Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Gabriel da Silva Oliveira.
The sanitization of hatching eggs is the backbone of the hygienic–sanitary management of eggs on farms and extends to the hatchery. Poultry production gains depend on the benefits of sanitizers. Obtaining the maximum yield from incubation free of toxic sanitizers is a trend in poultry farming.
- eggshells
- embryonic health
- hatchery
- hatching eggs
- microbiological safety
1. Introduction
In poultry, embryonic mortality from pathogenic microbial infection is preventable through simple, cheap and efficient preventive guidelines. In most countries, the sanitization of hatching eggs is the primary countermeasure to the attacks of pathogenic microorganisms on the embryo. Studies have shown that sanitizing hatching eggs with synthetic products such as hydrogen peroxide [1] and natural products such as clove essential oil [2] reduced the pathogenic microbiota in eggshells and increased the percentage of hatched chicks. These active materials are non-toxic, non-corrosive and non-damaging to the eggshell. However, unsatisfactory effects such as possible severe toxicity in embryos that led to their death were reported in eggs sanitized with formaldehyde [3]. Microfragments were found in the cuticle and the vertical crystalline layer in eggs sanitized with peracetic acid [4], and reduced hatchability was found in eggs sanitized with propolis [5].
The beneficial and non-beneficial effects of sanitizers in hatching eggs result from synchrony (favorable) or non-synchrony (unfavorable) factors, such as concentration and application time [4,6][4][6]. As mentioned earlier, it is clear that sanitizers, when applied to hatching eggs under certain conditions, can generate a repertoire of adverse effects that affect embryonic development. Embryonic health is undoubtedly an important aspect that influences the entire poultry sector. It is through a healthy embryo that a healthy chick will be born. In turn, if handled properly, this chick will become a healthy broiler that will reach the consumer’s table without undue influence on human health. At the same time, the poultry chain experiences significant economic gains for maintenance and growth. However, no sanitizers should be definitively rejected before being fully and continuously evaluated unless the compound is known to be lethally toxic to the point that humans cannot manipulate it with personal protective equipment. Human health must be a priority over all matters considered when choosing a sanitizer for hatching eggs.
Formaldehyde is the primary sanitizer in the routine sanitization of hatching eggs on European poultry farms (for example, Germany and Poland), as well as in Brazil and Egypt, among other countries [2,7,8,9,10][2][7][8][9][10]. However, it has genotoxic and cytotoxic properties [11] that subject poultry farmers and chicken embryos to a high risk of hazardous chemical exposure and possible irreversible bodily harm. Indoors, a short exposure not exceeding 0.1 mg/m3 (0.08 ppm) of formaldehyde is recommended to avoid damage to human health [12]. Cadirci [13] reported that the concentration required to reduce practically 100% of the microbial load of hatching eggshells is at least 600 mg/m3 (489 ppm) of formaldehyde, which is an excessively high concentration when compared to those recommended for human exposure. Therefore, formaldehyde needs to be removed from the routine sanitizing of hatching eggs.
2. Sanitizers and the Sanitization of Hatching Eggs
2.1. Objective, Optimal Timing and Methods for Sanitizing Hatching Eggs
Egg contamination triggers an embryonic health crisis and threatens the world’s poultry economy. This state of affairs can be alleviated by sanitizing hatching eggs, a relatively simple protocol in which the eggs must be submitted, soon after collection, to intervention in the high proliferation of pathogens in the eggshell and their possible mobility to the microenvironment of embryonic development, making the egg suitable for generating a chick. The ideal time to sanitize hatching eggs is up to 30 min after oviposition or collection (if it is immediate) [26,27,28][14][15][16]; otherwise, the probability of having no effect or worsening production results is very high. This is corroborated in [29][17], which reported improved hatchability of eggs sanitized immediately compared with those sanitized six hours after laying, probably due to microbial penetration. In this protocol, the contact of the sanitizer with the eggs occurs through gaseous or indirect means and by liquid or direct means (Figure 31):-
Fumigation: the release of sanitizing vapors on the surface of hatching eggshells in an enclosed space.
-
Spraying: the dispersion of a sanitizing mist on the surface of hatching eggs.
-
Immersion: the act of immersing hatching eggs in sanitizer until there is an interaction between them.
- Fumigation: the release of sanitizing vapors on the surface of hatching eggshells in an enclosed space.
