The traditional polymer circular economy (CE) continues to be challenging due to its reprocessing/recycle ability; also, at the same time, newly developed substitute materials have not expressed similar performance to conventional materials involved in contemporary applications. Hence, linear approaches such as “take-make-use-waste” have severely affected sustainability modules where non-renewable resources have been used at maximum levels. In addition, sustainability is termed along with the circular economy paradigm in recent times, although material sustainability differs from CE material. The circular economy mainly focuses on the economic, environmental and social impacts, whereas sustainability is more about an ecological importance. Globally, frameworks have been formed to enhance the sustainable environment. The United Nations (UN) has designed 17 sustainable development goals to be enforced in all countries in order to reach the goal of a sustainable society by 2030.
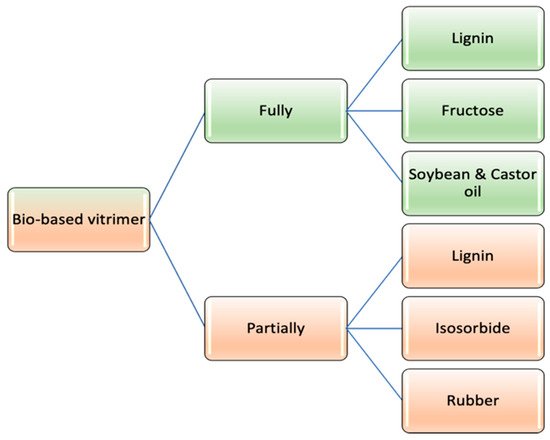
1. Classification of Bio-Vitrimers
Based on the involvement of renewable sources, vitrimeric materials can be classified as labelled in
Figure 13 [1][55], where fully bio-based vitrimers are synthesized using only bio-derived chemicals, whereas in the case of partial bio-vitrimers, the network formation can be achieved using petroleum based molecules along with bio-derived chemicals.
Figure 13.
Bio-based vitrimer categorization.
1.1. Fully Bio-Based Vitrimer
1.1.1. Lignin Derivatives
Lignin based materials have covered a large section of bio-vitrimeric materials, where in a very first report zinc catalyzed fully bio-based vitrimers were developed using epoxy monomers derived from sebacic acid, cured with lignin molecules decorated with ozone functional groups, and covalent network exchanges were achieved through transesterification reaction exchanges
[2][80]. An increment of lignin content is useful to enrich hydroxyl/ester groups in crosslink network, helpful to improve the mechanical and thermal properties. The generated epoxy vitrimer exhibits a prevailed shape memory and self-healing behaviors at 190 °C (5 min; healing efficiency = 70%), and at 80 °C (recovery ratio = 87–97%), respectively, and hence demonstrated fast stress relaxation at above 160 °C through transesterification reaction. Furthermore, recovering adhesive behavior was investigated through lap shear test, where aluminum sheets were joined together. The epoxy vitrimer adhesive joint exhibited sufficient strength (6.5 MPa) comparable to commercially available EPO glues (8 MPa), where after breaking, the separated sheets were joined together at 190 °C. The repaired material (recovered via transesterification exchange) shows 5 MPa shear strength
[2][80]. In addition, zinc acetylacetonate (Zn (acac)
2) prompted poly (ethylene glycol) diglycidyl ether (PEG-epoxy) were treated with lignin derived polycarboxylic acid (L-COOH).
