Cardiovascular disease is the leading cause of death and disability, accounting for approximately one in three deaths worldwide. It is the leading cause of death in the United States; coronary artery disease (CAD) and ischemic heart disease (IHD) remain the leading causes of death attributable to cardiovascular disease in men and women. The incidence of atherosclerotic cardiovascular disease is increasing due to an increase in cardiac risk factors such as obesity, diabetes, and hypercholesterolemia in addition to an aging population. However, age-adjusted mortality rates are decreasing, likely due to improved medical therapies allowing patients to live longer with IHD. Although cardiovascular disease is more common in men, women with CAD have a worse short and long-term prognosis. In addition, women with symptoms of angina or who have had an abnormal cardiac stress test are less likely to be referred for additional diagnostic testing and initiated on guideline directed medical therapies.
1. Etiologies
The pathogenesis of atherosclerosis begins in adolescence and early adulthood in the form of fatty streaks, long before manifesting as clinically overt disease
[1][8]. Lipid-rich atherosclerotic plaque may ulcerate or rupture causing thrombosis and ischemia. Atherosclerosis resulting in plaque rupture or erosion is by far the most common cause of myocardial infarction (MI) in men and women.
MI with nonobstructive coronary arteries (MINOCA) accounts for 5–10% of MI. Similarly, myocardial ischemia may occur with signs and symptoms of ischemia in the absence of significant
coronary artery disease (CAD)CAD which is classified as ischemia with no obstructive coronary artery disease (INOCA)
[2][9].
There is likely some degree of overlap between MINOCA and INOCA. MINOCA is defined by criteria for the universal definition of MI, in which there is rise and/or fall of cardiac troponin (cTn) value above the 99th percentile with signs and symptoms of myocardial ischemia. This can be manifested as new ischemic ECG changes, new ischemic wall motion abnormalities, the loss of viable myocardium on noninvasive imaging, or identification of coronary thrombus by angiography or autopsy without obstructive coronary artery stenosis (<50% stenosis) on angiography, and the absence of myocarditis or Takotsubo cardiomyopathy
[3][10]. In contrast to acute MI due to CAD, MINOCA commonly affects younger patients, particularly women with fewer traditional cardiac risk factors. The etiology of this condition is heterogenous and may result from plaque disruption by rupture or erosion, coronary vasospasm, spontaneous coronary artery dissection (SCAD), coronary artery embolization or coronary microvascular disease. However, in a majority of cases no etiology is identified
[4][11]. MINOCA can also present with cardiac arrest and heart failure and while the mortality rate tends to be lower than MI due to CAD overall, outcomes are significantly worse than age-matched controls compared with MI secondary to CAD
[5][12].
Women with IHD can present differently when compared to men. Women are less likely to present with classic chest pain (31% compared with 42% in men) and more often report dyspnea, weakness, back pain, palpitations, or loss of appetite. Importantly, patients with ACS who present without chest pain are more likely to be misdiagnosed and have a higher risk of death than those who present with classic chest pain symptoms. Moreover, the absence of chest pain has not been consistently correlated with ACS severity
[6][7][21,22].
2. Noninvasive Imaging Modalities
There are a variety of different non-invasive imaging modalities that play a role in the diagnosis, management, and prognostic assessment of women with IHD. Overall risk assessment and an understanding of the sex-specific differences in the presentation and pathophysiology of ischemia and performance of available diagnostics is imperative when deciding the optimal test for evaluating women for IHD. Female-specific considerations such as exposure of breast tissue to radiation and pregnancy status necessitate a patient-centered approach to diagnosis. Compared with men, women less frequently have obstructive coronary artery disease or undergo coronary angiography and are more likely to have coronary microvascular dysfunction
[8][9][7,23].
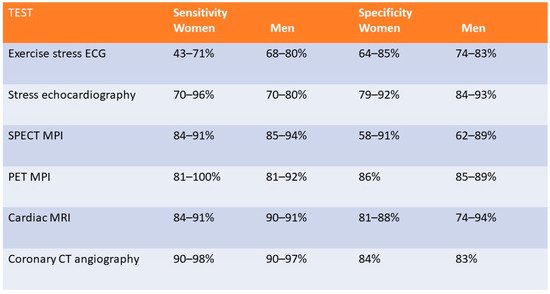
Figure 1 compares the sensitivity and specificity for the available diagnostic noninvasive modalities for the detection of IHD in men and women.
Figure 1.
Figure comparing the sensitivity and specificity of different non-invasive imaging modalities in men and women.
