1. Introduction
Pathogens facilitate the transmission of disease. Fungi, protozoans, and bacteria are only a few of the microorganisms that fall under this category. Pathogens that enter the body via food, drink, and the air affects over 15 million fatalities worldwide
[1][2][3][1,2,3]. Virulence and infectious dosage statistics for the COVID-19 virus, a worldwide pandemic, are only beginning to emerge. Rapid and sensitive pathogen detection methods are vital for the treatment of infectious illnesses, and the prevention of illness
[4][5][6][7][4,5,6,7]. Both fluids and aerosols, and surfaces, are covered in
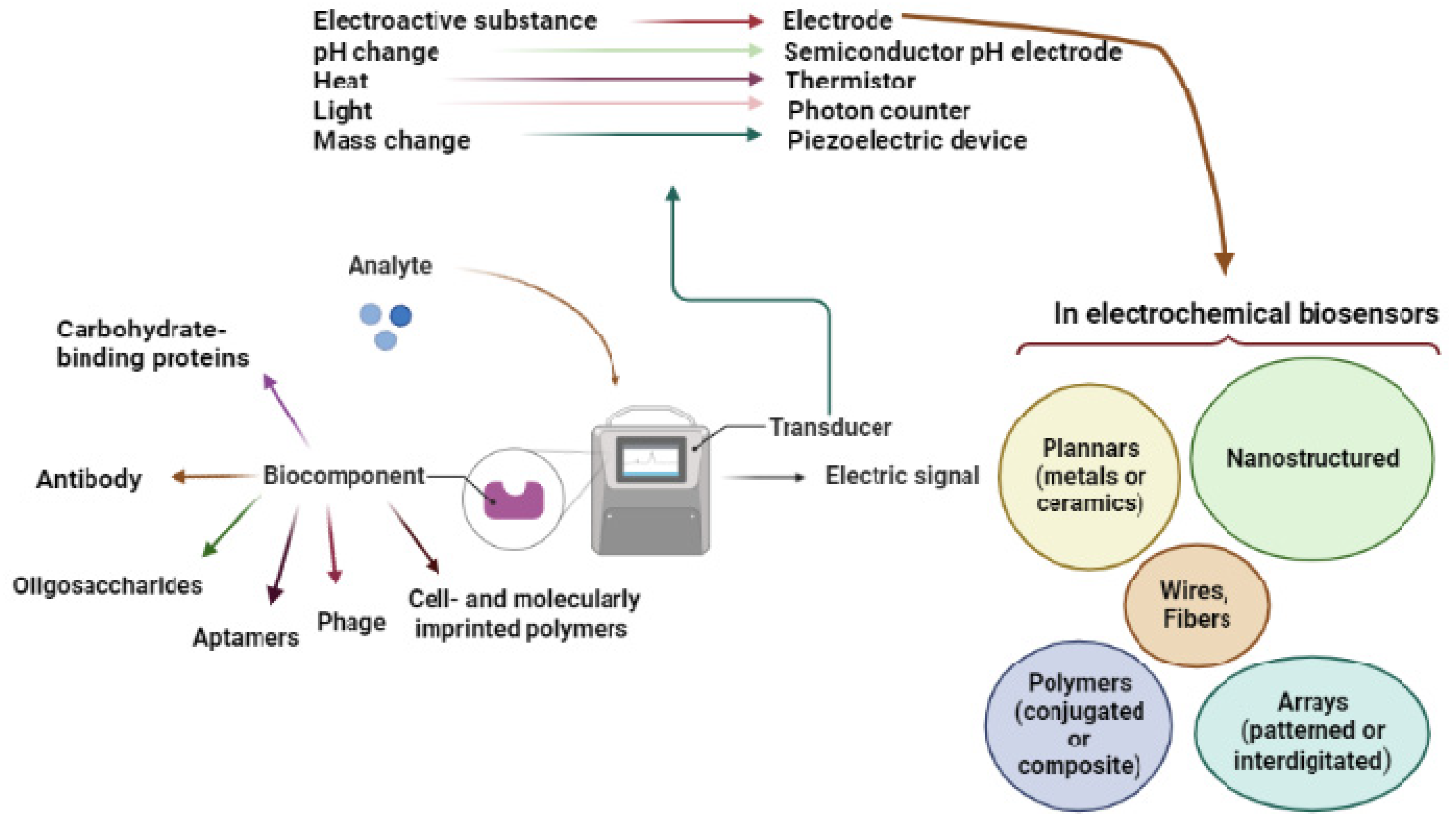
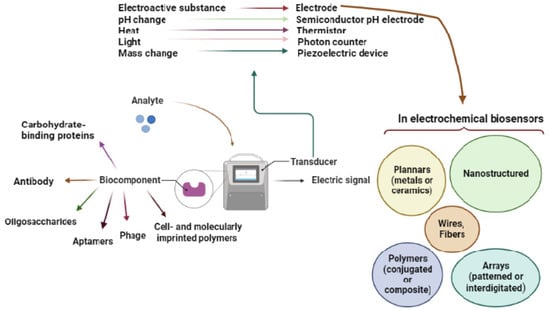
Figure 1.