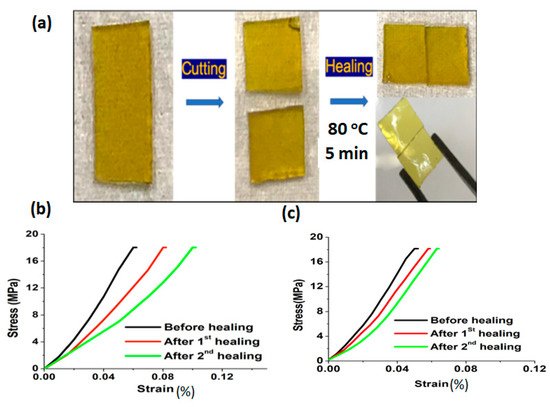
Rana et al. has reported a sustainable vitrimer, prepared by incorporating biomass-derived activated carbon (AC) filler into the epoxy matrix
[3][81]. The epoxy vitrimer has been prepared in single step reaction with bisphenol A diglycidyl ether (BADGE) and 2- aminophenyl disulfide (2-AFD) at 80 °C for 15 min and then added a different percentage of activated carbon dispersed solution into that. Subsequently, the mixture was poured into the mould and cured at 150 °C for 5 h. After that, the disulfide exchanges promoted temperature-dependent self-healing observed at 80 °C for 5 min in pristine epoxy vitrimer, and the material had demonstrated a lower temperature self-healing at 70 °C for 5 min upon the addition of activated carbon. Healing efficiency evaluated via flexural studies highlighted a prominent recovery in vitrimer biocomposites with 1 wt% of AC, where 85% and 70% efficiency was exhibited after two consecutive healings
(Figure 4). The obtained vitrimers were used as a coating material, displaying thermal self-healing via a transesterification process. The performed vitrimer was optimized with different percentages of PEG-epoxy/L-COOH (1:1, 1:1.5, 1:2)
[4][82]. The prepared sample relaxation was analyzed through stress relaxation studies, where 1:1.5 composition exhibited slower relaxation than the 1:1 and 1:2 samples. Thus, higher hydroxyl and carboxyl content contained epoxy vitrimer 1:1 and 1:2 samples were helpful to achieve a self-healing efficiency 90% and 100%, respectively, at 200 °C for 30 min. At the same time, epoxy 1:1.5 had resulted 60% healing efficiency at same conditions, with an extrapolated activation energy (Ea) of the epoxy vitrimer 1:1 of 54.42 kJ/mol.
Figure 4. (a) Self-healing of the epoxy vitrimer (i) pristine EP-p, (ii) cut into two pieces, and (iii) rejoined. Healing efficiency of vitrimer was calculated via the stress-strain relationship for (b) EP-p and (c) EP-1. Reproduced with permission [81].
To demonstrate removability and repairability, the developed vitrimer polymer was coated onto tin plates, where the coating demonstrates almost 90% self-healing efficiency. To avoid the presence of toxic catalyst, a catalyst free mechanism has been developed
[5][83]. In addition, lignin-based disulfide promoted catalyst free (vanillin-derived dialdehyde and amine monomers involved) bio-based vitrimer was demonstrated with prominent reprocessability and self-healing ability. The performed catalyst free vanillin vitrimer was optimized with different hardener molar ratios of tris (2-aminoethyl) amine and (4,4′-disulfanediyldianiline)
[6][84].
1.1.2. Fructose Derivatives
Fructose derived furan dialdehyde based vitrimers were developed when crosslinked with furan dialdehyde via imine exchange reactions, where vitrimeric materials demonstrate a fast stress relaxation (at r.t.) owing to imine reversible bonds (dynamic exchange). The activation energy (E
a = 64 KJ/mol) of the vitrimer network follows Arrhenius behaviour exhibited an energy lower than the reported polyimine dynamic
[7][8][9][10][11][12][13][85,86,87,88,89,90,91] and vitrimer
[14][41] networks activation energies, whereas T
g (−10 °C) of bio-based polyimine vitrimer was higher than the T
v (−60 °C). Reprocessing was demonstrated three times (at 120 °C for 10 min) without any significant loss of the mechanical properties
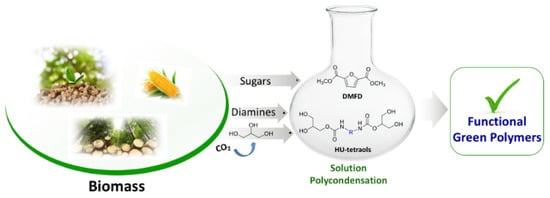
[15][50]. Subsequently, sugar derived dimethyl-2,5-furan dicarboxylate (DMFD) include polyester-hydroxy urethanes was prepared without isocyanate. Transcarbomylation reactions based covalent dynamic exchange was observed for the developed non-isocyanate polyester-urethane (NIPHEU) vitrimer
[16](Figure 5) [92].
Figure 5. A green strategy for the synthesis of nonisocyanate polyester-urethanes. Reproduced with permission [92].
The reported polymer exhibits good thermal stability including melting temperature (93 and 110 °C), as well as the onset degradation temperatures ranging from 170 to 220 °C, prompted by thermally-induced bond exchanges via transcarbamoylation mechanism.
