The enteric nervous system (ENS) is a part of the autonomic nervous system that intrinsically innervates the gastrointestinal (GI) tract. Whereas enteric neurons have been deeply studied, the enteric glial cells (EGCs) have received less attention. However, these are immune-competent cells that contribute to the maintenance of the GI tract homeostasis through supporting epithelial integrity, providing neuroprotection, and influencing the GI motor function and sensation. The endogenous cannabinoid system (ECS) includes endogenous classical cannabinoids (anandamide, 2-arachidonoylglycerol), cannabinoid-like ligands (oleoylethanolamide (OEA) and palmitoylethanolamide (PEA)), enzymes involved in their metabolism (FAAH, MAGL, COX-2) and classical (CB1 and CB2) and non-classical (TRPV1, GPR55, PPAR) receptors. The ECS participates in many processes crucial for the proper functioning of the GI tract, in which the EGCs are involved.
1. Introduction
The digestive system is the primary site of energy and nutrient absorption and plays a key role in metabolic homeostasis, i.e., “the capacity of organisms to maintain stable conditions on its composition and properties by compensating changes in their internal environment through the regulated exchange of matter and energy”.
[1]. Within the gut wall lies the largest endocrine and immune system of the body, as well as the enteric nervous system (ENS)
[2]. The gastrointestinal (GI) tract is connected with the central nervous system (CNS), through the extrinsic innervation of the autonomic nervous system (ANS) and stress hormones. Thus, the existence of an important brain-gut axis has been recognized
[3].
Whereas the neurons in the ENS have been widely studied throughout time, the enteric glial cells (EGCs) have received less attention
[4][5][6][4,5,6]. Numerous GI conditions have been found to be associated with alterations in the numbers and functions of these cells
[4][7][8][9][4,7,8,9].
The term nutraceutical was first defined in 1989. This term is a combination of the words “nutrition” and “pharmaceutical” and refers to “food components or active ingredients present in food that have positive effects for well-being and health, including the prevention and treatment of diseases”
[10]. The endogenous cannabinoid system (ECS) is a well-recognized modulator of the GI tract
[11][12][13][14][15][16][11,12,13,14,15,16]. The components of the ECS are found in many cell types within the GI tract, including the ENS. Not surprisingly, exogenously administered cannabinoids have profound effects that may be beneficial for the treatment of some GI conditions
[14][17][18][19][14,17,18,19], and adverse GI effects of their use have also been recognized (i.e., cannabinoid hyperemesis
[20][21][20,21] and small bowel intussusception,
[22]).
2. The Enteric Nervous System
The ENS constitutes a complex network of neurons and accompanying glial cells that control the major functions of the GI tract
[23]. In detail, the ENS is composed of intrinsic sensory neurons (intrinsic primary afferent neurons, IPANs), excitatory and inhibitory interneurons, and motor neurons. The complexity of the ENS contributes to the independency of its action: sensory neurons receive external inputs, then interneurons integrate the signals, and together with motor neurons generate outputs. Moreover, ENS may receive and process the signals from the CNS
[24].
Within the ENS, neuronal and glial cells are organized in myenteric and submucosal plexuses. The first one is located between the two layers of smooth muscle (circular and longitudinal muscle layer) and is involved in the coordination of GI motility, while neurons of the submucosal plexus (located between the mucosa and the muscle layers) participate in secretion and absorption of water and electrolytes
[2].
2.1. Enteric Neurons
IPANs possess mechano- or chemosensory activity, and besides the straight signal reception, they are able to receive and process the message of the intensity, duration, and pattern of stimuli. These neurons usually form a circumferential internetwork encircling the intestine. Within the group of IPANs, several classes may be listed, for example, according to their localization (myenteric/submucosal plexus) or the direction of signal transduction. Therefore, IPANs can receive, integrate and reinforce signals both locally and across the network (alike interneurons)
[25][26][25,26].
Interneurons, like IPANs, may be divided into ascending or descending. Furthermore, within the population of interneurons there are several classes that may be distinguished neurochemically and the proportion of interneurons in these classes may differ between the parts of the GI tract, that may reflect the regional diversity in the motor patterns in the intestines
[4][27][28][4,27,28].
The last group of neurons in the ENS are motor neurons, which are divided into two subgroups: inhibitory and excitatory. They participate in the control of intestinal motility as they contribute to the contractions and relaxations of the circular and longitudinal smooth muscles in a mechanism dependent on acetylcholine (ACh) (excitatory neurons), or nitric oxide (NO), vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating polypeptide (PACAP) (inhibitory neurons)
[2].
2.2. Enteric Glial Cells
Glial cells located in the GI tract are also known as enteric glial cells (EGCs). At first, they were simply considered as structural support for the ENS. It is now well recognized that they participate in several processes crucial for the GI tract
[29][30][29,30].
Hanani et al.
[31] classified EGCs into 4 subgroups based on their morphology. Type I EGCs, named “protoplasmic”, are star-shaped cells with short, irregularly branched processes, resembling protoplasmic astrocytes in the CNS. Type II (fibrous) EGCs are elongated glia with interganglionic fiber tracts. Type III (mucosal) EGCs possess long-branched processes. Finally, type IV (intermuscular) EGCs are the elongated glia accompanying the nerve fibers and encircling the smooth muscles.
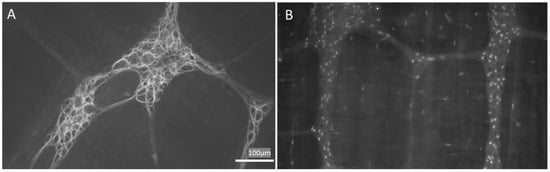
EGCs may also be subgrouped according to the molecular or functional differences due to the heterogeneities in receptors and channels expressed on their surface. In particular, several proteins are often used to identify EGCs, i.e., calcium-binding protein S100
[9][32][9,32], glial fibrillary acidic protein (GFAP)
[9][33][9,33] and the transcription factors: SOX-8, SOX-9, SOX-10
[34] (
Figure 1). Interestingly, Hanani et al.
