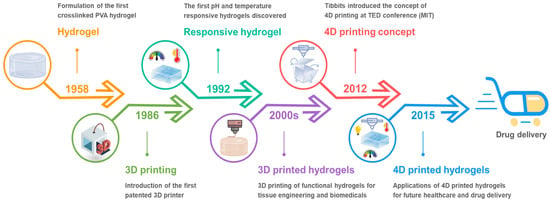
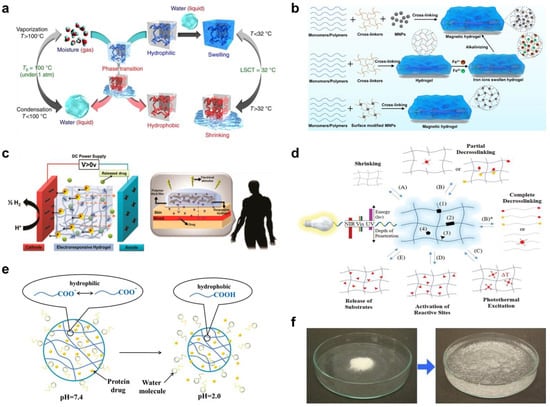
Figure 2. (a) Schematic diagram of thermal-responsive hydrogels. Reproduced with permission from [46]. Copyright © 2018 Springer Nature. (b) The preparation methods for magnetic-responsive hydrogels. Reproduced with permission from [40]. Copyright © 2021 Elsevier. (c) The mechanisms for electro-induced gel deswelling for drug delivery. Adapted with permission from [47]. Copyright © 2015 American Chemical Society. (d) Molecular architecture and responses of a photo-responsive hydrogel. Reproduced with permission from [48]. Copyright © 2019 Springer Nature. (e) Schematic illustration of pH-responsive hydrogels. Reproduced with permission from [49]. Copyright © 2019 Progress in Chemistry. (f) Dry SAP powder and swollen SAP hydrogel. Adapted with permission from [50]. Copyright © 2016 Elsevier.
The literature on LCST hydrogels is dominated by poly(N-isopropyl acrylamide) (pNIPAM) and its derivatives, because of its massive volume change at a relatively low critical temperature of around 32 °C
[23][24][25][17,18,19]. The biocompatibility and ease of processing of pNIPAM are demonstrated in a recent work by Allen and co-workers
[26][20] by culturing 3T3 fibroblasts on cell sheets produced from aligned electrospun fibers, demonstrating the promising prospect of pNIPAM for 4D printing toward drug delivery applications. Thermal responsive pNIPAM-based hydrogels with tunable responsiveness (critical temperature and swelling characteristics) have been developed by adjusting the number of repeating monomer units in (oligoethylene glycol methacrylate) (OEGMA)
[27][21], or by combining it with polymers such as poly[di(ethylene glycol) ethyl ether acrylate] (PDEGA)
[28][22]. In contrast, UCST hydrogels expand as the temperature rises. This positive thermal responsivity broadens the design space for future smart hydrogels. The most frequently employed UCST hydrogels are interpenetrating networks of polyacrylamide (pAAm) and polyacrylic acid (pAAc)
[29][30][23,24]. Recent research also showed that smart biomaterials, such as highly elastic protein elastic and elastin-mimetic proteins; resilin and resilin-mimetic proteins can be designed to exhibit tunable LCST and UCST transitions in physiological solutions
[31][32][25,26]. Extensive research by Dutta et al. and others
[31][33][34][35][25,27,28,29] has demonstrated that resilin and resilin-mimetic proteins can exhibit tunable multi-stimuli-responsiveness including both LCST and UCST.
Significant research efforts have also been focused on enhancing the biocompatibility and biodegradability of thermo-responsive hydrogels. Ye and co-workers
[36][30] have developed a supramolecular UCST hydrogel for sustained-release drug administration and tissue engineering scaffold using polyglycerol sebacate (pGS), a novel biodegradable elastomeric material with outstanding biocompatibility. Greater biodegradability can be achieved by the use of hydrolytically and enzymatically labile bonds
[36][30], or by introducing biodegradable monomers like benzomethylene dioxepane or methacrylate polylactide into their polymeric backbone
[37][38][31,32]. Natural polymers such as gelatine, cellulose, and chitosan can also be functionalised with poly(L-alanine-co-L-phenylalanine), poly(ethylene glycol), and glycerol phosphate to form smart hydrogels that are both thermal responsive and biodegradable
[39][40][33,34], promising for 4D printed drug delivery systems.
