This entry describes developments in remote management to prevent diabetes-related foot disease.
- diabetes, foot disease, remote management
Diabetes-related foot disease (DFD), which includes foot ulcers, infection and gangrene, is a leading cause of the global disability burden. About half of people who develop DFD experience a recurrence within one year. Long-term medical management to reduce the risk of recurrence is therefore important to reduce the global DFD burden. This review describes research assessing the value of sensors, wearables and telehealth in preventing DFD. Sensors and wearables have been developed to monitor foot temperature, plantar pressures, glucose, blood pressure and lipids. The monitoring of these risk factors along with telehealth consultations has promise as a method for remotely managing people who are at risk of DFD. This approach can potentially avoid or reduce the need for face-to-face consultations. Home foot temperature monitoring, continuous glucose monitoring and telehealth consultations are the approaches for which the most highly developed and user-friendly technology has been developed. A number of clinical studies in people at risk of DFD have demonstrated benefits when using one of these remote monitoring methods. Further development and evidence are needed for some of the other approaches, such as home plantar pressure and footwear adherence monitoring. As yet, no composite remote management program incorporating remote monitoring and the management of all the key risk factors for DFD has been developed and implemented. Further research assessing the feasibility and value of combining these remote monitoring approaches as a holistic way of preventing DFD is needed.
1. Introduction
Diabetes-related foot disease (DFD), including foot ulcers, infection and gangrene, is one of the 10 leading causes of the global disability burden [1][2][3]. About 40% of people who develop DFD experience a recurrence within one year, and thus DFD represents a chronic disease; the focus of research into this should be on avoiding remission and preventing major consequences, such as amputation and death [4]. Key risk factors for DFD recurrence and complications in people at risk of DFD include high plantar pressures, abnormal gait, hyperglycaemia, hypertension and
dyslipidemia [5][6][7]. Randomised controlled trials and meta-analyses show that foot disease is preventable by the control of these key reversible risk factors using interventions such as appropriate foot care, footwear and medical management [5][6][7][8]. A range of sensors and wearables have been developed or are currently under development for the remote monitoring of these key risk factors and this combined with telehealth management offers a way to remotely care for people at risk of DFD, as shown in Table 1. The implementation of these approaches could also minimize the risk to patients and staff of exposure to the current global SARS-CoV-2 pandemic [2][3].
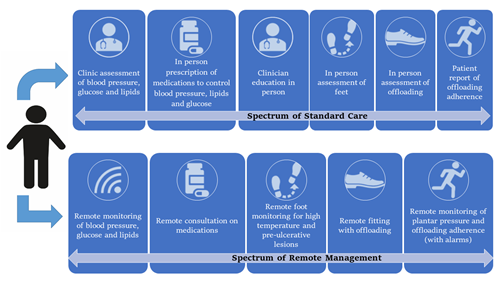
This review summarizes the potential application of remote monitoring systems using sensors and wearables to prevent DFD in the at-risk population, as shown in Figure 1 and Table 1. The challenges of implementing remote DFD prevention and how sensors and wearables could be applied to better prevent DFD are discussed below.
Table 1. Examples of sensors and wearables with potential value for preventing DFD.