3. Exercise Stress Testing
Exercise stress test electrocardiography (ETT) evaluates for inducible ischemia by the presence or absence of ST-segment changes (ST depressions or elevations) along with the assessment of hemodynamic response to exercise and overall cardiorespiratory fitness. Exercise is performed on a treadmill or bicycle ergometry with continuous ECG monitoring. This modality is widely accessible and inexpensive
[10][24]. However, the sensitivity and specificity to detect obstructive CAD is significantly lower in women, as shown in
Figure 1 [11][12][13][14][15][25,26,27,28,29]. A meta-analysis of the detection of obstructive CAD using ETT, demonstrated a sensitivity of 61% and specificity of 70% in women compared to 68% and 77%, respectively, in men
[12][26]. However, the ROMICAT study (Rule Out Myocardial Infarction Using Computer-Assisted Tomography) which examined 220 patients who underwent ETT demonstrated a sensitivity of 60% for >50% stenosis and a specificity of 94% with a negative predictive value of 78% in women, which was similar to 81% observed in men
[16][30]. The utility of ETT as an initial diagnostic strategy in low-risk, exercising women has been reinforced by results from the What Is the Optimal Method for Ischemia Evaluation in Women (WOMEN) trial, where ETT was shown to have similar 2-year outcomes while providing significant cost-savings
[17][31].
4. Exercise and Dobutamine Stress Echocardiography
Stress echocardiography allows for the visualization of abnormal myocardial contractility during exercise when compared to baseline, an indication of inducible myocardial ischemia. Exercise is preferred; however, a pharmacological stress agent such as dobutamine can be used to detect new or worsening left ventricular (LV) regional wall motion abnormalities (RWMA). In addition to the qualitative assessment of myocardial response to stress, quantitative measurements with techniques such as strain rate imaging (SRI) derived from tissue Doppler imaging (TDI) can be employed to assess for myocardial viability. Strain describes the shortening, thickening, and lengthening of the myocardium, which can be used as a measure of regional left ventricular function on echocardiography and can detect characteristic findings suggestive of ischemic myocardium such as reduced peak systolic strain, systolic lengthening, and post-systolic shortening
[18][32]. This results in a contraction variable which is independent of the passive tethering effects from other regions that can influence the interpretation of single point velocities measured by TDI and has been successfully used to differentiate between different myocardial viability states
[19][33]. Stress echocardiography is widely available and avoids exposure to ionizing radiation. Contraindications include uncontrolled hypertension, severe arrhythmias, significant left ventricular outflow tract obstruction, ACS, and symptomatic severe aortic stenosis
[20][34]. Overall, stress echocardiography has a higher sensitivity and specificity than ETT in women with a sensitivity of 70 to 96% and specificity 79 to 92%
[21][22][35,36]. The performance of stress echocardiography to detect inducible ischemia in men and women has been variable, with some studies showing statistical differences
[23][24][37,38] while other studies did not show significant variations in performance
[24][25][38,39]. A recent trial that included approximately 45% women observed a sensitivity of 95.4% and a specificity of 96% for the identification of obstructive CAD
[26][40].
5. SPECT- Myocardial Perfusion Imaging (Exercise and Pharmacological)
SPECT-myocardial perfusion imaging (MPI) provides information about global and regional LV systolic contractility, LV volume, as well as myocardial perfusion defects during exercise or pharmacological stress for the evaluation of patients with known or suspected CAD. Radionucleotide tracers, most commonly 99m-technetium (Tc99m)-labeled perfusion agents are injected intravenously at peak exercise or following vasodilator administration (agents such as adenosine, dipyridamole or regadenoson) or dobutamine administration. Both rest and stress images are typically obtained; however, in selected patients a stress-only protocol may be employed with rest images obtained only if stress images are abnormal
[27][41]. This modality demonstrates good performance in women, with a sensitivity reported of 84–91% and a specificity of 58–91%
[28][29][30][31][42,43,44,45]. SPECT-MPI can also be used to risk stratify patients by assessing the extent and severity of defect size and its degree of reversibility
[32][33][46,47]. However, limitations in women include false positive results from breast attenuation and reduced accuracy in patients with smaller sized hearts, which is more commonly seen in women
[34][48]. Additional consideration should also be given to radiation exposure, estimated at 11 mSV in this modality, particularly in younger patients. However, current estimates of radiation risk do not demonstrate a differential risk from SPECT-MPI in women relative to men
[35][36][49,50].
