Polyesters are synthetic resins formed by an esterification chemical reaction with some occurring naturally [22]. In addition, there are different orientations of polyesters and, hence, different classifications. The classifications aid in determining the processing, curing kinetics, and overall applications of the resin [23]. Saturated, unsaturated polyesters (UPs) and alkyd resins are the main classifications of polyesters; however, vinyl esters are also classified as polyesters since they have a di-ester group. Vinyl esters are based on the combination of an epoxy resin with an unsaturated polymer; they have excellent properties when compared to saturated, unsaturated-type polyesters and alkyd resins [24]. Developments of non-toxic polyester-based coatings have the potential to address a wide range of pollution problems, such as air pollution and water pollution, generated during the production of conventional polyester coatings [28]. The anticorrosion properties of polyester resin modified by nanocomposites intended for steel are of interest. The goal is to produce a bio-based polyester coating with minimal cost by implementing natural products as well as modifying with nanomaterials.
- anticorrosion
- polyester-based coatings
- organic coatings
- polymerization
- bio-based coatings
1. Polyesters
1.1. Unsaturated Polyesters
2.2. Saturated Polyesters
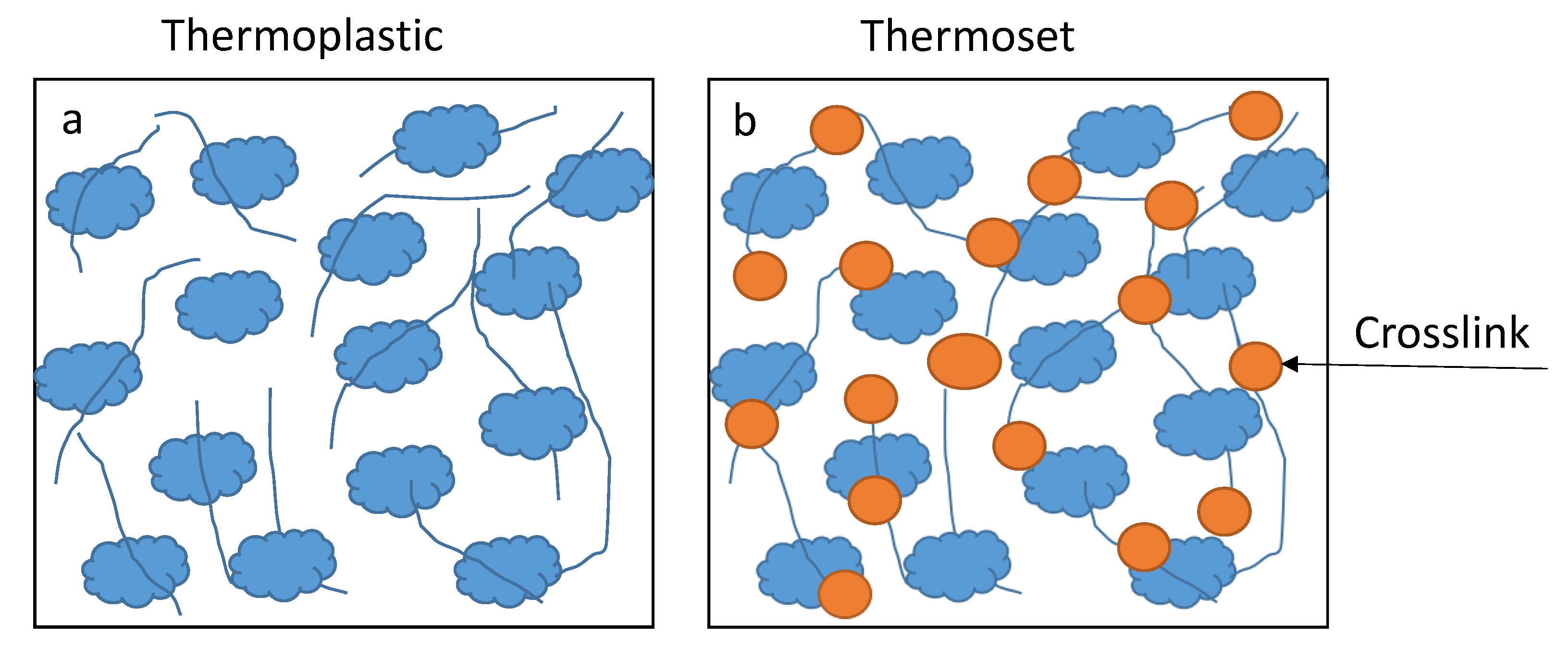
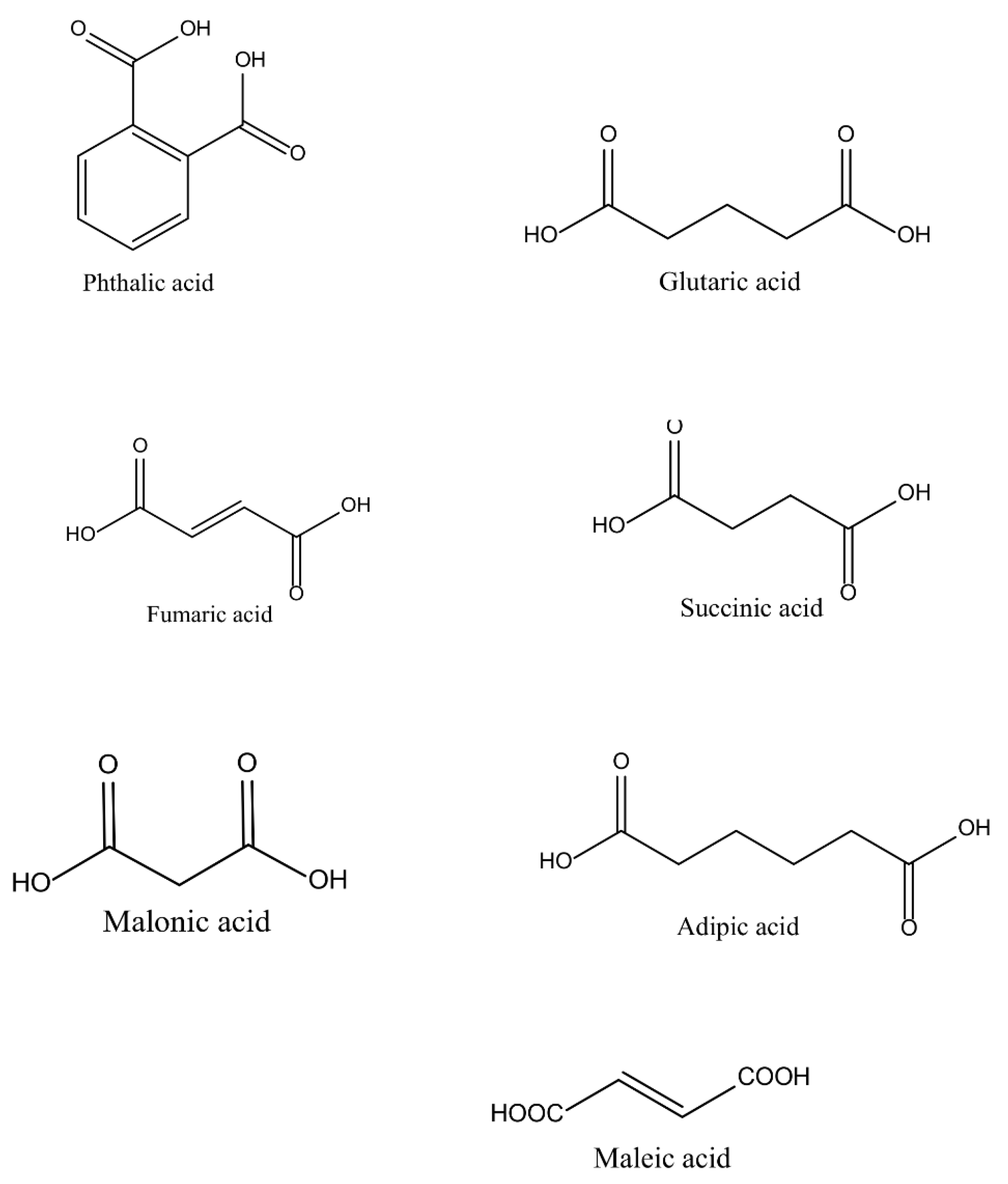
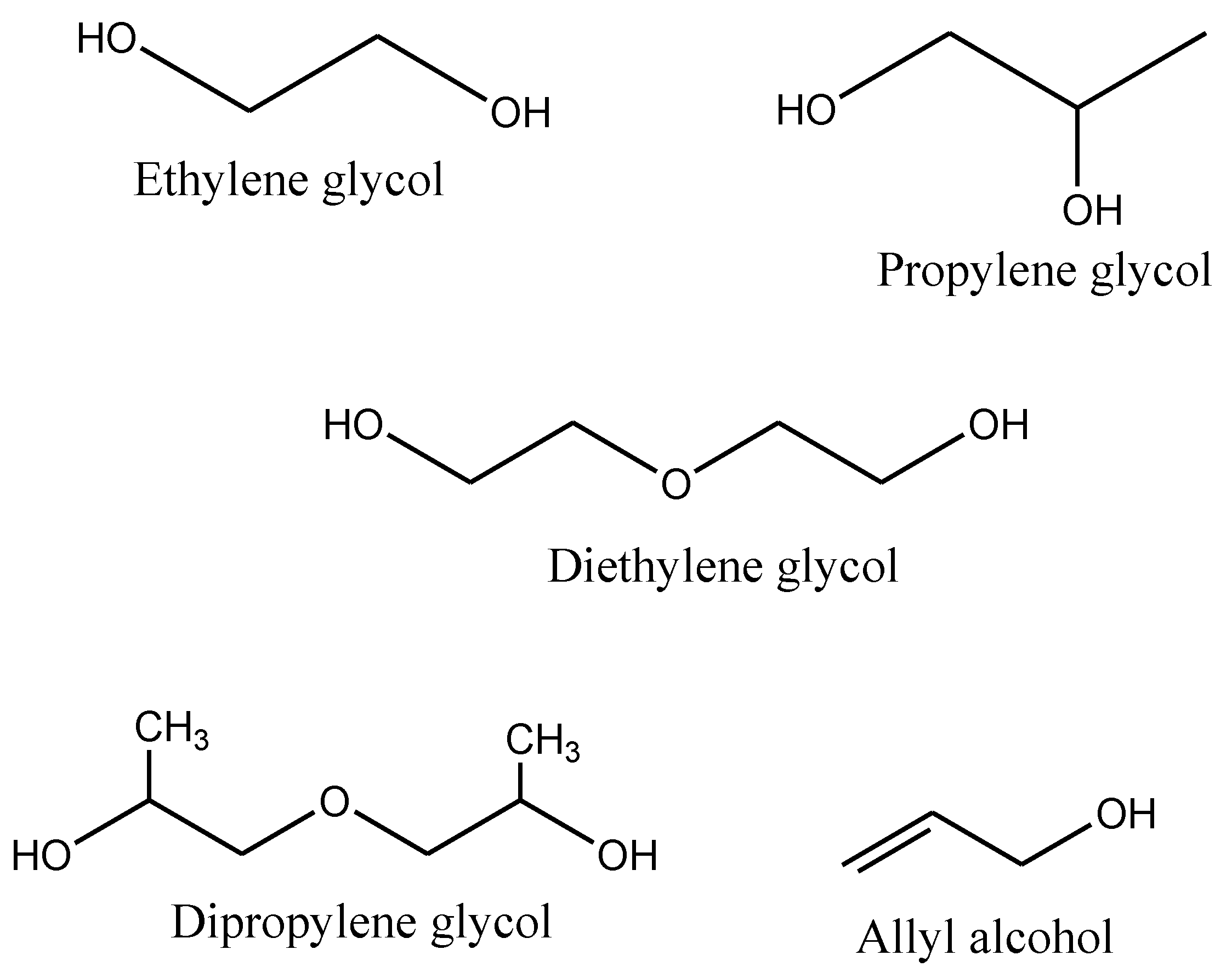
1.2. Saturated Polyesters
2.3. Alkyd Resins
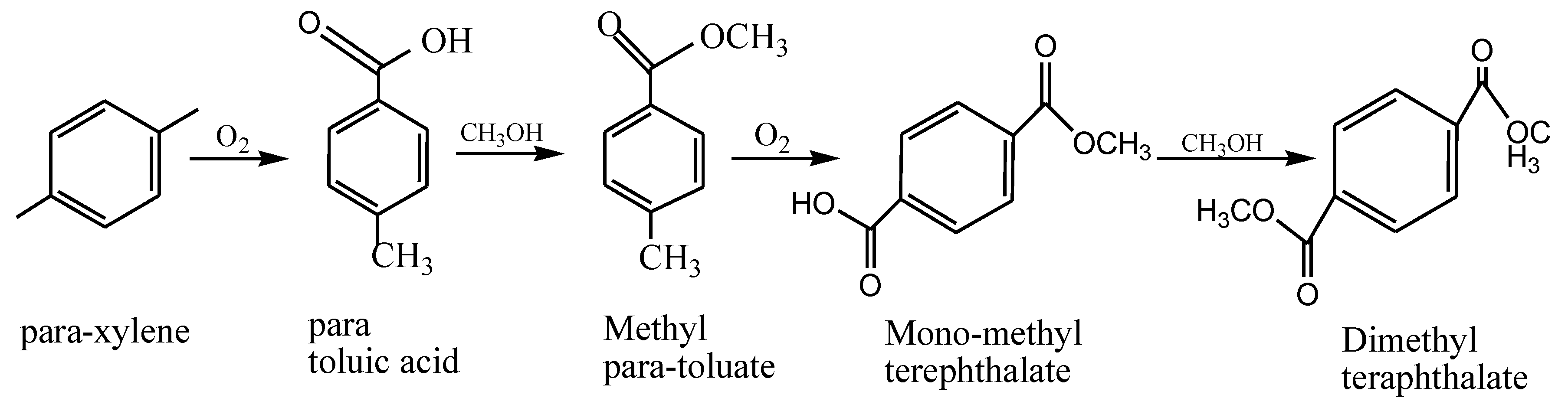
1.3. Alkyd Resins
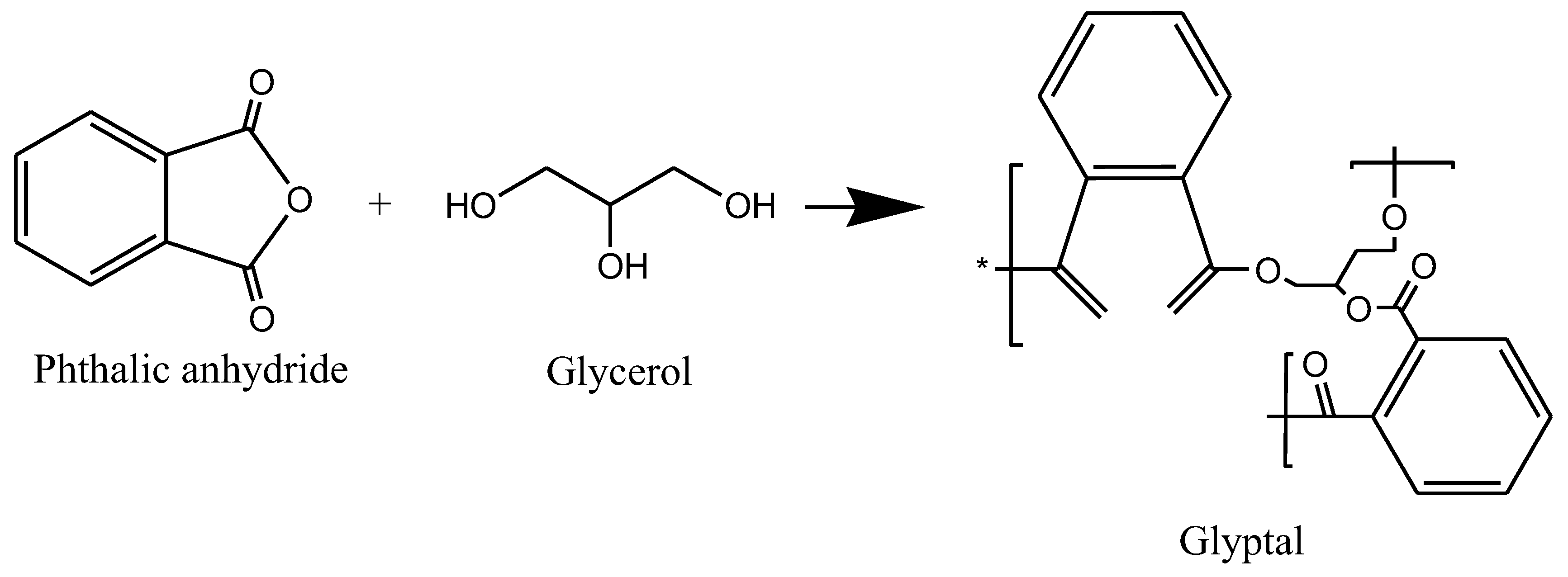
2.4. Vinyl Esters
1.4. Vinyl Esters
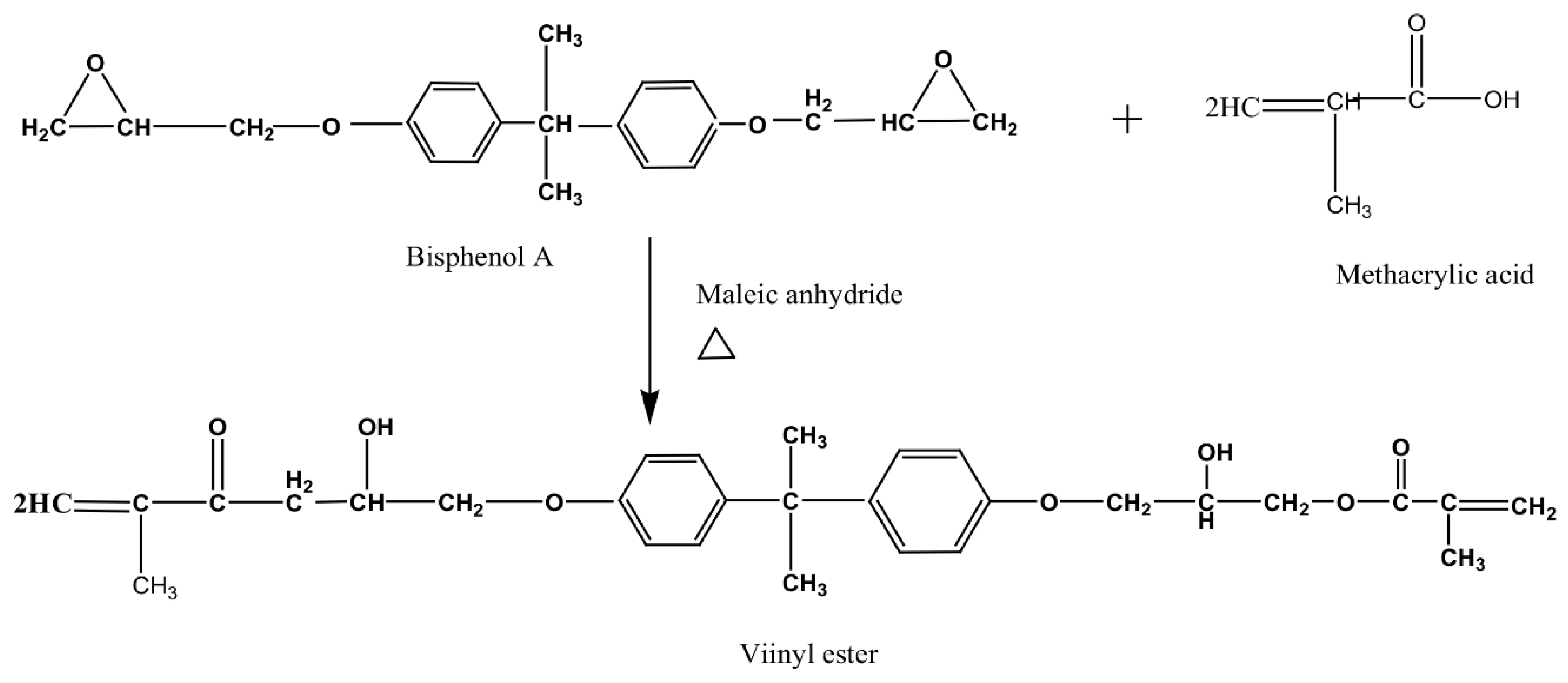