|
Risk factor |
Current Management Approach |
Sensors or Wearable Devices |
References |
Potential Value of Sensor/Wearable |
Potential Impact on Prevention |
|
Pre-ulcerative lesions |
Visits to podiatrist |
Home foot temperature monitor and mobile phone applications |
Offloading of “hot spots” following confirmed persistent temperature differences |
Reduced progression of at-risk sites prone to develop foot ulcers |
|
|
Elevated plantar pressures |
Offloading footwear |
Plantar pressure monitor |
[11] |
Warning systems to stimulate offloading and better design and modification of footwear |
Improved offloading with reduced ulcer development |
|
Elevated plantar pressures |
Patient education |
Footwear adherence monitor |
[11] |
Behaviour change support counselling informed by objective data |
Improved offloading adherence |
|
Hyperglycaemia |
Capillary glucose monitoring |
Continuous glucose monitor |
[12] |
Intensive glycaemic control |
Better informed management of hyper and hypoglycaemia and reduced progression of macro and microvascular disease |
|
Hypertension |
Outpatient blood pressure measurement |
Cuff-less blood pressure monitor |
[13]
|
Better implementation of anti-hypertensive medications and more frequent monitoring |
Better informed management of blood pressure and reduced progression of macro and microvascular disease and mortality |
|
Abnormal gait |
Not routinely managed |
Gait and activity monitor |
[14]
|
Gait retraining and encouraging remote physical activity |
Reduce gait abnormalities potentially reducing plantar pressures and ulcer incidence |
|
Peripheral artery disease |
Vascular laboratory assessment using ultrasound or Doppler |
Foot blood supply sensor |
[15]
|
Earlier identification of complications and prompt medical management |
Reduced progression of macro and microvascular disease |
Legend: The table outlines the risk factors for the development of diabetes-related foot disease and how sensors and wearables could be used to remotely monitor these factors. References are provided for the relevant research articles assessing the impact or implementation of such technologies for further reading.
Figure 1. Key aspects of existing standard care compared with a future remote prevention program for diabetes-related foot disease.
2. Remotely Monitoring Medical Management
The optimal control of glucose, blood pressure and lipids is frequently not well implemented among people that develop DFD [16]. People with DFD have an increased risk of all-cause mortality (relative risk (RR) 1.89, 95% confidence intervals (CI) 1.60, 2.23) and fatal myocardial infarction
(RR 2.22, 95% CI 1.09, 4.53) compared to people with diabetes without DFD [17]. In people with a history of diabetes-related foot ulcers, the risk of cardiovascular mortality is about 50% over 10 years and the annual mortality rate is estimated to be about 6% [18]. This emphasizes the importance of optimizing medical management in this population.
Glycaemic control is important for preventing both macro and microvascular complications, and a meta-analysis of past randomised trials suggests that intensive glycaemic control prevents amputations [19]. In clinical practice, diabetes management is usually informed by self-monitoring of blood glucose [20]. Wearable or implantable sensors are now available for the continuous monitoring of glucose [20]; these use enzymatic technology to monitor interstitial fluid rather than blood glucose[21]. These sensors can measure glucose up to every 5 min non-invasively for a period of about one week, after which most devices need to be replaced . Such sensors have been incorporated into closed loop systems which provide automated insulin delivery to people with type 1 diabetes with improvements in glycaemic control [22]. Recent meta-analyses of randomised trials comparing self-monitoring and the continuous automated monitoring of glucose in people with type 2 diabetes suggest that continuous monitoring facilitates better glycaemic control [23][24][25]. The use of such devices is now recommended by the North American guidelines for some patients, such as those with poor glycaemic control (HbA1c ≥ 9%) [26]. A recent trial showed that flash glucose monitoring (measuring interstitial fluid glucose) can be implemented in the primary care environment, although it may not be superior to traditional methods as measured by HbA1c at 12 months [12]. The application of continuous glucose monitoring for people with diabetes who are at a high risk of complications such as DFD may have substantial benefits, but access to this technology is currently limited to selected patients due to the current high expense of such monitoring systems.
High blood pressure is another important risk factor for complications in people with DFD.
Anti-hypertensive medications, such as angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, have been shown to reduce the incidence of cardiovascular events in people at risk of DFD, such as those with PAD [27]. Control of blood pressure is, however, frequently suboptimal in people at risk of DFD [28]. In a recent study of 2773 people with PAD, about 40% had a systolic blood pressure above the target level of 140 mmHg [28]. Currently, blood pressure is monitored through the assessment of pulsation linked with an inflatable cuff placed around the upper arm. Novel cuff-less wearable devices have now been developed for the estimation of blood pressure and may provide a more practical way of repeatedly monitoring blood pressure and facilitating better management [29]. These devices use varying methods, such as pulse transit time, laser Doppler flowmetry and artery vibration, to calculate blood pressure. Some of these devices are available commercially, such as from TMART Technologies Limited, China and Somnomedics, Germany, and some—but not all—have been shown to accurately measure blood pressure in small numbers of people with comparable results to classical cuff-dependent machines and also intra-arterial assessments [30][31][32]. The accuracy and value of these devices in improving the medical management of people at risk of DFD need further evaluation.