Figure 1. Overview of the biosensor and its components.
Immunoassays and deoxyribonucleic acid (DNA)-based assays are often used to identify and quantify infections
[8][9][8,9]. For example, toxin- and species-specific gene sequence data can influence the use of immunoassay or a DNA test at different stages of infection. Immunoassays are regularly used in medical diagnosis and food safety
[10]. Immunoglobulins (Igs) are created during and after infection, making them useful for pathogen identification (when the pathogen is gone). These tests involve both the biorecognition component and the target antibody. Immunoassays can be used to detect infections in the body if antigens are made available. Immunoassays can identify infections via antibodies and pathogen epitopes, making them extremely flexible
[8][10][11][8,10,11]. Because of the lack of antibodies and the need for extremely sensitive findings, or because the pathogen is present but does not create a significant number of antibodies, DNA-based tests are widely utilized in diagnostics
[8][10][8,10]. Detecting pathogens that have recently been present in a sample is essential for DNA-based testing to work. Toxins, antibodies, and genes that create toxins can be used to identify pathogens. Toxins, nucleic acids, and viruses are examples of pathogen detection targets. There are many biorecognition components to choose from, from antibodies to aptamers to imprinted polymers
[12][13][14][12,13,14]. Enzyme-linked immunosorbent assay (ELISA)
[15] and polymerase chain reaction (PCR)
[16][17][16,17] have been extensively studied for the detection of infections.
Because of its high sensitivity and specificity, applicability in monitoring, early detection of biothreat agents, and antimicrobial resistance profiling, PCR technology (conventional and real-time PCR) is most frequently utilized in pathogen detection
[18][19][18,19]. However, it has shortcomings, including the inability to distinguish between infections with identical genetic composition. For instance, when PCR has been used to identify
Listeria monocytogenes [20] and
Bacillus cereus [21], respectively, false signals of
Listeria innocua and
Bacillus thuringiensis have been recorded
[22]. The inability of PCR to distinguish between the DNA of dead and living cells is another significant drawback, and this issue is crucial for the food sector, regulatory bodies, and the customer
[19][22][19,22].
ELISA demonstrates the following benefits: (1) a straightforward process using affordable equipment; (2) high sensitivity and specificity because of an antigen-antibody response; (3) high efficiency since many analyses can be run simultaneously without extensive sample pre-treatment; (4) generally safe and environmentally benign because no radioactive materials or significant quantities of organic solvents are needed; and (5) as low-cost reagents are utilized, the assay is cost-effective. ELISA, however, has the following drawbacks: (1) antibody preparation is time-consuming and costly because it requires a sophisticated technique and expensive culture cell media to produce a particular antibody; (2) a high likelihood of erroneous positive or negative results exists because the surface of the microtiter plate immobilized with antigen has not been sufficiently blocked; (3) antibody instability exists because an antibody is a protein that needs to be transported and stored in a refrigerator; and (4) it has restricted use in foods with a solid matrix or that are viscous, such as peanut butter, jam, and honey
[22][23][22,23].
Even though label-free biosensors for pathogen detection can be useful for monitoring, they have seldom been reviewed. An analytical system is used in conjunction with a specific biorecognition element, such as a molecular probe, to measure one or more components of a sample. Although they can be extremely sensitive and robust, these testing methods are destructive. They need significant sample preparation and the addition of reagents, which prolongs the time it takes to obtain findings. The presence of background species in a sample can also inhibit bioanalytical techniques, such as PCR
[24][25][26][24,25,26], increasing the amount of error introduced into the measurement process
[27][28][27,28]. Plate-based bioanalytical systems have limitations and need continual real-time monitoring across several applications; hence, other bioanalytical processes should be investigated.