2. Polyester-Based Coatings
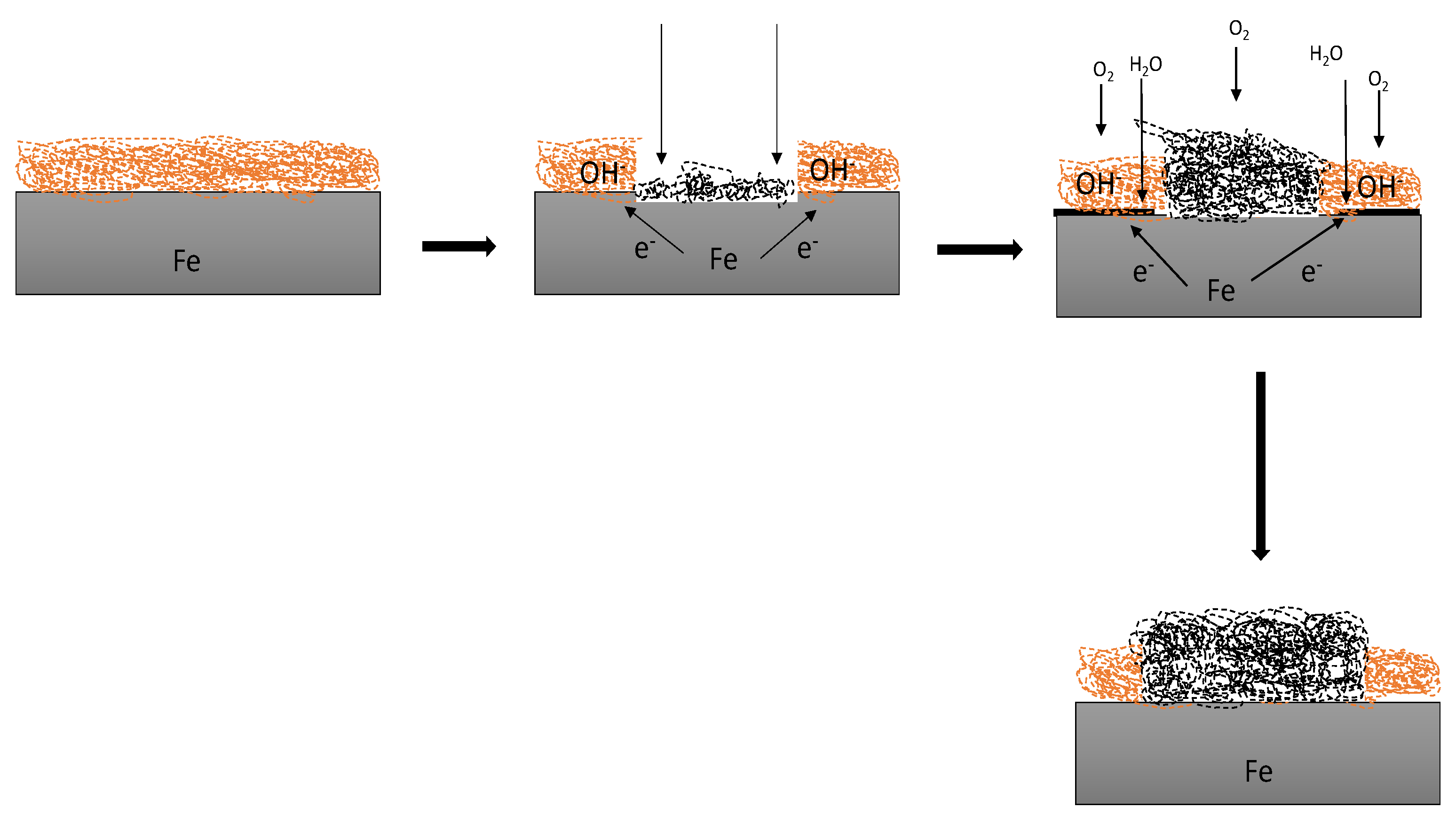
4. Applications
The applications of polyesters and vinyl esters depend on the properties of the resin. Different industries apply polyester and vinyl ester resin due to their excellent properties as already discussed. The different industries are outlined in Table 2 1 and the potential growth in the application of biodegradable polymers is depicted in Figure 158.