People at risk of DFD also require lipid control. The intensive lowering of low-density lipoprotein has proven efficacy in reducing major adverse cardiovascular and limb events in people at risk of DFD, such as those with PAD and diabetes [32]. Low-density lipoprotein sensors have also been built, although further development and testing is needed before they will be ready for widespread use [33].
Medication non-adherence is often defined as taking less than 80% of the prescribed
treatment [34]. Due to a variety of factors including cost and regimen complexity, adherence to diabetes treatment is often poor and is reported to vary from 23% to 77% across differing
populations [35][36]. In order to achieve optimal control of risk factors, it is important that patients adhere to prescribed medications. Sensors have now been developed that are capable of monitoring medication ingestion; for example, Proteus Discover provides data on medication taking and physical activity to both patients and practitioners[37]. It consists of an ingestible sensor, a wearable sensor patch, a patient mobile app and a provider Web portal. After being swallowed, the ingestible sensor is activated and sends a signal with a specific code that is detected by the patch. When the ingestible sensor pill is taken with medication, it can measure medication ingestion adherence. The patch also can measure activity, heart rate and step count. Data from the patch are transmitted to a mobile device to be viewed by the patient and then to the Cloud and onto a Web portal for a practitioner to view. The mobile device app prompts the patient to take their medication doses as scheduled. A previous study suggested that Proteus Discover can improve control of blood pressure, low-density lipoprotein and HbA1c [37]. Such sensors could have a role in people at risk of DFD, but this needs further testing and consultation with patients and other key stakeholders. There is a lack of
head-to-head clinical trials comparing the various types of sensors or monitors available for remote medical management described above; more importantly, the control arms in clinical trials of remote monitoring systems have varied substantially. Therefore, there is an ongoing need to assess the suitability of these sensors for optimizing medical management in people at risk of DFD.
3. Telehealth
For people with DFD, treatment and education typically occur in an outpatient clinic weekly or bi-weekly. Although remote monitoring methods for people with DFD using smartphone applications have been developed, these are still in their infancy and have not been widely tested or implemented [38][39][40]. Despite their potential application in remote DFD monitoring, the diagnostic accuracy of mobile phone images is reported to be poor and therefore should not be used as a
stand-alone diagnostic instrument for DFD [41]. This is a rapidly evolving area; therefore, novel mobile phone applications and remote monitoring methods may improve over time.
Telehealth is an established means of performing medical consultations [42]. The benefit of using telehealth for managing foot ulcers has been demonstrated in several meta-analyses and systematic reviews [43][44][45]. Most of the evidence comes from two clinical trials [46][47]: the first trial evaluated the effectiveness of a telehealth intervention made up of 2:1 online:standard outpatient consultations compared to a usual care intervention consisting of three standard outpatient clinic visits on ulcer healing in 374 people [46]. The authors reported no significant difference in ulcer healing or amputation between the two methods but did show an increased risk of mortality in the remote monitoring group (HR = 8.68, 95% CI: 6.9–10.88). This was a surprising finding that was not explained by any of the studied covariates [46].
A more recent cluster randomised controlled non-inferiority trial of 182 adults evaluated telehealth [94]. Weekly telemedicine consultations via an interactive Web-based ulcer record and a mobile phone for communication with the healthcare specialist in addition to outpatient clinic visits every 6 weeks was compared to visiting the outpatient clinic every second week [47]. The trial showed no difference in time to ulcer healing and a superiority in prevention against amputation (mean difference: 8.3%, 95% CI: 16.3%, −0.5%) in the intervention group [94]. An important factor to note in these trials was that the intervention arms all included some face-to-face consultations with a health care professional. Based on anecdotal evidence, at present, there appears to be a range of different approaches to telemedicine that are used globally, ranging from mobile phone-based consultations, hospital-based remote management consultations and the phone-based review of patients. However, how such approaches should be designed in line with face-to face care has not been well defined in the literature.
