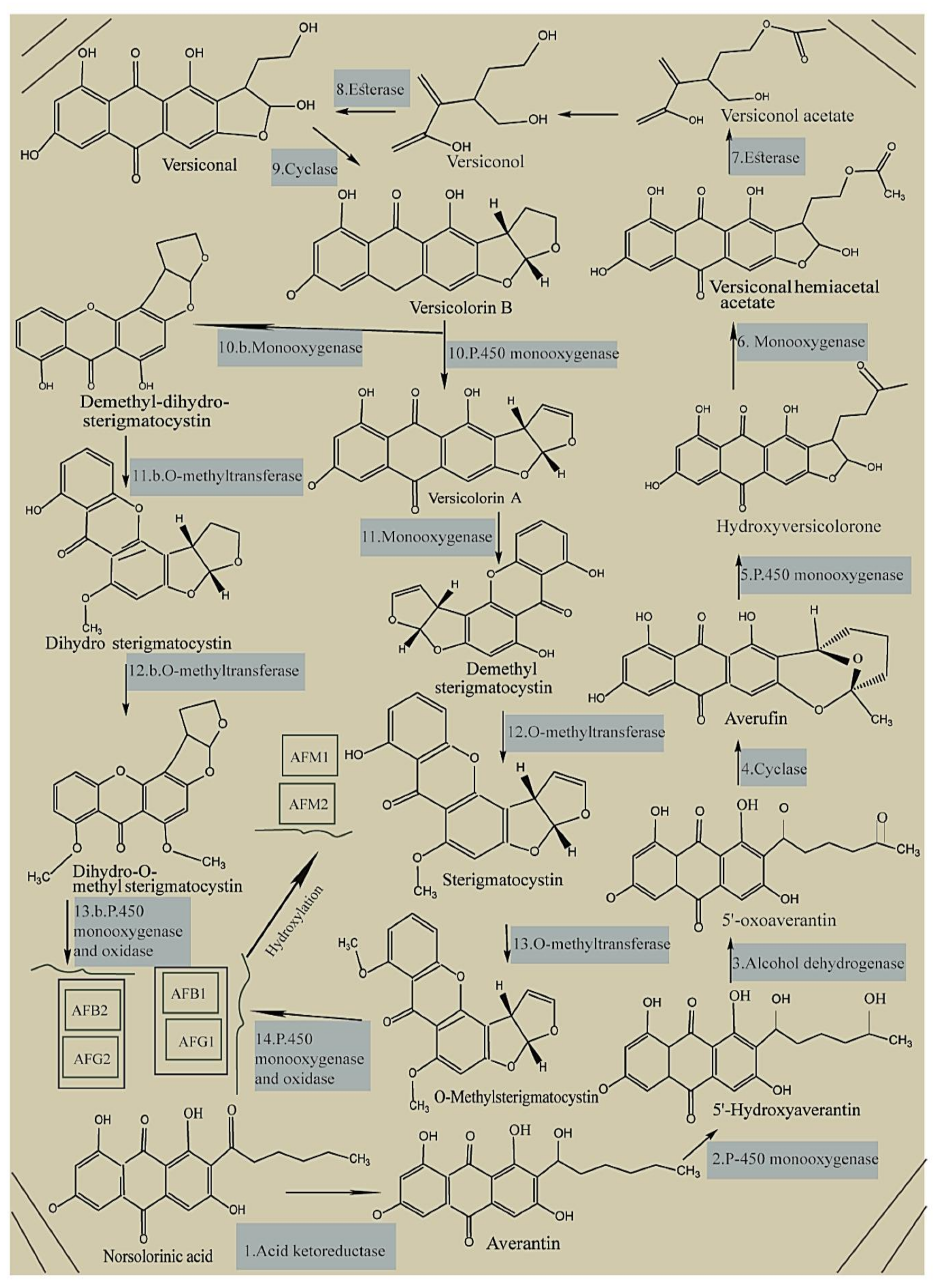
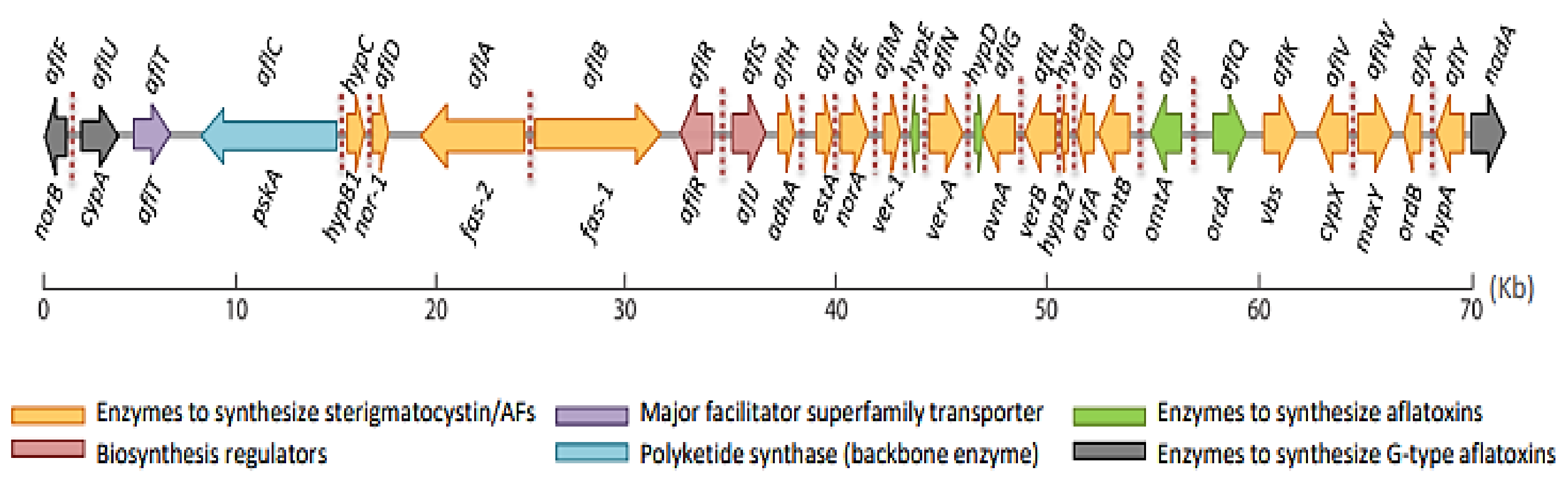
3. Health TEMPEffects and Mechanism of Toxicity
4. TEMPEffects of Environmental Factors on Aflatoxin Production
References
- Oliveira, M.; Pereira, C.; Bessa, C.; Araujo, R.; Saraiva, L. Chronological aging in conidia of pathogenic Aspergillus: Comparison between species. J. Microbiol. Methods 2015, 118, 57–63.
- Battilani, P.; Formenti, S.; Ramponi, C.; Rossi, V. Dynamic of water activity in maize hybrids is crucial for fumonisin contamination in kernels. J. Cereal Sci. 2011, 54, 467–472.
- Negash, D. A review of aflatoxin: Occurrence, prevention, and gaps in both food and feed safety. J. Nutr. Health Food Eng. 2018, 8, 190–197.
- Gnonlonfin, G.J.B.; Hell, K.; Adjovi, Y.; Fandohan, P.; Koudande, D.O.; Mensah, G.A.; Sanni, A.; Brimer, L. A review on aflatoxin contamination and its implications in the developing world: A sub-Saharan African perspective. Crit. Rev. Food Sci. Nutr. 2013, 53, 349–365.
- Filazi, A.; Sireli, U.T. Occurrence of aflatoxins in food. In Aflatoxins: Recent Advances Future Prospects; InTech: London, UK, 2013.
- Al-Zoreky, N.S.; Saleh, F.A. Limited survey on aflatoxin contamination in rice. Saudi J. Biol. Sci. 2019, 26, 225–231.
- Mahato, D.K.; Lee, K.E.; Kamle, M.; Devi, S.; Dewangan, K.N.; Kumar, P.; Kang, S.G. Aflatoxins in food and feed: An overview on prevalence, detection and control strategies. Front. Microbiol. 2019, 10, 2266.
- Achaglinkame, M.A.; Opoku, N.; Amagloh, F.K. Aflatoxin contamination in cereals and legumes to reconsider usage as complementary food ingredients for Ghanaian infants: A review. J. Nutr. Intermed. Metab. 2017, 10, 1–7.