6. Stress Positron Emission Tomography
Stress myocardial PET imaging provides similar information to SPECT-MPI imaging while providing information about CFR; which can aid in the diagnosis of CMD; and quantifying global and regional myocardial blood flow (MBF). Quantification of MBF requires accurate measurement of the total tracer activity transported by arterial blood and delivered to the myocardium over a certain period of time. Time-activity curves are acquired (arterial isotope activity versus time) using image regions located in the arterial pool (LV; left atrium or aorta)
[37][51]. The test is also performed in a similar manner whereby rest CT and PET images are attained using radiotracers; either rubidium-82 or 13N-ammonia; which are injected intravenously. These tracers have a very short half-life; therefore; stress images are obtained within 30–80 min following rest images depending on the washout period of the tracer used. As a result; pharmacological stress is typically performed due to their short duration of action
[38][52]. The use of PET imaging relative to SPECT is primarily limited by availability. This modality has been shown to be superior to SPECT with respect to image quality; interpretive certainty; and diagnostic accuracy in both men and women
[39][53]. Radiation dose is significantly less than SPECT imaging; estimated at 2–3 mSV depending on the tracer with a similar sensitivity and specificity in men and women
[40][41][54,55]. A large meta-analysis including 1442 patients reported an overall sensitivity of 92% and specificity of 85%
[42][56]. A study of 409 patients underwent a baseline PET scan which was repeated 2–3 years later to assess the clinical outcomes of pharmacologic therapy and lifestyle modifications as well as their impact on noninvasive imaging. Patients were sorted into three categories: “poor” treatment without diet or lipid-lowering drugs; or who were actively smoking; “moderate” treatment on American Heart Association diet and lipid-lowering drugs or on strict low-fat diet without lipid-lowering drugs; and “maximal” treatment with strict low-fat diet; regular exercise; and lipid-active drugs dosed to specific target goals of low-density lipoproteins; high-density lipoproteins and triglycerides. Over five years; coronary events occurred in 6.6%, 20.3%, and 30.6% of patients on maximal; moderate; and poor treatment; respectively (
p = 0.001). Size and/or severity of perfusion abnormalities significantly decreased for patients receiving maximal treatment and increased for patients undergoing moderate and poor treatment (
p = 0.003 and 0.0001; respectively)
[43][57] Nuclear imaging may also be used detect inflammation within plaque using specific molecular targets. PET-CT imaging using radiotracers such as 18F-fluorodeoxyglucose (18F-FDG) and 68Gallium (68Ga-DOTATATE) allows for combined anatomic identification of coronary plaques coupled with molecular inflammation which has been as-sociated with atherosclerotic plaque progression
[44][45][58,59]. Increased coronary 18F-fluoride (18F-NaF) uptake has been associated with more rapid progression of coronary calcification and has been used to identify and localize ruptured and high-risk coronary plaque
[46][47][60,61]. Feasibly their use could permit the identification of patients at increased risk of adverse events or determine which lesions are vulnerable and may benefit from revascularization.
7. Coronary CT Angiography
8. Stress Cardiac Magnetic Resonance Imaging
Stress CMR can provide accurate assessment of myocardial ischemia, viability, and function. The absence of ionizing radiation with CMR combined with its high contrast and spatial resolution are advantageous, especially in younger or pregnant women. Stress CMR images are obtained with the use of vasodilators (typically adenosine, regadenoson or dipyridamole) to induce hyperemia followed by a gadolinium-based contrast agent injected peripherally; serial T1-weighted CMR images are then acquired. The contrast enters normally perfused myocardial regions more quickly and in higher concentrations which can be detected as a greater increase in T1-signal relative to abnormally perfused regions
[60][85]. Although not widely available, CMR has the potential to quantify MBF and detect CMD defined by invasive coronary reactivity testing
[61][86]. Limitations of stress CMR include limited availability and the inability to include an exercise component. Additionally, there is concern for nephrogenic systemic fibrosis in patients with advanced CKD; however, currently used agents have a low estimated risk (<0.07%) of this complication
[62][87]. Finally, patient tolerability and implanted ferromagnetic devices or objects may preclude its use in certain patients.
9. Safety of Non-Invasive Imaging Modalities for Ischemia in Women and Pregnancy
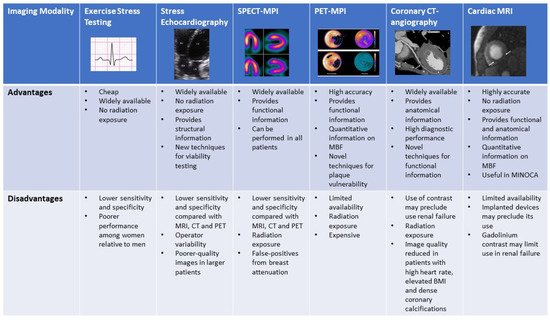
A range of effective, non-invasive modalities are available for evaluation of ischemia in women. Exposure of breast tissue to radiation and pregnancy or breastfeeding status are important female-specific considerations which can influence selection. Figure 27 compares the advantages and disadvantages of each modality.
Figure 27. Figure comparing the advantages and disadvantages of the various non-invasive imaging modalities.
Figure comparing the advantages and disadvantages of the various non-invasive imaging modalities.
Exercise stress ECG testing can be considered an option in all women regardless of pregnancy status given its safety profile
[63][100]. Exercise stress echocardiography is similarly safe for use in pregnancy and avoids exposure to ionizing radiation. Pharmacological stress echocardiography also appears to be similarly safe in pregnancy with no significant maternal or fetal risk; dobutamine and dipyridamole are considered category B agents favored over adenosine, which is category C
[64][101].