Drug delivery systems based on pH have emerged as useful carriers for many drugs, including anti-tumor compounds, and are related to the acidic extracellular microenvironment in damaged tissues and some acidic organelles like lysosome and endosome. Here we report on the cytotoxic properties of MOMO, a synthetic precursor of the protozoan antimicrobial and anticancer agent climacostol, which is efficiently activated in mild extracellular acidosis to obtain a great quantity of climacostol in biologically active (Z)-configuration. Biological effects observed on free-living ciliates, tumour cell lines, and Drosophila melanogaster demonstrated that MOMO is well-tolerated in a physiological environment, while its cytotoxicity is rapidly and efficiently triggered at pH 6.3. In addition, the cytostatic versus cytotoxic effects of acidified-MOMO can be modulated in a dose-dependent manner. While these results demonstrate the pH-dependence of MOMO effects, methoxymethyl ether (MOM) protection emerges as a potential prodrug strategy for the design of new efficient pH-sensitive molecules as pharmacologically cytotoxic compounds.
- Climacostomum virens
- secondary metabolites
- predator-prey intercactions
- resorcinolic lipids
- ciliates
- natural products
1. Introduction
During the synthesis of climacostol, the choice of the methoxymethyl ether (MOM) protecting group, which can be removed in a weakly acidic environment, allowed the researchers to efficiently obtain the toxin in the biologically active (Z)-configuration climacostol [1] (Figure 1).
Figure 1. Molecular structures of (A) climacostol, and (B) MOMO.
2. Influence and application
This procedure was also applied to design a prodrug strategy based on pH activation, potentially useful to deliver climacostol to acidic pathological multicellular (tumors) or unicellular (parasitophorous of Leishmania and digestive vacuole of Plasmodium) targets. In fact, the MOM-protected climacostol, which the authors called MOMO, progressively shifts to active climacostol if exposed to pH values lower than 7. This shifting was initially verified in a native biological system (against ciliates other than C. virens) where ciliates exposed to MOMO in a physiological culture medium (SMB) at pH = 6.8 did not reveal any sign of toxicity. On the contrary, MOMO resuspended in SMB at pH = 6.3 (not harmful itself for ciliates) induced the same necrotic effect as climacostol [2].
In addition, it was demonstrated that the activity of MOMO could be modulated in a dose-dependent manner to induce cytotoxic or cytostatic effects. The biological activity of MOMO on some tumor cell lines confirmed that this molecule proved toxic in acidic conditions (pH < 6.8), by yielding active climacostol. In these conditions, MOMO induces apoptosis in melanoma cells confirming that it behaves with the same action mechanism as climacostol (Figure 2).
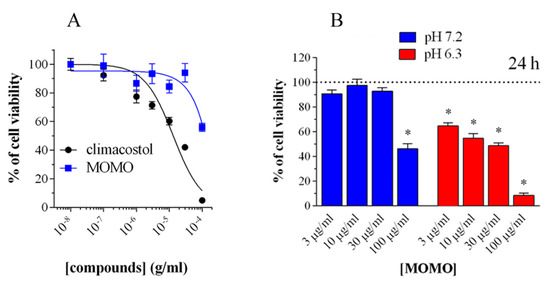
Figure 2. Cytotoxicity of methoxymethyl ether (MOM)-protected climacostol (MOMO) in melanoma cells. (A) 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay on mouse B16-F10 cells, treated with increasing concentrations of MOMO and climacostol for 24 h. (B) Increasing concentrations of MOMO in physiologic (pH 7.2) and acidic (pH 6.3) culture medium for 24 h of incubation. Data are representative of at least 6 independent experiments. * p < 0.0001 relative to the respective control. Modified from [2] and reproduced under the Creative Commons attribution 4.0 license: http://creativecommons.org/licenses/by/4.0/.
Finally, the safety of MOMO was also assessed in vivo using the fruit fly Drosophila melanogaster which is considered a very potent tool to detect the potential damaging effect of new compounds. Drosophila flies fed directly with acidic nutrients containing increasing concentrations of MOMO showed a significant reduction in oviposition and survival of larvae, which also exhibited apoptotic cells in the gut, as revealed by TUNEL labeling and cleaved-caspase 3 immunostaining. In addition, mitosis was also reduced in brain tissue of larvae, thus suggesting that active MOMO exhibited prolonged toxic effects after oral intake and gut absorption.
Overall, our data revealed that MOMO efficiently targets different tissues of the developing fly with high metabolic/proliferating activity, such as midgut and brain.
References
- Fiorini, D.; Giuli, S.; Marcantoni, E.; Quassinti, L.; Bramucci, M.; Amantini, C.; Santoni, G.; Buonanno, F.; Ortenzi, C.; A Straightforward diastereoselective synthesis and evaluation of climacostol, a natural product with anticancer activities. Synthesis 2010, 9, 1550–1556, 10.3389/fchem.2019.00463.
- Catalani, E.; Buonanno, F.; Lupidi, G.; Bongiorni, S.; Belardi, R.; Zecchini, S.; Giovarelli, M.; Coazzoli, M.; De Palma, C.; Perrotta, C.; et al.Clementi, E.Prantera, G.Marcantoni, E.Ortenzi, C.Fausto, A.M.Picchietti, S.Cervia, D. The natural compound climacostol as a prodrug strategy based on pH activation for efficient delivery of cytotoxic small agents. Frontiers in Chemistry 2019, 7, 463-484, 10.3389/fchem.2019.00463.