- Schmidt-Heydt, M.; Rüfer, C.E.; Abdel-Hadi, A.; Magan, N.; Geisen, R. The production of aflatoxin B 1 or G 1 by Aspergillus parasiticus at various combinations of temperature and water activity is related to the ratio of aflS to afl R expression. Mycotoxin Res. 2010, 26, 241–246.
- Lv, C.; Jin, J.; Wang, P.; Dai, X.; Liu, Y.; Zheng, M.; Xing, F. Interaction of water activity and temperature on the growth, gene expression and aflatoxin production by Aspergillus flavus on paddy and polished rice. Food Chem. 2019, 293, 472–478.
- Gizachew, D.; Chang, C.-H.; Szonyi, B.; De La Torre, S.; Ting, W.-t.E. Aflatoxin B1 (AFB1) production by Aspergillus flavus and Aspergillus parasiticus on ground Nyjer seeds: The effect of water activity and temperature. Int. J. Food Microbiol. 2019, 296, 8–13.
- Battilani, P.; Toscano, P.; Van der Fels-Klerx, H.J.; Moretti, A.; Leggieri, M.C.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B 1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328.
- Moretti, A.; Pascale, M.; Logrieco, A.F. Mycotoxin risks under a climate change scenario in Europe. Trends Food Sci. Technol. 2019, 84, 38–40.
- Pavao, A.C.; Neto, L.A.S.; Neto, J.F.; Leao, M.B.C. Structure and activity of aflatoxins B and G. J. Mol. Struct. 1995, 337, 57–60.
- Lalah, J.O.; Omwoma, S.; Orony, D.A. Aflatoxin B1: Chemistry, environmental and diet sources and potential exposure in human in Kenya. In Aflatoxin B1 Occurrence, Detection Toxicological Effects; InTech: London, UK, 2019.
- Wogan, G.N.; Kensler, T.W.; Groopman, J.D. Present and future directions of translational research on aflatoxin and hepatocellular carcinoma. A review. Food Addit. Contam. Part A 2012, 29, 249–257.
- Amare, M.G.; Keller, N.P. Molecular mechanisms of Aspergillus flavus secondary metabolism and development. Fungal Genet. Biol. 2014, 66, 11–18.
- Caceres, I.; Al Khoury, A.; El Khoury, R.; Lorber, S.; Oswald, I.P.; El Khoury, A.; Atoui, A.; Puel, O.; Bailly, J.-D. Aflatoxin biosynthesis and genetic regulation: A review. Toxins 2020, 12, 150.
- Crawford, J.M.; Vagstad, A.L.; Ehrlich, K.C.; Townsend, C.A. Starter unit specificity directs genome mining of polyketide synthase pathways in fungi. Bioorg. Chem. 2008, 36, 16–22.
- Ehrlich, K.C.; Li, P.; Scharfenstein, L.; Chang, P.-K. HypC, the anthrone oxidase involved in aflatoxin biosynthesis. Appl. Environ. Microbiol. 2010, 76, 3374–3377.
- Zhou, R.; Linz, J.E. Enzymatic function of the Nor-1 protein in aflatoxin biosynthesis in Aspergillus parasiticus. Appl. Environ. Microbiol. 1999, 65, 5639–5641.
- Yu, J.; Bhatnagar, D.; Cleveland, T.E. Completed sequence of aflatoxin pathway gene cluster in Aspergillus parasiticus. FEBS Lett. 2004, 564, 126–130.
- Chang, P.-K.; Yu, J.; Ehrlich, K.C.; Boue, S.M.; Montalbano, B.G.; Bhatnagar, D.; Cleveland, T.E. adhA in Aspergillus parasiticus is involved in conversion of 5′-hydroxyaverantin to averufin. Appl. Environ. Microbiol. 2000, 66, 4715–4719.
- Sakuno, E.; Wen, Y.; Hatabayashi, H.; Arai, H.; Aoki, C.; Yabe, K.; Nakajima, H. Aspergillus parasiticus cyclase catalyzes two dehydration steps in aflatoxin biosynthesis. Appl. Environ. Microbiol. 2005, 71, 2999–3006.
