The sympathetic nervous system (SNS) originates in the ventral brainstem, where sympathetic premotor neurons are found. They are found predominantly in the rostral ventrolateral medulla (RVLM) and in the rostral ventromedial medulla (RVMM). These neurons project to the intermediolateral nucleus (IML, also known as the sympathetic preganglionic nucleus), which then projects to the dorsal root ganglia (DRG) for terminal output to peripheral organs which control heart rate, blood pressure, respiration, glycemia, vigilance and other physiological responses. When negative emotions are induced under chronic stress, the sympathetic nervous system is continuously activated and increases the release of catecholamines (such as epinephrine and norepinephrine). In a spontaneous colon tumor model, ablation of sympathetic premotor neurons in APCmin/+ mice reduces the number of polyps in the mouse intestine. Sympathetic denervation also leads to decreased tumorigenesis in a spontaneous prostate tumor mouse model. These results suggest that loss of SNS function may slow tumorigenesis.
1. Stress Activates SNS-Related Neural Circuits
The
rostral ventrolateral medulla (RVLM)RVLM is associated with tumor growth. Recently, Zhang and colleagues found that pharmacogenetic tools, DREADDs (Designer Receptors Exclusively Activated by Designer Drugs), used to manipulate catecholamine neurons in the RVLM, regulate CD8
+ immune cells and promote immune evasion
[1][24]. Under anxious states, neurons in the RVLM undergo a similar activation by receiving signals from upstream anxiety-regulation brain regions, indicating that anxiety-promoted tumor progression may be achieved via activation or inhibition of neural circuits projecting to the RVLM from anxiety-associated brain regions
[2][26]. In addition, the
rostral ventromedial medulla (RVMM)RVMM also controls temperature and pain. It receives complex inputs from the whole brain, including antinociception information from the PAG and thermogenic information from the dorsal media hypothalamus (DMH). However, it is not clear whether the RVMM or RVMM-associated circuits are involved in the regulation of cancer progression during the stress response.
Anxiety is a state of arousal that occurs in response to stress. The amygdala, including the
basolateral amygdala (BLA
), the
central amygdala (CeA)CeA, the medial amygdala (MeA) and the
bed nucleus of the stria terminalis (BNST)BNST (extend amygdala), is considered to be an important brain area for processing stress
[3]. Experimental activation of the amygdala and its downstream projection targets, including the lateral hypothalamus (LHA), the
locus coeruleus (LC
), the
periaqueductal grey (PAG)PAG and other regions, results in an anxious state
[4][27]. Tumor studies have suggested the association between the activity of the amygdala and cancer: a study of cancer patients found that the left amygdala volume is larger in patients with a psychiatric history compared to those with no such history
[5][28]. Investigations using fMRI have shown that amygdala activity in breast cancer patients is associated with peripheral inflammatory factors and that social support reduces amygdala activity and lowers levels of inflammatory markers
[6][7][29,30]. Therefore, these studies may indicate the association between cancer and amygdala activity. To explain these findings, it is thought that amygdala activation is highly involved in sympathetic activity, and that neurons in the amygdala that project to areas containing sympathetic premotor neurons have anatomical and functional overlap with those regions which elicit anxiety responses. It is known that activation of somatostatin
+ GABAergic neurons in the CeA regulates blood pressure and other sympathetic functions by projecting to the RVLM (sympathetic premotor area) or the nucleus of the solitary track (NTS, peripheral sensory center)
[8][31]. This means that activation of the CeA directly leads to sympathetic activation. The BNST is also involved in stress-induced anxiety
[9][32], and there are direct or indirect projections from the BNST to the medulla, which regulate sympathetic function
[10][11][12][13][33,34,35,36].