| Industry | Type of Polyester | Properties | Reference | |||||
|---|---|---|---|---|---|---|---|---|
| Transportation |
|
| [148,149,150] | [48][49][50] | ||||
| Electrical |
|
| [151,152,153] | [51][52][53] | ||||
| Industrial applications |
|
| [154,155,156,157,158] | [54][55][56][57][58] | ||||
| Construction | Ethylene tetrafluoroethylene (ETFE) |
| [159,160] | [59][60] |
References
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–2650.
- Lenz, R.W.; Marchessault, R.H. Bacterial polyesters: Biosynthesis, biodegradable plastics and biotechnology. Biomacromolecules 2005, 6, 1–8.
- Liu, Y.Q.; Liu, Y.; Tay, J.H. The effects of extracellular polymeric substances on the formation and stability of biogranules. Appl. Microbiol. Biotechnol. 2004, 65, 143–148.
- Roberts, C.W. Fire-retardant components of self-extinguishing polyesters. Polym. Eng. Sci. 1963, 3, 111–116.
- Xu, G.; Gong, L.; Yang, Z.; Liu, X.Y. What makes spider silk fibers so strong? From molecular-crystallite network to hierarchical network structures. Soft Matter 2014, 10, 2116–2123.
- Kim, M.N.; Lee, B.Y.; Lee, I.M.; Lee, H.S.; Yoon, J.S. Toxicity and biodegradation of products from polyester hydrolysis. J. Environ. Sci. Health Part A 2001, 36, 447–463.
- Levin, B.C. A summary of the NBS literature reviews on the chemical nature and toxicity of the pyrolysis and combustion products from seven plastics: Acrylonitrile–butadiene–styrenes (ABS), nylons, polyesters, polyethylenes, polystyrenes, poly (vinyl chlorides) and rigid polyurethane foams. Fire Mater. 1987, 11, 143–157.
- Yang, S.H.; Fu, P.; Liu, M.Y.; Wang, Y.D.; Zhang, Y.C.; Zhao, Q.X. Synthesis, characterization of polytridecamethylene 2, 6-naphthalamide as semiaromatic polyamide containing naphthalene-ring. Express Polym. Lett. 2010, 4, 442–449.
- Bledzki, A.K.; Reihmane, S.A.; Gassan, J. Thermoplastics reinforced with wood fillers: A literature review. Polym. Plast. Technol. Eng. 1998, 37, 451–468.
- Massy, J. Thermoplastic and thermosetting polymers. In A Little Book about BIG Chemistry; Springer: Cham, Switzerland, 2017; pp. 19–26.
- Dholakiya, B. Unsaturated polyester resin for specialty applications. Polyester 2012, 7, 167–202.
- Makhlouf, A.S.H. (Ed.) Handbook of Smart Coatings for Materials Protection (No. 64); Elsevier: Amsterdam, The Netherlands, 2014.
- Hernandez-Izquierdo, V.M.; Krochta, J.M. Thermoplastic processing of proteins for film formation—A review. J. Food Sci. 2008, 73, R30–R39.
- Guzowski, J.; Korczyk, P.M.; Jakiela, S.; Garstecki, P. The structure and stability of multiple micro-droplets. Soft Matter 2012, 8, 7269–7278.
- Makhlouf, A.; Satha, H.; Frihi, D.; Gherib, S.; Seguela, R. Optimization of the crystallinity of polypropylene/submicronic-talc composites: The role of filler ratio and cooling rate. Express Polymer Lett. 2016, 10, 237–247.
- Li, X.P.; Zhao, L.Y.; Liu, Z.Z. Topological optimization of continuum structure based on ANSYS. In Proceedings of the MATEC Web of Conferences, Shanghai, China, 21–23 October 2016; Tianjin University of Technology: Tianjin, China; Volume 95, p. 07020.