Merging of targeted biorecognition elements with very sensitive transducer components improves pathogen detection and quantification in biosensors. The International Union of Pure and Applied Chemistry (IUPAC) has said that, to create an effective biosensor, an element that may be directly connected to the biorecognition element must be included
[29]. Although the biosensor can measure everything from droplet sizes to continuous flow forms, it must also be a self-contained, integrated instrument. Biosensors that can detect pathogens in real-time without sample preparation may now be used in several settings. A wide range of matrices and conditions may now be analyzed using biosensors, including food and body fluids, as well as surfaces of objects
[30]. Biosensors allow both sample preparation-free and label-free approaches
[31][32][33][34][31,32,33,34]. Examples of molecular species known as “reporters” include organic dyes and quantum dots. Biorecognition elements or secondary binding stages can be used to directly affix labels to a target or through a succession of sample preparation operations or secondary binding stages
[35]. Consequently, label-free biosensors do not rely on a reporting species to detect the target species
[36][37][36,37]. Using a label-free assay means fewer sample preparation steps and lower costs than using a label-based assay, both of which are key factors in applications with limited preparation facilities or trained personnel
[36][37][38][36,37,38].
Pathogen biosensing transducers of many sorts have been examined
[13][26][38][13,26,38]. Either mechanical or optical transducers, such as cantilever biosensors or surface plasmon resonance (SPR)-based sensors can be used to detect infections
[39][40][41][39,40,41]. A transducer consisting of conducting or semiconducting materials is used. An electrochemical approach can be used to convert the chemical energy released by pathogens and electrode-immobilized biorecognition components into electricity. Biosensors based on electrochemical processes are able to detect pathogens without the need for sample preparation, enabling in situ detection of pathogens on surfaces, quick and low-cost pathogen detection platforms, multiplexing pathogen detection, and wireless data collection actuation gathering (
Table 1)
[39][42][43][44][45][46][39,42,43,44,45,46].
Table 2 shows the chronological order of using biosensors to detect bacteria or viruses.
Table 1. Pathogen-based biorecognition elements.
Table 2. Chronological table of biosensor usage for bacterial or viral detection.
Application of real (sometimes complex) samples at the point-of-care (POC) and in the field is one of the issues that researchers are still attempting to solve for all types of biosensors. The type of instrument required to advance electrochemical biosensors to point-of-care has been made available by screen-printing technology
[81]. Because of their reliability, reproducibility, mass production, and low cost, screen-printed electrodes (SPEs), which first debuted in the 1990s, have significantly contributed to the advancement of electrochemical biosensors
[81][82][83][81,82,83]. SPEs have been found to be flexible tools that could be molded into many shapes, manufactured of various materials, and modified with a range of biological components, including enzymes, antibodies, DNA, synthetic recognition elements, and others
[82]. Additionally, when using the enhanced electrocatalytic characteristics of nanoparticles, modifications with a variety of nanomaterials and synthetic recognition elements have been used to increase sensitivity
[82]. For instance, nanomaterials (carbon nanotubes, graphene, gold nanoparticles, etc.) applied on an SPE’s working electrode (WE) can greatly increase surface activity due to their superior electrocatalytic capabilities and substantially larger specific surface area. This technique can be carried out automatically on the planar SPE by a mass-producible dispenser or just before mixing the modifier with the ink while printing. One benefit of this improvement is that it can aid in the direct detection of some conductive analytes. In addition, it is frequently used to enhance the immobilization of the recognition element, which is frequently a biomolecule, to facilitate analyte identification and signal transduction
[84].
Additionally, the appealing characteristics of carbon—chemical inertness, low background currents, and a broad potential window—have attracted a large amount of attention to SPEs. In addition to carbon, which is still the most affordable option, other metals such as gold also have advantages. The suitability of gold SPEs in electrochemical biosensors has been greatly increased by the affinity between thiol moieties and gold, which enables SPEs with gold working electrodes to be easily adjusted with the production of self-assembled monolayers
[81].