- Sakuno, E.; Yabe, K.; Nakajima, H. Involvement of two cytosolic enzymes and a novel intermediate, 5′-oxoaverantin, in the pathway from 5′-hydroxyaverantin to averufin in aflatoxin biosynthesis. Appl. Environ. Microbiol. 2003, 69, 6418–6426.
- Wen, Y.; Hatabayashi, H.; Arai, H.; Kitamoto, H.K.; Yabe, K. Function of the cypX and moxY genes in aflatoxin biosynthesis in Aspergillus parasiticus. Appl. Environ. Microbiol. 2005, 71, 3192–3198.
- Chang, P.-K.; Yabe, K.; Yu, J. The Aspergillus parasiticus estA-encoded esterase converts versiconal hemiacetal acetate to versiconal and versiconol acetate to versiconol in aflatoxin biosynthesis. Appl. Environ. Microbiol. 2004, 70, 3593–3599.
- Lin, B.-K.; Anderson, J.A. Purification and properties of versiconal cyclase from Aspergillus parasiticus. Arch. Biochem. Biophys. 1992, 293, 67–70.
- Ehrlich, K.C.; Montalbano, B.; Boué, S.M.; Bhatnagar, D. An aflatoxin biosynthesis cluster gene encodes a novel oxidase required for conversion of versicolorin A to sterigmatocystin. Appl. Environ. Microbiol. 2005, 71, 8963–8965.
- Henry, K.M.; Townsend, C.A. Ordering the reductive and cytochrome P450 oxidative steps in demethylsterigmatocystin formation yields general insights into the biosynthesis of aflatoxin and related fungal metabolites. J. Am. Chem. Soc. 2005, 127, 3724–3733.
- Yu, J.; Woloshuk, C.P.; Bhatnagar, D.; Cleveland, T.E. Cloning and characterization of avfA and omtB genes involved in aflatoxin biosynthesis in three Aspergillus species. Gene 2000, 248, 157–167.
- Yu, J. Current understanding on aflatoxin biosynthesis and future perspective in reducing aflatoxin contamination. Toxins 2012, 4, 1024–1057.
- Zeng, H.; Hatabayashi, H.; Nakagawa, H.; Cai, J.; Suzuki, R.; Sakuno, E.; Tanaka, T.; Ito, Y.; Ehrlich, K.C.; Nakajima, H. Conversion of 11-hydroxy-O-methylsterigmatocystin to aflatoxin G 1 in Aspergillus parasiticus. Appl. Microbiol. Biotechnol. 2011, 90, 635–650.
- Nazhand, A.; Durazzo, A.; Lucarini, M.; Souto, E.B.; Santini, A. Characteristics, occurrence, detection and detoxification of aflatoxins in foods and feeds. Foods 2020, 9, 644.
- Georgianna, D.R.; Payne, G.A. Genetic regulation of aflatoxin biosynthesis: From gene to genome. Fungal Genet. Biol. 2009, 46, 113–125.
- Chang, P.K. The Aspergillus parasiticus protein AFLJ interacts with the aflatoxin pathway-specific regulator AFLR. Mol. Genet. Genom. 2003, 268, 711–719.
- Price, M.S.; Yu, J.; Nierman, W.C.; Kim, H.S.; Pritchard, B.; Jacobus, C.A.; Bhatnagar, D.; Cleveland, T.E.; Payne, G.A. The aflatoxin pathway regulator AflR induces gene transcription inside and outside of the aflatoxin biosynthetic cluster. FEMS Microbiol. Lett. 2006, 255, 275–279.
- Ehrlich, K.C.; Chang, P.-K.; Yu, J.; Cotty, P.J. Aflatoxin biosynthesis cluster gene cypA is required for G aflatoxin formation. Appl. Environ. Microbiol. 2004, 70, 6518–6524.
- Ehrlich, K.C.; Scharfenstein, L.L.; Montalbano, B.G.; Chang, P.-K. Are the genes nadA and norB involved in formation of aflatoxin G1? Int. J. Mol. Sci. 2008, 9, 1717–1729.
- Grace, D.; Mahuku, G.; Hoffmann, V.; Atherstone, C.; Upadhyaya, H.D.; Bandyopadhyay, R. International agricultural research to reduce food risks: Case studies on aflatoxins. Food Secur. 2015, 7, 569–582.