- Thair, L.; Jassim, I.K.; Al-Khuzaie, S.R.; Hammody, J.F.; Kalil, M.H. Corrosion protection of carbon steel oil pipelines by unsaturated polyester/clay composite coating. Am. Acad. Sci. Res. J. Eng. Technol. Sci. 2016, 18, 108–119.
- Blank, W.J.; Berndlmaier, R.; Miller, D. Additives for high solids and water-borne coatings. In Proceedings of the Presented at the International Waterborne, Higher-Solids, and Powder Coatings Symposium, New Orleans, LA, USA, 18–20 February 1998.
- Grigore, M.E. Methods of recycling, properties and applications of recycled thermoplastic polymers. Recycling 2017, 2, 24.
- Xia, Y.; Larock, R.C. Vegetable oil-based polymeric materials: Synthesis, properties, and applications. Green Chem. 2010, 12, 1893–1909.
- Ali Fathima Sabirneeza, A.; Subhashini, S. A novel water-soluble, conducting polymer composite for mild steel acid corrosion inhibition. J. Appl. Polym. Sci. 2013, 127, 3084–3092.
- Jafarzadeh, S.; Claesson, P.M.; Sundell, P.E.; Tyrode, E.; Pan, J. Active corrosion protection by conductive composites of polyaniline in a UV-cured polyester acrylate coating. Prog. Org. Coat. 2016, 90, 154–162.
- Lee, Y.H.; Lee, S.J.; Park, J.W.; Kim, H.J. Synthesis and properties of flexible polyester with urethane polyol for automotive pre-coated metals. J. Adhes. Sci. Technol. 2016, 30, 1537–1554.
- Rammelt, U.; Reinhard, G. Characterization of active pigments in damage of organic coatings on steel by means of electrochemical impedance spectroscopy. Prog. Org. Coat. 1994, 24, 309–322.
- Mariam Fadzlina, R. Synthesis of Polyaniline Modified with Palm Oil Based Alkyd as Counter Electrode in Solar Cell Application/Mariam Fadzlina Ramli. Ph.D. Thesis, Universiti Malaya, Kuala Lumpur, Malaysia, 2020.
- Jaffe, M.; Easts, A.J.; Feng, X. Polyester fibers. In Thermal Analysis of Textiles and Fibers; Woodhead Publishing: Sawston, UK, 2020; pp. 133–149.
- Gradinaru, V.; Treweek, J.; Overton, K.; Deisseroth, K. Hydrogel-tissue chemistry: Principles and applications. Annu. Rev. Biophys. 2018, 47, 355.
- Mukhtar, A.; Ullah, H.; Mukhtar, H. Fatty acid composition of tobacco seed oil and synthesis of alkyd resin. Chin. J. Chem. 2007, 25, 705–708.
- Isaac, I.O.; Ekpa, O.D.; Ekpe, U.J.; Odiongenyi, A.O. The effects of polybasic acid type on kinetics of the preparation of cottonseed oil based alkyd resins. IOSR J. Pharm. Biol. Sci. Ver. I 2015, 10, 231.
- Lambourne, R.; Strivens, T.A. (Eds.) Paint and Surface Coatings: Theory and Practice; Elsevier: Amsterdam, The Netherlands, 1999.
- Hofland, A. Alkyd resins: From down and out to alive and kicking. Prog. Org. Coat. 2012, 73, 274–282.
- Kushwaha, P.K.; Kumar, R. Studies on water absorption of bamboo-polyester composites: Effect of silane treatment of mercerized bamboo. Polym. Plast. Technol. Eng. 2009, 49, 45–52.
- Blayo, A.; Gandini, A.; Le Nest, J.F. Chemical and rheological characterizations of some vegetable oils derivatives commonly used in printing inks. Ind. Crop. Prod. 2001, 14, 155–167.
- Uchiyama, M.; Satoh, K.; Kamigaito, M. Stereospecific cationic RAFT polymerization of bulky vinyl ethers and stereoblock poly (vinyl alcohol) via mechanistic transformation to radical RAFT polymerization of vinyl acetate. Giant 2021, 5, 100047.
- Zhang, X.; Bitaraf, V.; Wei, S.; Guo, Z.; Zhang, X.; Wei, S.; Colorado, H.A. Vinyl ester resin: Rheological behaviors, curing kinetics, thermomechanical, and tensile properties. AIChE J. 2014, 60, 266–274.
- Dai, J.; Ma, S.; Wu, Y.; Zhu, J.; Liu, X. High bio-based content waterborne UV-curable coatings with excellent adhesion and flexibility. Prog. Org. Coat. 2015, 87, 197–203.
- Lee, Y.H.; Kim, H.J.; Park, J.H. Synthesis and characterization of polyester-based nanocomposites coatings for automotive pre-coated metal. Prog. Org. Coat. 2013, 76, 1329–1336.
- Atta, A.M.; Nassar, I.F.; Bedawy, H.M. Unsaturated polyester resins based on rosin maleic anhydride adduct as corrosion protections of steel. React. Funct. Polym. 2007, 67, 617–626.