There has been limited study of the value of telehealth consultations in preventing rather than treating DFD. The COVID-19 pandemic has provided a stimulus for studies testing the use of remote monitoring technologies and telehealth consultations in preventing DFD (see Table 2).
Table 2. Currently available and required evidence for the remote assessment and prevention of diabetes-related foot disease.
|
Remote Monitoring |
Available Evidence |
Current Limitations of Available Evidence |
Relevant Studies |
|
Home foot temperature monitor |
A number of small RCTs show a decreased incidence of foot ulcers in people performing home-based temperature monitoring |
Lack of a widely tested and user-friendly way of identifying “hot spots” Generalizability from prior smaller studies in select populations |
|
|
Plantar pressure monitor |
Possible to monitor plantar pressure remotely and use patient alarms to warn patients of impending sites of tissue breakdown as reported in one small RCT |
Unclear if technology can be further developed to be more user-friendly and whether the findings are applicable and would be effective on a widespread basis |
[11] |
|
Footwear adherence monitor |
Technology has been developed to accurately measure footwear adherence |
Need for widespread testing of value of using devices Patients’ views on use of adherence monitoring is still unclear |
[11] |
|
Continuous glucose monitor |
Highly developed area of monitoring and tested in multiple RCTs with proven benefit in improving glycaemic control (HbA1c) |
Whether this remote monitoring improves outcomes in people at risk of developing DFD remains unclear |
[12] |
|
Cuff-less blood pressure monitor |
Technology developed to assess this reported to be accurate in a small number of studies |
Currently unclear whether these devices can be used on a widespread scale |
[13] |
|
Foot blood supply and sensation assessment |
Technology still in the early developmental stages for monitoring
|
The benefit of these devices in improving clinical outcomes need to be further evaluated in RCTs |
[15–82][15] |
Legend: PAD= peripheral artery disease, RCT= randomised controlled trial, HbA1c= glycated haemoglobin A1c, DFD = diabetes-related foot disease
References
- Zhang, Y.; Lazzarini, P.A.; McPhail, S.M.; Van Netten, J.J.; Armstrong, D.G.; Pacella, R.E. Global Disability Burdens of Diabetes-Related Lower-Extremity Complications in 1990 and 2016. Diabetes Care 2020, 43,
- Anand, S.S.; Caron, F.; Eikelboom, J.W.; Bosch, J.; Dyal, L.; Aboyans, V.; Abola, M.T.; Branch, K.R.; Keltai, K.; Bhatt, D.L.; et al. Major Adverse Limb Events and Mortality in Patients With Peripheral Artery Disease: The COMPASS Trial. J. Am. Coll. Cardiol. 2018, 71, 2306–2315.
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242.
- 964–974.
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375.
- Heart Protection Study Collaborative Group. Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20,536 people with peripheral arterial disease and other high-risk conditions. J. Vasc. Surg. 2007, 45, 645–654.
- Golledge, J.; Ward, N.C.; Watts, G.F. Lipid management in people with peripheral artery disease. Curr. Opin. Lipidol. 2019, 30, 470–476.
- van Netten, J.; Raspovic, A.; Lavery, L.A.; Monteiro-Soares, M.; Rasmussen, A.; Sacco, I.C.N.; Bus, S.A. Prevention of foot ulcers in the at‐risk patient with diabetes: A systematic review. Diabetes Metab. Res. Rev. 2020, 36, e3270.
- Rogers, L.C.; Lavery, L.A.; Joseph, W.S.; Armstrong, D.G. All Feet On Deck-The Role of Podiatry During the COVID-19 Pandemic: Preventing hospitalizations in an overburdened healthcare system, reducing amputation and death in people with diabetes. J. Am. Podiatr. Med. Assoc. 2020, volume, page range.
- de Stegge, W.B.A.; Mejaiti, N.; Van Netten, J.J.; Dijkgraaf, M.G.W.; Van Baal, J.G.; Busch-Westbroek, T.E.; Bus, S.A. The cost-effectiveness and cost-utility of at-home infrared temperature monitoring in reducing the incidence of foot ulcer recurrence in patients with diabetes (DIATEMP): Study protocol for a randomized controlled trial. Trials 2018, 19, 520.