- Spraying: the dispersion of a sanitizing mist on the surface of hatching eggs.
- Immersion: the act of immersing hatching eggs in sanitizer until there is an interaction between them.
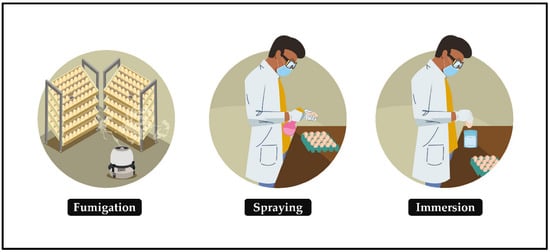
Figure 31.
Main methods of sanitizing hatching eggs.
2.2. Formaldehyde
Formaldehyde (liquid or gaseous; also called paraformaldehyde-polymerized phase) has been linked to reduced eggshell microbiota and increased hatchability percentage. There are also reports that it did not affect any of these variables (Table 1). Nevertheless, it is also associated with reports of toxicity and permanent harmful damage to embryos and chicks when applied to hatching eggs (Table 1). Although these effects depend on the concentration, length of time and method of application of formaldehyde and the period in which the egg is exposed [13], formaldehyde itself is carcinogenic because it impairs and inhibits DNA repair [30][18]. Therefore, its use is unjustifiable regarding embryonic life safety, health and protection. Poultry production should value lower risks to bird life (whether during development or after hatching), which will benefit the highest priority condition of preserving human health. Given the possible future restrictions on using formaldehyde in the poultry industry, other sanitizers must be readily available and approved by competent bodies to meet the global poultry demand.Table 1.
Some reports of the effects of formaldehyde on hatching eggs.
| Study (Reference) | Effect on Eggshell Microbial Count * | Effect on Hatchability * |
| [31][19] | Non-evaluated | No effects |
| [32][20] | No effects | No effects |
| [33][21] | - | No effects |
| [34][22] | Reduced | Increased |
| [35][23] | Reduced | Non-evaluated |
| [36][24] | - | Increased |
| [37][25] | Reduced | Non-evaluated |
| [38][26] | Reduced | No effects |
| [2] | Reduced | Increased |
| [39][27] | Non-evaluated | Increased |
| Study (Reference) | Some Reports of Adverse Effects on Embryos and Chicks | |
| [40][28] | Underweight, underdeveloped and malformed embryos. | |
| [41][29] | Increased embryonic mortality in the early stage. | |
| [42,43,44][30][31][32] | Reduced chick survival rate in the first post-hatch week. | |
| [45][33] | Increased embryonic mortality in early, mid and late stages. | |
| [46][34] | Reduced chick quality score as a result of slow activities and high number of unclosed navels. | |
* Effect compared to a negative control (non-sanitized eggs) and in the absence of negative control compared to the other sanitizers tested.
References
- Al-Shemery, N.J.; Kamaluddin, Z.N. Effect of Using Different Concentrations of Hydrogen Peroxide and Formalin Compared to Formaldehyde Evaporation in Sterilization of Hatching Eggs of Broiler. Euphrates J. Agric. Sci. 2018, 10, 36–41.
- Oliveira, G.S.; Nascimento, S.T.; dos Santos, V.M.; Silva, M.G. Clove Essential Oil in the Sanitation of Fertile Eggs. Poult. Sci. 2020, 99, 5509–5516.
- Gholami-Ahangaran, M.; Shahzamani, S.; Yazdkhasti, M. Comparison of Virkon S and Formaldehyde on Hatchabil-ity and Survival Rate of Chicks in Disinfection of Fertile Eggs. Rev. Med. Vet. 2016, 167, 45–49.
- Soares, C.E.S.; Cartabiano-Leite, C.E.; Ferreira, W.X.; Maiorka, A.; Dahlke, F.; Scussel, V.M.; de Dea Lindner, J. Peracetic Acid: Effect on the Chicken Eggshell Cuticle and Decontaminating Action on Filamentous Fungi. Jokull J. 2021, 71, 82–96.
- Oliveira, G.S.; dos Santos, V.M.; Nascimento, S.T.; Rodrigues, J.C. Alternative Sanitizers to Paraformaldehyde for Incubation of Fertile Eggs. Poult. Sci. 2020, 99, 2001–2006.