- Ramesh, K.; Ramesh, S.; Vengadaesvaran, B.; Arof, A.K. Silicone-Polyester Blended Coatings for Corrosion Protection: Coating. Int. J. Fundam. Phys. Sci. 2011, 1, 83–86.
- Ramesh, K.; Osman, Z.; Arof, A.K.; Vengadaeswaran, B.; Basirun, W.J. Structural and corrosion protection analyses of coatings containing silicone-polyester resins. Pigment. Resin Technol. 2008, 37, 37–41.
- Piazza, D.; Silveira, D.S.; Lorandi, N.P.; Birriel, E.J.; Scienza, L.C.; Zattera, A.J. Polyester-based powder coatings with montmorillonite nanoparticles applied on carbon steel. Prog. Org. Coat. 2012, 73, 42–46.
- Chen, X.; Wu, L.; Zhou, S.; You, B. In situ polymerization and characterization of polyester-based polyurethane/nano-silica composites. Polym. Int. 2003, 52, 993–998.
- Xue, D.; Van Ooij, W.J. Corrosion performance improvement of hot-dipped galvanized (HDG) steels by electro-deposition of epoxy-resin-ester modified bis- ethane (BTSE) coatings. Prog. Org. Coat. 2013, 76, 1095–1102.
- Winter, H.H. August. The solidification rheology of amorphous polymers− Vitrification as compared to gelation. In Macromolecular Symposia; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; Volume 374, p. 1600113.
- Gopi, D.; Govindaraju, K.M.; Kavitha, L.; Basha, K.A. Synthesis, characterization and corrosion protection properties of poly (N-vinyl carbazole-co-glycidyl methacrylate) coatings on low nickel stainless steel. Prog. Org. Coat. 2011, 71, 11–18.
- Hollamby, M.J.; Fix, D.; Dönch, I.; Borisova, D.; Möhwald, H.; Shchukin, D. Hybrid polyester coating incorporating functionalized mesoporous carriers for the holistic protection of steel surfaces. Adv. Mater. 2011, 23, 1361–1365.
- Tanoğlu, M.; Seyhan, A.T. Investigating the effects of a polyester preforming binder on the mechanical and ballistic performance of E-glass fiber reinforced polyester composites. Int. J. Adhes. Adhes. 2003, 23, 1–8.
- Barratt, S.R.; Ennos, A.R.; Greenhalgh, M.; Robson, G.D.; Handley, P.S. Fungi are the predominant micro-organisms responsible for degradation of soil-buried polyester polyurethane over a range of soil water holding capacities. J. Appl. Microbiol. 2003, 95, 78–85.
- Bagherpour, S. Fibre Reinforced Polyester Composites; InTech: London, UK, 2012; pp. 135–166.
- O’Brien, D.J.; Baechle, D.M.; Yurchak, O.B.; Wetzel, E.D. Effect of processing conditions and electrode characteristics on the electrical properties of structural composite capacitors. Compos. Part A Appl. Sci. Manuf. 2015, 68, 47–55.
- Hendriks, C.F.; Janssen, G.M.T. Testing regulations and procedures for environmental auditing of recycled aggregates. Heron 2001, 46, 135–143.
- Sanada, K.; Tada, Y.; Shindo, Y. Thermal conductivity of polymer composites with close-packed structure of nano and micro fillers. Compos. Part A Appl. Sci. Manuf. 2009, 40, 724–730.
- Teng, H. Overview of the development of the fluoropolymer industry. Appl. Sci. 2012, 2, 496–512.
- Leivo, E.; Wilenius, T.; Kinos, T.; Vuoristo, P.; Mäntylä, T. Properties of thermally sprayed fluoropolymer PVDF, ECTFE, PFA and FEP coatings. Prog. Org. Coat. 2004, 49, 69–73.
- Ueberschlag, P. PVDF piezoelectric polymer. Sens. Rev. 2001, 21, 118–126.
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27.
- Robinson, L.A. Structural Opportunities of ETFE (Ethylene Tetra Fluoro Ethylene). Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2005.
- Lamnatou, C.; Moreno, A.; Chemisana, D.; Reitsma, F.; Clariá, F. Ethylene tetrafluoroethylene (ETFE) material: Critical issues and applications with emphasis on buildings. Renew. Sustain. Energy Rev. 2018, 82, 2186–2201.
- Hu, J.; Chen, W.; Zhao, B.; Yang, D. Buildings with ETFE foils: A review on material properties, architectural performance and structural behavior. Constr. Build. Mater. 2017, 131, 411–422.
- Sauerbrunn, S.; Weddle, B. Thermoset Curing Schedule and its Effect on the Final Properties. In Proceedings of the 2005 Annual Meeting, Hyatt Regency Savannah, Savannah, Georgia, 10–11 September 2007.