1.1.3. Soybean and Castor Oil
Owing to their abundant availability and better biocompatibility, plants oil derived polymers have often been discussed, with epoxidized soybean oil, a soybean oil derived commercially available at low cost. The design of a plant oil derived vitrimer with high glass transition temperature and mechanical strength represents a significant challenge. Liu and coworkers demonstrated a fully bio-based epoxy vitrimer, where the performed epoxy vitrimer was prepared from ESO and rosin derived Fumaropimaric acid (FPA) with zinc acetylacetonate catalyst, demonstrating exchange via transesterification reactions. The materials properties were optimized based on the involvement of FPA in network (ESO-FPA1.0, ESO-FPA0.8 and ESO-FPA0.6), with the ESO-FPA1.0 cured network exhibiting the higher Tg (65 °C). FPA derived ESO-FPA1.0 in vitrimer network attained a higher tensile strength (16.62 MPa) than the conventional citric acid cured ESO (0.6 MPa) networks. This might be due to the presence of a rigid hydrogenated phenanthrene ring and more reactive groups of FPA, resulted in higher crosslinking density. Shape memory behavior was described at 80 °C (which was above T
g) for 30 min and deformation of the shape was analyzed at 160 °C for 30 min, resulting in the shape fixity ratio (R
f) and shape recovery ratio (R
r) of 98% and 89%, respectively. The performed vitrimer material was degraded with ethanol at 120 °C for 2 h, where ethanol hydroxyl groups were reacted with the ESO-FPA ester groups, and curing happened without the involvement of a catalyst. However, recycled vitrimer exhibits a reduction in T
g (65 °C to 30 °C) and it reproduces only 88% (16 MPa to 10.5 MPa) of its initial mechanical strength
[17][93]. In addition, a catalyst free vegetable oil derived (epoxidized soybean oil (ESO) cured with 4,4′-dithiodiphenylamine (APD)) epoxy vitrimer network was demonstrated. Varying percentage of crosslinked networks were investigated including the different curing time at 180 °C. However, the ESOV-28 (28 h cured) specimen demonstrates prominent tensile strength owing to its high crosslink density. All the performed samples displayed a similar thermal stability (without constrain of gel fraction); hence, described catalyst-free bio vitrimer material promoted their exchanges via disulfide exchanges and reprocessed efficiently at 180 °C for 10 min under 20 MPa pressure
[18][94].
1.2. Partially Bio-Based Vitrimers
1.2.1. Lignin Based Derivatives
Lignin-derived eugenol-based epoxy vitrimers have been prepared by Zhang and coworkers, where transesterification reaction promoted covalent adaptive network exchanges were achieved. The synthesized eugenol epoxy was further reacted with succinic anhydride (SA) in the presence of zinc catalyst
[19][95]. The performed eugenol derived epoxy vitrimer demonstrates self-healing at 190 °C, and shape memory and fast stress relaxation at 80 °C and 200 °C, respectively. Hence recycling of the material was achieved through physical and chemical principles, where the epoxy vitrimers were pocessed at 160 °C for 1h in hot press, and further the chemical degradation/decomposition of the polymer took place at 160 °C in the presence of ethanol
(Figure 6). In another study, lignin derived vanillin based di-aldehyde monomer was treated with conventional diamine to obtain the corresponding bio-based vitrimers, where imine covalent exchange enables the physical reprocessing and chemical recycling. The dynamic imine metathesis reaction promotes self-healing at 180 °C for 1h. Cut sample shows optimal mechanical recovery after healing at 150 °C for 1 h (healing efficiency = 74.5%). At the same temperature, physical reprocessing was performed for 10 min, and the efficiency of reprocessed vitrimer was evaluated obtaining a 71.2% tensile strength and 72.8% elongation at break after three hot-pressing cycles. The chemical recycling was observed under an acidic environment at 50 °C, and the obtained aldehyde monomer can be reused again for the preparation of vitrimers
[20][96].
Figure 6. Eugenol-derived biobased epoxy. Reproduced with permission [95].
1.2.2. Isosorbide Derivative
Isosorbide derived monomers were reacted with aromatic diamines (4,4′-disulfanediyldianiline (MDS)) to obtain a ADCAN induced vitrimer material, where covalent network exchange takes place through a disulfide metathesis reaction. The resulting material demonstrate excellent thermomechanical properties in comparison to the conventional epoxy cured using 4,4′-methylenedianiline (MDA). The material exhibited a prominent reprocessing/self-healing at 100 °C for 60 min and shape memory at 80 °C for 1 min. The materials degrade in 5 wt% NaOH aqueous solution, owing to the presence of isosorbide
[21][72].