2.2. Magnetic Responsive Hydrogels
The magnetic field has also been studied as a potential external trigger for controlling the properties of smart hydrogels. The ability to activate remote actuation with a fast response time and biocompatibility even at high field strength makes electromagnetic a favorable stimulus, especially in vivo applications. To achieve magnetic responsiveness in hydrogels, exogenous additives like paramagnetic or ferromagnetic are included in the polymeric matrix, allowing for rapid and large actuation behaviors in response to magnetic fields
[41][35]. Magnetic additives such as metal alloys (e.g., iron and neodymium alloy), metal oxides (e.g., ferrous ferric oxide), and functionalized magnetic nanoparticles can be coupled with pNIPAM, pAAm and gelatin in a chemically or physically crosslinked polymer network to form magnetically responsive smart hydrogels
[42][43][44][36,37,38]. More complex magnetic responsive hydrogels with stronger interactions between the magnetic particles and the polymer network were also created via covalent and coordination bonds
[45][39]. These hydrogel systems, in general, do not require extra crosslinkers during synthesis and gelation occurs spontaneously when mixed
[18]. The preparation methods for magnetic responsive hydrogels are illustrated in Figure 2b [40].
There are two main action modes in magnetic responsiveness in hydrogels for controlled drug release, that are changing the direction of the magnetic field to arrange perpendicularly or parallelly to the drug diffusion direction and switching on/off the magnetic field to trigger release
[46][47][48][41,42,43]. Several magnetic responsive smart hydrogels are currently being tested in vivo in animal models and have the potential to translate to clinical drug delivery applications
[49][50][44,45].
2.3. Electrical Responsive Hydrogels
Inspired by artificial muscle biomimicry, electrical responsive hydrogels can expand or contract under a solvent-induced or an externally applied electrical field
[51][52][51,52]. The use of an external electrical field as a stimulus has particular advantages for drug delivery due to quick, precise, and programmable responsiveness
[53][54][53,54].
For the controlled release of drug, electrical responsive hydrogels are fundamentally based on the mobility of ions in response to an electrical field and the rearrangement of the ion concentration profile at the hydrogel-swelling media interface. Equilibrium is achieved through the balance of fixed charges on the polymer backbone and counterions attracted by the surrounding swelling media. As a result, the ion concentration is not uniformly distributed inside and outside the gel, creating an osmotic pressure that causes swelling or deswelling
[19] (Figure 2c) [47].
Electrical responsive hydrogels are typically polyelectrolytes with ionizable groups along their side chains or polymeric backbone
[55][56][55,56]. Numerous synthetic polyelectrolytes and related copolymers have been utilized to fabricate electrical responsive hydrogels, including poly (vinyl alcohol) (PVA)
[57], poly(sodium maleate-co-sodium acrylate)
[58], PVA/pAAc
[59], pAAc/poly(N-vinylpyrrolidone)
[60], and sulfonated polystyrene (s-PS)
[61]. There are also natural polyelectrolytes, including proteins
[62][63][62,63], polysaccharides
[64], and polypeptides
[65], that respond to electrical stimuli
[66][67][68][66,67,68]. They can be combined with synthetic polymers to create hybrid electrical responsive hydrogels, for instance, fibrin protein blended with pAAc
[69], chitosan coupled with poly(N,N-dimethylacrylamide)
[70], and alginate combined with poly(methacrylic acid)
[71] for 4D printed drug delivery systems.
2.4. Photo-Responsive Hydrogels
The use of light as a stimulus is particularly advantageous for remotely inducing the expansion and contraction of 4D printed hydrogels for controlled delivery of therapeutic agents. The two primary mechanisms of photo-responsive hydrogels are based on reversible crosslinking and photothermal excitation
[20](Figure 2d) [48]. Both approaches can be accomplished by including photoactive moieties into the hydrogel matrix
[20][48]. For reversible crosslinking, the presence of photoactive moieties such as azobenzene or o-nitrobenzyl groups can induce the photocleavage or photoisomerization of hydrogel matrices upon illumination, resulting in reversible contraction–expansion of polymer chains
[72][73][72,73]. A second way to achieve light-induced deformation is by employing photothermal nanomaterials, which rapidly convert light irradiation to heat dissipation, to control the reversible dehydration–hydration processes of the photo-responsive hydrogels
[74][75][74,75].