- Ming, A.; Walter, I.; Alhajjar, A.; Leuckert, M.; Mertens, P.R. Study protocol for a randomized controlled trial to test for preventive effects of diabetic foot ulceration by telemedicine that includes sensor-equipped insoles combined with photo documentation. Trials 2019, 20, 521.
- Manji, K. Pressure-Sensing Insoles in the Neuropathic Ulcer Treatment Pathway (PINUP). Available online: https://clinicaltrials.gov/ct2/show/NCT02586519?term=insoles&cond=Diabetic+Foot&draw=2&rank=8 (accessed on 14 April Year).
- Furler, J.; O’Neal, D.; Speight, J.; Blackberry, I.; Manski-Nankervis, J.-A.; Thuraisingam, S.; De La Rue, K.; Ginnivan, L.; Doyle, R.; Holmes-Truscott, E.; et al. Use of professional-mode flash glucose monitoring, at 3-month intervals, in adults with type 2 diabetes in general practice (GP-OSMOTIC): A pragmatic, open-label, 12-month, randomised controlled trial. Lancet Diabetes Endocrinol. 2020, 8, 17–26.
- Persell, S.D.; Peprah, Y.A.; Lipiszko, D.; Lee, J.Y.; Li, J.J.; Ciolino, J.D.; Karmali, K.N.; Sato, H. Effect of Home Blood Pressure Monitoring via a Smartphone Hypertension Coaching Application or Tracking Application on Adults With Uncontrolled Hypertension: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e200255–e200255.
- Ferreira, J.S.S.P.; Júnior, R.H.C.; Silva, E.Q.; Veríssimo, J.L.; Monteiro, R.L.; Pereira, D.S.; Suda, E.Y.; Sartor, C.; Sacco, I. Study protocol for a randomized controlled trial on the effect of the Diabetic Foot Guidance System (SOPeD) for the prevention and treatment of foot musculoskeletal dysfunctions in people with diabetic neuropathy: The FOotCAre (FOCA) trial I. Trials 2020, 21, 73.
- Iacopi, E.; Coppelli, A.; Riitano, N.; Abbruzzese, L.; Pieruzzi, L.; Goretti, C.; Piaggesi, A. Adherence to guideline recommended medical therapies in type 2 diabetic patients with chronic critical limb ischemia. Diabetes Res. Clin. Pract. 2019, 158, 107898.
- Brownrigg, J.R.W.; Davey, J.; Holt, P.J.E.; Davis, W.A.; Thompson, M.M.; Ray, K.K.; Hinchliffe, R.J. The association of ulceration of the foot with cardiovascular and all-cause mortality in patients with diabetes: a meta-analysis. Diabetologia 2012, 55, 2906–2912.
- Mader, J.K.; Haas, W.; Aberer, F.; Boulgaropoulos, B.; Baumann, P.; Pandis, M.; Horvath, K.; Aziz, F.; Köhler, G.; Pieber, T.R.; et al. Patients with healed diabetic foot ulcer represent a cohort at highest risk for future fatal events. Sci. Rep. 2019, 9, 10325.
- Hasan, R.; Firwana, B.; Elraiyah, T.; Domecq, J.P.; Prutsky, G.; Nabhan, M.; Prokop, L.J.; Henke, P.; Tsapas, A.; Montori, V.M.; et al. A systematic review and meta-analysis of glycemic control for the prevention of diabetic foot syndrome. J. Vasc. Surg. 2016, 63, 22S–28S.
- Colagiuri, S.; Dickinson, S.; Girgis, S.; Colagiuri, R. National Evidence Based Guideline for BloodGlucose Control in Type 2 Diabetes. Diabetes Aust. NHMRC 2009, volume, page range.
- Klonoff, D.C.; Ahn, D.; Drincic, A. Continuous glucose monitoring: A review of the technology and clinical use. Diabetes Res. Clin. Pract. 2017, 133, 178–192.