The hypothalamus, including the DMH and the LHA, is a downstream output target of the amygdala and cortex, which is also involved in encoding anxiety information. It is thought to play an important role in regulating sympathetic activity during stress. This area is a crucial hub for projections to regions containing sympathetic premotor neurons. During stress, the amygdala inhibits the ventral DMH, disinhibits the GABAergic projection from the ventral DMH to the medulla, where the sympathetic premotor neurons are activated, resulting in sympathetic functions
[14][37]. Orexin/hypocretin neurons within the LHA are thought to be involved in stress. Activation of the orexin system induces anxiety-like behavior
[15][38]. In addition, the orexin system is found to be associated with breast cancer in animal models: activation of LHA orexin neurons in a mouse model of breast cancer leads to sleeping disruption and metabolic abnormality complicated by tumors, and this effect occurs via the sympathetic system as it can be blocked by 6-hydroxydopamine, a selective catecholaminergic neurotoxin
[16][39].
An earlier comparative study of animal models showed that periaqueductal gray (PAG) activity is associated with breast tumor growth
[17][40]. Indeed, the PAG is closely related to the regulation of cancer pain
[18][19][41,42]. The important descending pain pathway, the PAG-RVMM projection, which extends to the dorsal horn, is the primary pathway for pain suppression
[20][43]. Regulation of nociception is influenced by anxiety circuits which are modulated by the amygdala. GABAergic neurons in the amygdala project to PAG GABAergic neurons and locally innervate adjacent glutamatergic neurons. Following chronic inhibitory stress, inhibitory signaling by these amygdala projections relieves GABAergic inhibition of glutamatergic neurons in the PAG, thereby regulating nociception
[21][44]. In addition, the PAG is also involved in sympathetic functions: activation of the lateral/dorsolateral PAG is known to increase heart rate and arterial pressure
[22][45]. Therefore, the PAG may be involved in the regulation of tumor progression through pain regulation pathways and sympathetic pathways.
The m
edia prefrontal cortex (mPFC
) is at the top of the response initiation hierarchy during the stress response. It has functional links that govern the amygdala and hippocampus
[14][37]. The mPFC is considered the region that suppresses anxiety. For instance, activation of glutamatergic projections from the mPFC to the amygdala causes anxiolytic effects, whereas inhibition results in anxiogenic effects
[23][46]. At the same time, activation of these regions inhibits stress-induced sympathetic activity
[24][47]. However, no direct connection between sympathetic premotor neurons and the mPFC has been found. Reward signals may lead to mPFC activation
[25][48], and activation of reward circuits involving the ventral tegmental area (VTA) is thought to reduce negative emotion
[26][49]. Studies investigating tumors have found that activation of the VTA also promotes immune function, resulting in inhibition of tumor growth in mice
[27][50]. O
neur previous study found that activation of the dopaminergic projections from the VTA to the mPFC reduces anxiety levels in stressed animals, and tumor growth slows down as anxiety levels decrease
[28][51]. At the same time, anxiety-related sympathetic hormone levels also decrease, indicating the importance of the mPFC in tumor regulation and treatment.
2. Sympathetic Nerve Fibers Release Neurotransmitters to Promote Tumor Progression
Sympathetic nerve fibers originate from the DRG and project to nearly all organs and tissues, including solid tumors. In addition to the original neuronal fibers in pathological tissues, newly formed neuronal fibers also develop during the early cancer states
[29][30][25,52]. Long-term, continuous, specific activation of sympathetic nerve fibers of the mice around tumors using NaChBac-channel viruses significantly increases catecholaminergic neurotransmitter levels and promotes cancer growth and metastasis, with adrenalectomy, indicating an important role of sympathetic nerve fibers in cancer progression
[31][53]. The major secretions of sympathetic nerves are norepinephrine (NE) and neuropeptide Y (NPY).