Numerous nanomaterials, including inorganic nanomaterials (e.g., gold and neodymium oxide)
[76], carbon-based materials
[77], and black phosphorus
[74], have been introduced into photo-responsive hydrogels. Researchers have demonstrated photoresponsivity by incorporating gold nanorods into pNIPAM-AAc hydrogels
[78], others reported smart hydrogels (agarose and pNIPAM) containing single-walled nanocarbons (SWNTs) and single-walled nanohorns (SWNHs) that show marked phase transitions upon NIR irradiation
[79][80][79,80]. Additive manufacturing of such intelligent photo-responsive hydrogels is still in its infancy yet promising for 4D printed drug delivery systems.
2.5. pH Responsive Hydrogels
Apart from physical stimuli, physiological conditions (e.g., the inherently low pH of the stomach, or the slightly alkaline condition of the blood) have been exploited to initiate swelling-controlled drug release from hydrogel carriers
[81]. Systems that are capable of responding to a dynamic pH environment are useful for healthcare applications, as various places throughout the human body experience pH variations during a disease condition. The dynamic pH ranges in various tissues and cellular compartments in the human body are detailed in
Table 1 [82].
Table 1.
pH in various tissues and cellular compartments [82].
The pH sensitivity of a hydrogel network can be modified by adjusting the hydrophilicity and ionic character of the internal pendant functional groups
[21] (Figure 2e) [49]. It has been demonstrated that hydrogels containing acidic moieties swell as the acid groups deprotonate at higher pH. Cationic groups, on the other hand, generate more swelling at lower pH values
[83]. Thus, there are two major types of pH-responsive hydrogels: anionic and cationic hydrogels. Anionic hydrogels contain pendant groups that ionize at a pH greater than their acid dissociation constant (pKa) and expand at higher (primarily basic) pH values. Due to the presence of physical interactions between the polymer chains, their polymer networks remain folded at pH values less than their pKa (low-pH environment). Conversely, cationic hydrogels expand when the pH level falls below pKa and contract when the pH value rises over pKa
[84].
Anionic hydrogels are frequently constructed of crosslinked polymer networks containing carboxyl groups (polyacrylic acid
[85], polymethacrylic acid
[86], and polycarboxymethyl agarose
[87]) and their copolymers
[88]. Monomers bearing amine and amide groups, such as AAm
[89], dimethylaminoethyl methacrylate (DMAEMA)
[90], and 2-(diethylamino)ethyl methacrylate (DEAEMA)
[91], as well as their copolymers
[92], are commonly used as constituents of cationic hydrogels. Emerging hydrogels made of biopolymers such as alginate
[93], gelatine
[94], chitosan
[95], and albumin
[96], resilin-mimetic proteins
[68], silk
[97], soy protein
[98], and their blends and composites could also exhibit pH responsiveness with superior biocompatibility and biodegradability compared to their synthetic counterparts. With the dynamic pH ranges in various tissues and cellular compartments in the human body, 4D printed pH-responsive hydrogels are advantageous for precisely controlled drug release into a specific area of the human body under a specific condition.
2.6. Water Responsive Hydrogels
Water responsive hydrogels, or superabsorbent polymers, are crosslinked three-dimensional interconnected macromolecular networks possessing extremely high liquid swelling capacity
(Figure 2f), providing an effective vehicle for therapeutic agents to be encapsulated and released in biological environments. Water-responsive hydrogels are often composed of ionic monomers and are weakly crosslinked. As a result, they exhibit an extraordinary capacity for water absorption
[99][100][99,100]. Water-responsive hydrogels have been the most commercially successful members of the hydrogel family and are widely used in healthcare products, including pharmaceuticals, personal hygiene, wound dressing, and drug delivery applications
[101][102][101,102].
At present, the majority of water responsive hydrogels are mostly synthetic or petrochemical in origin, and they are predominantly composed of acrylic monomers, most frequently acrylamide (AAm)
[103], acrylic acid (AAc)
[104], and their copolymers
[105]. In recent years, the trend towards substituting “greener” alternatives in water responsive hydrogels is more and more pronounced due to the low degradability and biocompatibility of the synthetic incumbents. As a result, emerging bio-based water responsive hydrogels are being made from renewable raw materials such as cellulose
[106][107][106,107], soy protein
[108][109][108,109], starch
[110], natural gums
[111], chitin
[112] and their hybrids and composites, providing a customizable and effective route toward 4D printed hydrogels for drug delivery applications.