- Nogueira, W.C.L.; Pena, A.C.S.; de Souza, C.N.; Azevedo, I.L.; Fariafilho, D.E.; Almeida, A.C. Disinfection of Fertile Eggs of Free-Range Poultry with Essential Oils. Rev. Bras. Saude Prod. Anim. 2019, 20, e0822019.
- Al-Shammari, K.I.; Batkowska, J.; Gryzi, M.M. Assessment of Ultraviolet Light Effect in Hatching Eggs Disinfection on Hatchability Traits of Two Breeds of Quails and Chickens. Acta Sci. Pol. Zootec. 2015, 14, 33–44.
- Badran, A.M.M.; Osman, A.M.R.; Yassein, D.M.M. Comparative Study of the Effect of Some Disinfectants on Em-bryonic Mortality, Hatchability, and Some Blood Components. Egypt. Poult. Sci. J. 2018, 38, 1069–1081.
- Tebrün, W.; Motola, G.; Hafez, M.H.; Bachmeier, J.; Schmidt, V.; Renfert, K.; Reichelt, C.; Brüggemann-Schwarze, S.; Pees, M. Preliminary Study: Health and Performance Assessment in Broiler Chicks Following Application of Six Different Hatching Egg Disinfection Protocols. PLoS ONE 2020, 15, e0232825.
- Oliveira, G.S.; Nascimento, S.T.; dos Santos, V.M.; Dallago, B.S.L. Spraying Hatching Eggs with Clove Essential Oil Does Not Compromise the Quality of Embryos and One-Day-Old Chicks or Broiler Performance. Animals 2021, 11, 2045.
- Zhang, L.; Steinmaus, C.; Eastmond, D.A.; Xin, X.K.; Smith, M.T. Formaldehyde Exposure and Leukemia: A New Meta-Analysis and Potential Mechanisms. Mut. Res./Rev. Mut. Res. 2009, 681, 150–168.
- World Health Organization. WHO Guidelines for Indoor Air Quality: Selected Pollutants; World Health Organization: Geneva, Switzerland, 2010; pp. 103–156.
- Cadirci, S. Disinfection of Hatching Eggs by Formaldehyde Fumigation-a Review. Eur. Poult. Sci. 2009, 73, 116–123.
- Araújo, W.A.G.; Albino, L.F.T. Incubação Comercial ; Transworld Research Network.: Trivandrum, India, 2011.
- Oliveira, G.D.S.; dos Santos, V.M.; Nascimento, S.T. Essential Oils as Sanitisers for Hatching Eggs. Worlds Poult. Sci. J. 2021, 77, 605–617.
- Oliveira, G.D.S.; dos Santos, V.M.; McManus, C. Propolis: Effects on the Sanitisation of Hatching Eggs. Worlds Poult. Sci. J. 2022, 78, 261–272.
- Mousa-Balabel, T.M.; Mohamed, R.A.; Al-Midani, S.A.; El-Samad, M.S.A. Impact of Boiler Breeders Hatching Eggs Disinfection Time on Some Hatchability Parameters. Int. J. Sci. Basic Appl. Res. 2016, 30, 230–240.
- Ge, J.; Yang, H.; Lu, X.; Wang, S.; Zhao, Y.; Huang, J.; Xi, Z.; Zhang, L.; Li, R. Combined Exposure to Formaldehyde and PM2.5: Hematopoietic Toxicity and Molecular Mechanism in Mice. Environ. Int. 2020, 144, 106050.
- Hrnčár, C.; Prachárová, S.; Bujko, J. The Effect of Disinfection of Hatching Eggs on Hatchability of Oravka Chickens. Sci. Pap. Anim. Sci. Biotechnol. 2012, 45, 411–414.
- Durmus, I. Determining Effects of Use of Various Disinfecting Materials on Hatching Results and Total Bacterial Count. Asian J. Anim. Vet. Adv. 2012, 7, 739–744.
- Fidan, E.D.; Turkyilmaz, M.K.; Ahmet Nazligul, A. The Effects of Different Storage and Fumigation Lengths on Hatchability and Hatching Weight in Japanese Quails (Coturnix coturnix Japonica). J. Anim. Vet. Adv. 2012, 11, 1400–1404.