The focus of recent research into the regulation of stress-related cancer progression has been NE and NE signaling since Thaker and colleagues found that NE and β-adrenergic receptor (β-AR) signaling induced by elevated chronic stress promotes tumor growth and angiogenesis in mice
[32][54]. NE release during the stress response is thought to contribute to increased DNA damage and cause tumorigenesis
[33][34][55,56]. NE activates arrestin-β and the PKA system, further resulting in p53 inactivation and inhibits p53-mediated DNA damage repair
[35][57]. Spontaneous tumor model studies have also demonstrated the negative effects of stress-induced DNA damage on tumor therapy
[36][58]. The adrenergic receptor antagonist ICI 118,551 and β-AR knockout blocks cancer development caused by chronic restraint, thereby reducing the proportion of pancreatic ductal adenocarcinoma (PDAC) in LSL-
Kras+/G12D;
Pdx1-Cre (KC) mice, a spontaneous pancreatic tumor model, while the agonist isoproterenol promotes PDAC
[37][59]. NE-β-AR signaling activates many biological reactions and cell-signaling-related proteins, such as Src and CREB, and also activates L-type voltage-dependent calcium channels (VDCC)
[37][38][39][59,60,61]. These reactions promote cancer proliferation. NE-β-AR signaling is also necessary for angiogenesis as it results in an energy acquisition switch from oxidative phosphorylation to glycolysis in endothelial cells, and thus angiogenesis
[40][62]. The immune response is closely related to the development and treatment of cancer. NE-β-AR signaling stimulates macrophage development, differentiation, polarization to M2, infiltration, and therefore promotes cancer metastasis
[41][42][43][44][17,63,64,65].
NPY is another neurotransmitter released by sympathetic nerve fibers in response to stress. Levels of NPY remain elevated longer than NE does during stress responses and sympathetic activation
[45][66]. However, in contrast to NE-receptors signaling, NPY has not been adequately investigated in tumor studies. In vitro studies have shown that NPY can activate Y5R or the Y2R–Y5R complex to promote cell proliferation via the Erk pathway
[46][47][67,68]. In addition, Y2R activated by NPY in endothelial cells promotes angiogenesis
[48][49][69,70]. Macrophages, which express large amounts of Y1R, are also affected by NPY. Activation of Y1R in macrophages leads to the release of NO and cytokines, including IL-4, IL-6, IL-12, and TNF-α, which promote inflammation and angiogenesis
[50][71] (
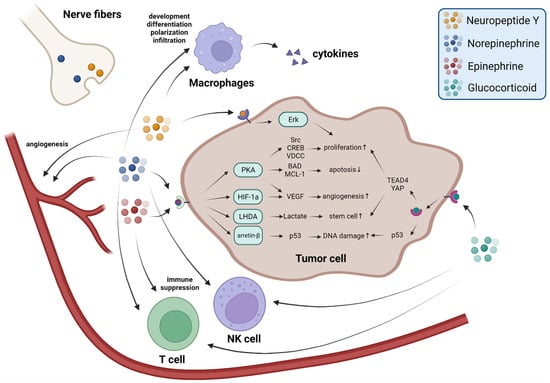
Figure 12).
Figure 12. Stress-related neurotransmitters and hormones promote cancer progression in multiple ways. During the stress response, NE and NPY are released from nerve fibers, epinephrine is released from the adrenal gland medulla, GCs are released from the adrenal gland cortex and arrive at the tumor through the circulation. Their function occurs via receptors on cancer cells, blood vessels, and immune cells to promote cancer progression in multiple ways.
3. The Adrenal Medulla Secretes Epinephrine to Promote Tumor Progression
The adrenal glands are activated in response to stress. They are controlled by sympathetic projections to the adrenal medulla. In response to stress, two hormones, epinephrine and NE, are released and enter the circulation
[51][72].
The adrenal medulla predominantly releases epinephrine (~75%)
[52][73]. During acute stress, epinephrine is released in large quantities, improving the ability to deal with danger. Epinephrine and NE share receptors, so epinephrine also has a negative impact on cancer development. Epinephrine leads to cell proliferation by adrenergic receptors
[53][74] and binding to β-ARs activates the PKA system and further regulates BAD and MCL-1 proteins to inhibit apoptosis
[54][55][75,76]. Epinephrine-β-AR signaling also promotes cancer stem-like traits through a cascade of responses produced by lactate, which is metabolized by LHDA
[56][77]. Epinephrine also promotes angiogenesis: activation of epinephrine-β-ARs-HIF-1α results in increased VEGF secretion
[57][78]. An immunological study found that the elevation of epinephrine caused by social disruption suppresses CD8
+ T-cell proliferation as well as macrophage-derived IFN-γ
[58][79] (
Figure 12).