- Brown, S.A.; Kovatchev, B.P.; Raghinaru, D.; Lum, J.W.; Buckingham, B.A.; Kudva, Y.C.; Laffel, L.M.; Levy, C.J.; Pinsker, J.E.; Wadwa, R.P.; et al. Six-Month Randomized, Multicenter Trial of Closed-Loop Control in Type 1 Diabetes. NEJM 2019, 381, 1707–1717.
- Janapala, R.N.; Jayaraj, J.S.; Fathima, N.; Kashif, T.; Usman, N.; Dasari, A.; Jahan, N.; Sachmechi, I. Continuous Glucose Monitoring Versus Self-monitoring of Blood Glucose in Type 2 Diabetes Mellitus: A Systematic Review with Meta-analysis. Cureus 2019, 11, e5634.
- Tweden, K.S.; Deiss, D.; Rastogi, R.; Addaguduru, S.; Kaufman, F. Longitudinal Analysis of Real-World Performance of an Implantable Continuous Glucose Sensor over Multiple Sensor Insertion and Removal Cycles. Diabetes Technol. Ther. 2019, In press.
- Soupal, J.; Petruželková, L.; Grunberger, G.; Hásková, A.; Flekač, M.; Matoulek, M.; Mikeš, O.; Pelcl, T.; Horová, E.; Škrha, J.; et al. Glycemic Outcomes in Adults With T1D Are Impacted More by Continuous Glucose Monitoring Than by Insulin Delivery Method: 3 Years of Follow-Up From the COMISAIR Study. Diabetes Care 2019, 43, 37–43.
- Fonseca, V.; Grunberger, G.; Anhalt, H.; Bailey, T.S.; Blevins, T.; Garg, S.K.; Handelsman, Y.; Hirsch, I.B.; Orzeck, E.A.; Roberts, V.L.; et al. Continuous glucose monitoring: a consensus conference of the american association of clinical endocrinologists and american college of endocrinology. Endocr. Pract. 2016, 22,
- 1008–1021.
- Östergren, J.; Sleight, P.; Dagenais, G.; Danisa, K.; Bosch, J.; Qilong, Y.; Yusuf, S. Impact of ramipril in patients with evidence of clinical or subclinical peripheral arterial disease. Eur. Heart J. 2004, 25, 17–24.
- Manapurathe, D.T.; Moxon, J.V.; Krishna, S.M.; Rowbotham, S.; Quigley, F.; Jenkins, J.; Bourke, M.; Bourke, B.; Jones, R.E.; Golledge, J. Cohort Study Examining the Association Between Blood Pressure and Cardiovascular Events in Patients With Peripheral Artery Disease. J. Am. Heart Assoc. 2019, 8, e010748。
- Islam, S.M.S.; Cartledge, S.; Karmakar, C.; Rawstorn, J.C.; Fraser, S.F.; Chow, C.; Maddison, R. Validation and Acceptability of a Cuffless Wrist-Worn Wearable Blood Pressure Monitoring Device Among Users and Health Care Professionals: Mixed Methods Study. JMIR mHealth uHealth 2019, 7, e14706.
- Pellaton, C.; Vybornova, A.; Fallet, S.; Marques, L.; Grossenbacher, O.; De Marco, B.; Chapuis, V.; Bertschi, M.; Alpert, B.S.; Solà, J. Accuracy testing of a new optical device for noninvasive estimation of systolic and diastolic blood pressure compared to intra-arterial measurements. Blood Press. Monit. 2020, In press.
- Krisai, P.; Vischer, A.S.; Kilian, L.; Meienberg, A.; Mayr, M.; Burkard, T. Accuracy of 24-hour ambulatory blood pressure monitoring by a novel cuffless device in clinical practice. Hear. 2018, 105, 399–405.
- Chunta, S.; Suedee, R.; Lieberzeit, P.A. Low-Density Lipoprotein Sensor Based on Molecularly Imprinted Polymer. Anal. Chem. 2015, 88, 1419–1425.