2. Transduction Elements
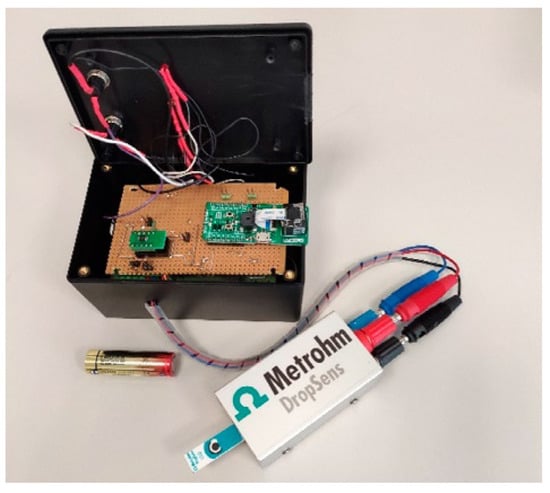
The working electrode is often the principal transduction element when employing an electrochemical biosensor. Conventionally, a three-electrode potentiostat system employs three electrodes; however, conductometry and impedance measurements often utilize two electrodes (working and auxiliary). Manufacture of electrodes can use a range of materials and procedures. Electrons and holes pass through an electrode to transfer charge. Electrodes are made from conductive and semiconducting materials such as gold (Au) and carbon (C). Different manufacturing methods may be utilized to make electrodes of different sizes. For instance, Fortunati et al. recently quantified the SARS-CoV-2 spike protein using an Internet of Things-Wifi (IoT-WiFi) smart and portable electrochemical immunosensor (
Figure 2) with integrated machine learning characteristics. Based on the immobilization of monoclonal antibodies against the SARS-CoV-2 S1 subunit on screen-printed electrodes (SPEs) functionalized with gold nanoparticles, the immunoenzymatic sensor was developed. The working electrode diameter of the SPEs was 4 mm, and their dimensions were 3.4 × 1.0 × 0.05 cm. The counter electrode was made of carbon, while the reference electrode and electrical connections were silver
[80].
Figure 2. The smart portable wireless potentiostat for Rapid Quantification of SARS-CoV-2 Spike Protein. Reprinted with permission from ref.
[80].
An electrode’s material type, manufacturing method, and design all play a role in categorizing it. The form factor may be used to classify electrode designs into planar, wire, nanostructured, and array-based types of structures. A biosensor’s ability to detect a specific biological agent, and its sensitivity and dynamic range, are determined by the electrode’s shape and characteristics and the material, production technique, and design of the biosensor. As a result, the entire cost of the biosensor is significantly affected
[85][89].
2.1. Metal Electrodes
The detection of pathogens has traditionally relied on gold (Au) and platinum (Pt) electrodes. A cutting process is commonly used to create thick metal electrodes. Traditional microfabrication methods, such as physical vapor deposition and screen printing, are frequently used to make thin-film metal electrodes
[86][87][90,91]. Transducer elements are often built using Teflon, polyether ketone (PEK), and glass as insulating polymers or ceramic substrates for the resulting conductive components. Pathogen detection applications have yet to utilize 3D printing technologies such as inkjet printing
[88][89][90][92,93,94]. Selective laser melting and microextrusion printing have also been employed to manufacture electrochemical sensors and electrodes. The detection limits of unstructured metal electrodes can vary widely. These biosensors for bacteria have detection limits of 1 to 10
4 CFU/mL, for example, using unstructured metal electrodes
[91][92][93][95,96,97].
2.2. Ceramic Electrodes
Pathogens in food may be detected using semiconducting and conducting ceramics such as indium tin oxide (ITO), polysilicon, and titanium dioxide (TiO
2). A silicon electrode was used by Das et al. to detect
Salmonella typhimurium (
S. typhimurium)
[61]. Antibody-functionalized indium tin oxide (ITO) electrodes developed by Barreiros dos Santos et al. can detect
E. coli, according to researchers
[94][98]. Specifically, ITO’s high conductivity and transparency directly correlate with biosensor response and pathogen surface coverage
[95][96][99,100]. Due to ITO’s high conductivity and transparency, biosensor response and pathogen surface coverage are linked
[97][101].