- Shahein, E.H.A.; Sedeek, E.K. Role of Spraying Hatching Eggs with Natural Disinfectants on Hatching Characteris-tics and Eggshell Bacterial Counts. Egypt. Poult. Sci. J. 2014, 34, 213–230.
- Keïta, A.; Huneau-Salaün, A.; Guillot, A.; Galliot, P.; Tavares, M.; Puterflam, J. A Multi-Pronged Approach to the Search for an Alternative to Formaldehyde as an Egg Disinfectant without Affecting Worker Health, Hatching, or Broiler Production Parameters. Poult. Sci. 2016, 95, 1609–1616.
- Bekele, M.W.; Leta, M.U. Effect of Egg Storage Temperature and Fumigation on Hatchability of Cobb 500 and Hub-bard Broiler Strains. Afr. J. Agric. Res. 2016, 11, 3418–3424.
- Clímaco, W.L.D.S.; Melo, É.D.F.; Vaz, D.P.; Saldanha, M.M.; Pinto, M.F.V.D.S.; Fernandes, L.C.C.; Baião, N.C.; Oliveira, L.G.D.; Sant’Anna, F.M.D.; Souza, M.R.D.; et al. Eggshell Microbiology and Quality of Hatching Eggs Subjected to Different Sanitizing Procedures. Pesq. Agrop. Bras. 2018, 53, 1177–1183.
- Pees, M.; Motola, G.; Hafez, M.H.; Bachmeier, J.; Brüggemann-Schwarze, S.; Tebrün, W. Use of Electron Irradiation versus Formaldehyde Fumigation as Hatching Egg Disinfectants-Efficacy and Impact on Hatchability and Broiler Performance. Tierärztliche Prax. Ausg. G Großtiere/Nutztiere 2020, 48, 406–413.
- Hrnčár, C.; Hanusová, E.; Hanus, A.; Arpášová, H.; Kokoszyński, D.; Bujko, J. The Effect of Various Disinfectants on Hatching Results in Chickens. Sci. Pap. Anim. Sci. Biotechnol. 2021, 54, 193–196.
- Zeweil, H.S.; Rizk, R.E.; Bekhet, G.M.; Ahmed, M.R. Comparing the Effectiveness of Egg Disinfectants against Bacte-ria and Mitotic Indices of Developing Chick Embryos. J. Basic Appl. Zool. 2015, 70, 1–15.
- Baylan, M.; Akpınar, G.C.; Canogullari, S.D.; Ayasan, T. The Effects of Using Garlic Extract for Quail Hatching Egg Disinfection on Hatching Results and Performance. Rev. Bras. Cienc. Avic. 2018, 20, 343–350.
- Batkowska, J.; Al-Shammari, K.I.A.; Gryzinska, M.M.; Brodacki, A.; Wlazlo, L.; Nowakowicz-Debek, B. Effect of Us-ing Colloidal Silver in the Disinfection of Hatching Eggs on Some Microbial, Hatchability and Performance Traits in Japanese Quail (Coturnix Cot. Japonica). Eur. Poult. Sci. 2017, 81, 211.
- Batkowska, J.; Al-Shammari, K.I.A.; Lukasz, W.; Nowakowicz-Debek, B.; Gryzinska, M. Evaluation of Propolis Ex-tract as a Disinfectant of Japanese Quail (Coturnix coturnix Japonica) Hatching Eggs. Poult. Sci. 2018, 97, 2372–2377.
- Wlazlo, L.; Drabik, K.; Al-Shammari, K.I.A.; Batkowska, J.; Nowakowicz-Debek, B.; Gryzińska, M. Use of Reactive Oxygen Species (Ozone, Hydrogen Peroxide) for Disinfection of Hatching Eggs. Poult. Sci. 2020, 99, 2478–2484.
- Bekhet, G.M. Impact of Hatching Egg Disinfection on Hatching Characteristics and Chick Embryos. Indian J. Anim. Res. 2021, 55, 353–358.
- Taşdemir, A.N.; Onbaşılar, E.E.; Yalçın, S.; Boyalı, B.; Aygören, H.; Tülek, E.; Sarıçam, S.; Akan, M. Effects of Oregano Juice on Eggshell Microbial Load, Layer Embryo Development, Hatching Results, and Growth at the First 2 Weeks after Hatch. Trop. Anim. Health Prod. 2021, 53, 404.
More