- Delamater, A.M. Improving Patient Adherence. Clin. Diabetes 2006, 24, 71.
- Winkler, A.; Teuscher, A.U.; Mueller, B.; Diem, P. Monotoring adherence to prescribed medication in type 2 diabetic patients treated with sulfonylureas. Swiss. Med. Wkly. 2002, 132, 379–385.
- Paes, A.H.P.; Bakker, A.; Soe-Agnie, C.J. Impact of dosage frequency on patient compliance. Diabetes Care 1997, 20, 1512.
- Frias, J.P.; Virdi, N.; Raja, P.; Kim, Y.; Savage, G.; Osterberg, L.; Exeter, C.; Da Silva, E.; Naik, A. Effectiveness of Digital Medicines to Improve Clinical Outcomes in Patients with Uncontrolled Hypertension and Type 2 Diabetes: Prospective, Open-Label, Cluster-Randomized Pilot Clinical Trial. J. Med. Internet Res. 2017, 19, e246.
- Ploderer, B.; Brown, R.; Seng, L.; Lazzarini, P.A.; Van Netten, J.; He, Q.; Strong, D.; Margolis, D.; Mamillapalli, C. Promoting Self-Care of Diabetic Foot Ulcers Through a Mobile Phone App: User-Centered Design and Evaluation. JMIR Diabetes 2018, 3, e10105.
- Boodoo, C.; Perry, J.A.; Hunter, P.J.; Duta, D.I.; Newhook, S.C.; Leung, G.; Cross, K.; Fatehi, F.; Yderstraede, K. Views of Patients on Using mHealth to Monitor and Prevent Diabetic Foot Ulcers: Qualitative Study. JMIR Diabetes 2017, 2, e22.
- Basatneh, R.; Najafi, B.; Armstrong, D.G. Health Sensors, Smart Home Devices, and the Internet of Medical Things: An Opportunity for Dramatic Improvement in Care for the Lower Extremity Complications of Diabetes. J. Diabetes Sci. Technol. 2018, 12, 577–586.
- van Netten, J.; Clark, D.; Lazzarini, P.A.; Janda, M.; Reed, L.F. The validity and reliability of remote diabetic foot ulcer assessment using mobile phone images. Sci. Rep. 2017, 7, 9480.
- Perednia, D.A.; Allen, A. Telemedicine technology and clinical applications. JAMA 1995, 273, 483–488.
- Hazenberg, C.E.V.B.; Aan de Stegge, W.B.; Van Baal, S.G.; Moll, F.L.; Bus, S.A. Telehealth and telemedicine applications for the diabetic foot: A systematic review. Diabetes/Metab. Res. Rev. 2020, 36, e3247–e3247.
- Singh, T.P.; Vangaveti, V.N.; Kennedy, R.L.; Malabu, U.H. Role of telehealth in diabetic foot ulcer management—A systematic review. Aust. J. Rural Health 2016, 24, 224–229.
- Tchero, H.; Noubou, L.; Becsangele, B.; Mukisi-Mukaza, M.; Retali, G.-R.; Rusch, E. Telemedicine in Diabetic Foot Care: A Systematic Literature Review of Interventions and Meta-analysis of Controlled Trials. Int. J. Low. Extrem. Wounds 2017, 16, 274–283.
- Rasmussen, B.S.B.; Froekjaer, J.; Bjerregaard, M.R.; Lauritsen, J.M.; Hangaard, J.; Halekoh, U.; Henriksen, C.W.; Yderstraede, K.B. A Randomized Controlled Trial Comparing Telemedical and Standard Outpatient Monitoring of Diabetic Foot Ulcers. Diabetes Care 2015, 38, 1723–1729.
- Smith-Strom, H.; Igland, J.; Ostbye, T.; Tell, G.S.; Hausken, M.F.; Graue, M.; Skeie, S.; Cooper, J.G.; Iversen, M.M. The Effect of Telemedicine Follow-up Care on Diabetes-Related Foot Ulcers: A Cluster-Randomized Controlled Noninferiority Trial. Diabetes Care 2018, 41, 96–103.