2.3. Polymer Electrodes
Pathogen-detecting electrodes have also been made from polymers. Polymers are not just good for human health and the environment, but are also relatively low-cost. Various biorecognition element immobilization techniques are also compatible with polymer electrodes
[98][99][102,103]. Implantable and wearable biosensors require electrode–tissue mechanical matching, which is made possible by polymers’ mechanical properties. An (organic) conjugated polymer (CP) electrode or a polymer composite can be categorized as a type of polymer electrode, and they have long been used for pathogen detection
[100][101][102][104,105,106].
Remarkable transparency, biocompatibility, low oxidation potential, outstanding conductivity, ease of fabrication, low cost, and a small band gap (e.g., 1.6 eV) are some of the special qualities of organic CPs
[103][104][105][107,108,109]. Poly (acetylene), poly (pyrrole), poly(thiophene), poly(terthiophene), poly(aniline), poly(fluorine), poly (3-alkylthiophene), poly tetrathiafulvalene, poly naphthalene, and poly (p-phenylene sulfide), poly(para-phenylene vinylene) are examples of common types of organic CPs
[103][105][107,109].
E. coli and human influenza A virus were detected using spin-coated films coated with poly (3,4-ethylene dioxythiophene)
[76]. Organic CPs’ semiconducting nature gives them unique optical and optoelectronic capabilities. Thus, synthetic chemists’ capacity to modify the chemical structures of polymerized monomers allows for the design and tweaking of CPs for particular purposes
[105][109].
Polymer composite electrodes, which comprise a nonconductive polymer combined with a conductive one, frequently conduct or convey scattered words. Dispersed phases such as graphite or gold nanoparticle (AuNP)-graphene or carbon nanotubes (CNTs) have been widely employed in conjunction with different polymers, including poly ethyleneimine (PEI), poly allylamine (PA), and chitosan (PAA)
[106][107][108][109][110,111,112,113].
Researchers have developed a poly allylamine/CNT polymer composite electrode that may be used for anodic stripping voltammetry to detect bacteria including
E. coli,
S. typehimurium, and
Campylobacter at concentrations as low as 10
3–10
5 cells/mL
[109][113].
S. typhimurium was detected using AuNP-coated synthetic polymer composite electrodes made of poly (amidoamine), carbon nanotubes, and chitosan
[106][110][110,114].
Polymer composite electrodes have a detection limit of 1–10
3 CFU/mL, which is equivalent to that of metal and polymer electrodes. Nanomaterials may be disseminated throughout the polymer in polymer composite electrodes rather than using electrode nanostructuring methods. Polymer electrodes have risen in popularity because of the growing need for flexible biosensors. Electrodes consisting of a polymer or a film that may be affixed to a flexible substrate, such as paper, are among the most recent biosensor technologies being researched
[111][115], because 3D printing processes are compatible with conjugated polymers and composites of polymers
[112][113][116,117]. Additionally, polymer electrodes are becoming attractive candidates for wearable biosensors that can conform to the wearer’s body
[114][115][118,119]. Regarding polymer electrodes, the most common form factor is a thin film, but nanowires and nanofibers can also be used
[115][116][119,120].
2.4. The shape and Design of the Electrodes
Electrodes made from Au have been utilized to detect infections of all forms and sizes. Advanced masks and programmable tool paths can be utilized to create electrodes using lithography and 3D printing
[117][118][121,122]. In addition to complicated form factors, electrode patterning may be used to fabricate electrode arrays using lithographic, 3D printing, and assembly techniques
[118][122]. Biosensor sensitivity and multiplexing have been improved by electrode arrays, which include interdigitated microelectrodes and other patterned electrodes. There are alternating, parallel fingers on the electrodes in an interdigitated array microelectrode (IDAM) with excellent response time
[119][123]. For pathogen detection, Au interdigitated microelectrode arrays are a popular choice.
S. typhimurium can be detected using electrochemical impedance spectroscopy (EIS) using interdigitated Au micro electron arrays, such as those used by Dastider et al.
[70]. Detection of
S. typhimurium has also been performed utilizing interdigitated arrays of ceramic electrodes such as ITO
[66][120][66,124]. Electrode arrays with geometries other than interdigitated designs can be made using the aforementioned emerging manufacturing processes for electrochemical sensing applications. Arrays of silver (Ag) microelectrodes may be created using aerosol jet additive manufacturing
[121][125].