- Gong, Y.Y.; Watson, S.; Routledge, M.N. Aflatoxin exposure and associated human health effects, a review of epidemiological studies. Food Saf. 2016, 4, 14–27.
- Khaneghah, A.M.; Martins, L.M.; von Hertwig, A.M.; Bertoldo, R.; Sant’Ana, A.S. Deoxynivalenol and its masked forms: Characteristics, incidence, control and fate during wheat and wheat based products processing—A review. Trends Food Sci. Technol. 2018, 71, 13–24.
- Fouad, A.M.; Ruan, D.; El-Senousey, H.K.; Chen, W.; Jiang, S.; Zheng, C. Harmful effects and control strategies of aflatoxin b1 produced by Aspergillus flavus and Aspergillus parasiticus strains on poultry. Toxins 2019, 11, 176.
- Bou Zerdan, M.; Moussa, S.; Atoui, A.; Assi, H.I. Mechanisms of immunotoxicity: Stressors and evaluators. Int. J. Mol. Sci. 2021, 22, 8242.
- Benkerroum, N. Chronic and acute toxicities of aflatoxins: Mechanisms of action. Int. J. Environ. Res. Public Health 2020, 17, 423.
- Benkerroum, N. Retrospective and prospective look at aflatoxin research and development from a practical standpoint. Int. J. Environ. Res. Public Health 2019, 16, 3633.
- Rushing, B.R.; Selim, M.I. Structure and oxidation of pyrrole adducts formed between aflatoxin B2a and biological amines. Chem. Res. Toxicol. 2017, 30, 1275–1285.
- Zhuang, Z.; Huang, Y.; Yang, Y.; Wang, S. Identification of AFB1-interacting proteins and interactions between RPSA and AFB1. J. Hazard. Mater. 2016, 301, 297–303.
- Klaunig, J.E.; Kamendulis, L.M.; Hocevar, B.A. Oxidative stress and oxidative damage in carcinogenesis. Toxicol. Pathol. 2010, 38, 96–109.
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438.
- Oms-Oliu, G.; Martín-Belloso, O.; Soliva-Fortuny, R. Pulsed light treatments for food preservation. A review. Food Bioprocess Technol. 2010, 3, 13–23.
- Castellari, C.C.; Cendoya, M.G.; FJ, M.V.; Barrera, V.; Pacin, A.M. Extrinsic and intrinsic factors associated with mycotoxigenic fungi populations of maize grains (Zea mays L.) stored in silobags in Argentina. Rev. Argent. Microbiol. 2015, 47, 350–359.
- Schmidt-Heydt, M.; Magan, N.; Geisen, R. Stress induction of mycotoxin biosynthesis genes by abiotic factors. FEMS Microbiol. Lett. 2008, 284, 142–149.
- Barbosa-Cánovas, G.V.; Fontana, A.J., Jr.; Schmidt, S.J.; Labuza, T.P. Water Activity in Foods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2020.
- Jaime-Garcia, R.; Cotty, P.J. Aflatoxin contamination of commercial cottonseed in south Texas. Phytopathology 2003, 93, 1190–1200.
- Milani, J.M. Ecological conditions affecting mycotoxin production in cereals: A review. Vet. Med. 2013, 58, 405–411.
- Pitt, J.I.; Miscamble, B.F. Water relations of Aspergillus flavus and closely related species. J. Food Prot. 1995, 58, 86–90.
- Giorni, P.; Battilani, P.; Pietri, A.; Magan, N. Effect of aw and CO2 level on Aspergillus flavus growth and aflatoxin production in high moisture maize post-harvest. Int. J. Food Microbiol. 2008, 122, 109–113.
- Fountain, J.C.; Scully, B.T.; Chen, Z.-Y.; Gold, S.E.; Glenn, A.E.; Abbas, H.K.; Lee, R.D.; Kemerait, R.C.; Guo, B. Effects of hydrogen peroxide on different toxigenic and atoxigenic isolates of Aspergillus flavus. Toxins 2015, 7, 2985–2999.
- Chulze, S.N. Strategies to reduce mycotoxin levels in maize during storage: A review. Food Addit. Contam. 2010, 27, 651–657.