2. Bio-Based Vitrimer Composites
The addition of carbon based nanofillers are helpful to obtain the thermal and photo induced transesterification based covalent exchanges in the bio-based epoxy networks. The addition of nanofillers is also helpful to rapid up the shape recovery, where NIR induced shape memory was observed even after fifth cycle (Recovery ratio = 100%), as well as a reduction in recovery time was observed due to light energy absorbed capacity of CNTs (which get convert into the thermal energy; E
a = 40.73 to 54.91 kJ/mol)
[22][97]. The addition of nanofiller is not only helpful to improve the mechanical and thermal properties, it is also helpful to improve the adhesive fracture energy, as well as stress relaxation. The transcarbonation exchange based materials demonstrate prominent mechanical properties, as well as exhibiting reprocessing, self-healing and shape memory properties (
Table 1), where tensile strength can be controlled based on the ratio between 1,3-Propanediol (PD) and bis (6-membered cyclic carbonate) (BCC). This change in tensile strength was observed due to their crosslink density, where PD soft segments were included in less; hence, the paper cellulose fiber network made the hydrogen bond interaction with covalently crosslinked vitrimer network helpful to enhance the mechanical properties. The reported material exhibits self-healing and shape memory at 150 °C, whereas, separately cut samples were reconnected together at 170 °C at 4 MPa, resulted a 80% healing efficiency
[23][98].
Table 1. Different bio-based vitrimers.
| Bio-Based Derivatives |
Material |
Recycling/
Reprocessing |
Self-Healing |
Shape Memory |
Ref. |
| Lignin based |
Epoxy derived from eugenol with succinic anhydride |
190 °C for 1 h,
Low efficiency | a,# |
190 °C for 1 h,
90% | b |
80 °C for less than minute, 100% | c |
[19] | [95] |
| Dialdehyde monomer derived from vanillin with conventional diamine |
150 °C for 10 min,
71.2% | a, | * |
150 °C for 1 h,
74.5% | b |
- |
[20] | [96] |
| Dialdehyde derived from vanillin and amine monomers |
60 °C for 20 min,
Maximum efficiency | a,# |
Addition of ethylene diamine |
- |
[6] | [84] |
| Epoxy derived from sebacic acid and ozone crafted lignin |
- |
190 °C for 5 min,
70% | b |
80 °C for less than minute, 87–97% | c |
[2] | [80] |
| Fructose |
Furan dialdehyde and fatty acid-based diamine/ triamine |
120 °C for 10 min, Nearly 100% | a, | * |
- |
- |
[15] | [50] |
| Soybean & Castor oil |
Fumaropim-aric acid (FPA) derived from Rosin and epoxidized soybean oil (ESO) |
120 °C for 2 h, 88% | a, | * |
180 °C for 60 min,
Nearly 100% |
80 °C for 30 min, 89% |
[17] | [93] |
| 4,4′-dithiodiphenylamine (APD) cured Epoxidized soybean oil (ESO) |
180 °C for 10 min under 20 MPa,
80% | a,~ |
- |
- |
[18] | [94] |
| Vinylogus urethane vitrimer derived from aminate DL-limonene (AL) |
160 °C, 6 MPa for 30 min |
- |
70 °C for 1 min, 100% |
[24] | [99] |
| Isosorbide |
Isosorbide derived epoxy and aromatic diamines |
100 °C for 1 h,
82.6% | a, | * |
100 °C for 1 h,
100% | b |
80 °C for 1 min 100% |
[21] | [72] |
| Natural rubber |
Dodecanedioic acids (DAs) and aniline trimer (ACAT) derived epoxidized natural rubber |
200 °C for 20 min, 88% | a,# |
NIR and 200 °C for 30 min, 80% | b |
NIR and 80 °C for less than minute, 95% | c |
[25] | [100] |
| Composite |
Cellulose paper from 1,3-Propanediol (PD) and bis (6-membered cyclic carbonate) (BCC) and |
HCl at 90 °C for 36 h |
160 °C for 10 s, 75% | b, | * | ,^ | and 170 °C for 2 h, 4 MPa, 80% | b, | * | ,$ |
150 °C for less than minute | c |
[23] | [98] |
| Carbon nano tubes (MWNT) with epoxy/cashew nutshell liquid |
- |
- |
NIR and 60 °C for less than minute, 100% | c |
[22] | [97] |
[a]-recovery efficiency. [b]-healing efficiency. [c]-shape recovery ratio. [*]-tensile strength. [#]-stress-strain. [^]-scratch. [$]-cut and overlapped. [~]-elongation at break.