[35] and others
[36], showed that EGCs are interconnected and electrically coupled by gap junctions and form an extensive functional glial network
[37].
Figure 1. Appearance of enteric glial cells (EGCs). (A,B) are images obtained from the myenteric plexus of the rat distal colon; immunoreactivity to GFAP (A) and Sox-10 (B) are characteristic of EGCs. GFAP: glial fibrillary acidic protein. Images obtained by L.L.-G. (NeuGut-URJC).
EGCs play a role in intercellular communication, intestinal barrier formation and support, as well as control of the GI motility, immune response, and visceral sensitivity (
Table 1).
Table 1.
Functions of the enteric glial cells in the gastrointestinal tract.
|
Aspect
|
Function
|
Localization
|
Mediators
|
References
|
|
Epithelial barrier
|
Intestinal barrier
formation and support
Enhancing epithelial healing
Neuropods formation
|
Mucosa
|
proEGF
TGF-β
S-nitrosoglutathione
15d-PGJ2
NGF-β *
Artemin *
|
[38][39][40][41][42][43][44][45][38,39,40,41,42,43,44,45]
|
|
Intestinal motility
|
Control of GI motility #
|
Myenteric plexus
|
ATP
|
[46][47][48][46,47,48]
|
|
Enteric neurotransmission
|
Neuronal communication
|
ENS
|
ATP
NFG
GSH
|
[49]
|
|
Immune response
|
Activation of EGCs
|
ENS
|
MHC II class
IL-1β
IL-6
TGF-β
proEGF
GSH
PGE2
|
[50][51][52][53][54]][62][63][68][69][70][50,51[55][56][57][64],52[,5365,5458][59][60,56],57[,58,5961,60][66][67],55[,61,62,63,64,65,66,67,68,69,70]
|
|
Visceral sensitivity
|
Sensitizing/activating nociceptors
|
ENS
|
ATP
GABA
IL-1β
neurotrophins
|
[8][71][72][8,71,72]
|
* Mediators released by enteroendocrine cells; # EGC loss results in impaired GI motility. Abbreviations: 15d-PGJ2, 15-deoxy-Δ12,14-prostaglandin J2; ATP, adenosine triphosphate; EGC, enteric glial cell; EGF, Epidermal growth factor; ENS, enteric nervous system; GABA, gamma amino butyric acid; GI, gastrointestinal; GSH, glutathione; IL, interleukin; MHC, major histocompatibility complex; NGF, nerve growth factor; PGE2, prostaglandin E2; proEGF, proepidermal growth factor; TGF, Transforming growth factor.
3. The Endocannabinoid System
The term cannabinoid comprises a group of at least 66 biologically active terpenophenols which are found in cannabis (
Cannabis sativa) and their synthetic analogs
[73][127]. Cannabinoids are molecules that act on the endogenous cannabinoid system (ECS), also known as the endocannabinoid system, and are usually divided into three main groups: phytocannabinoids (cannabinoids found in plants), endocannabinoids (endogenous compounds found in animals that modulate cannabinoid receptors); and synthetic cannabinoids (synthetic compounds that may or may not be structurally related that also produce agonistic effects in cannabinoid receptors)
[74][128].
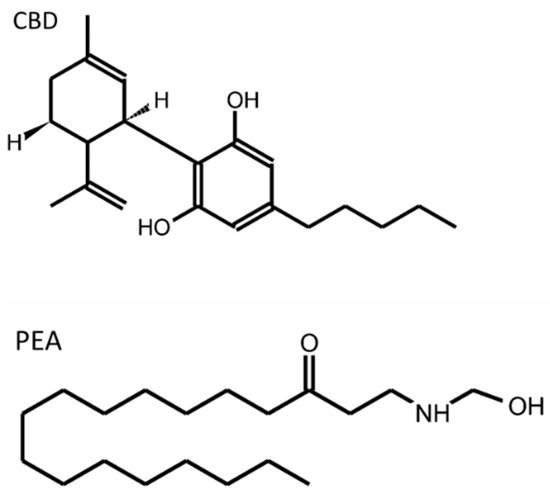
Figure 2 shows the molecular structure of the two cannabinoid compounds that have been more deeply studied in relation to EGCs.
The ECS is composed of cannabinoid receptors (CB1, CB2), their endogenous ligands (endocannabinoids, ECBs), and the enzymes involved in the biosynthesis and degradation of cannabinoids.
Cannabinoid receptors belong to the G-protein coupled receptors (GPCR) family. Their activation results in the inhibition of adenyl cyclase activity and suppression of voltage gated Ca
2+ channels
[75][129]. Noteworthy, CB receptors possess more than one endogenous agonist: anandamide (
N-arachidonoyl ethanolamine, AEA) and 2-arachidonoyl glycerol (2-AG). ECBs are derivatives of the arachidonic acid, synthesized on demand from the membrane phospholipids in the post-synaptic cells in response to increased levels of intracellular Ca
2+, released immediately after synthesis, and then diffuse throughout the cellular membrane without being stored in vesicles. The action of ECBs is mediated through CB1 or CB2 receptors. Noteworthy, ECBs exhibit different selectivity and affinity: AEA is a partial agonist of CB1 with very weak activity at CB2 receptors, while 2-AG is characterized as a potent agonist of both receptors. Besides these compounds, there are other ECBs that remain less known: 2-arachidonyl glyceryl ether (2-AGE, a CB1 selective agonist)
[76][130], N-arachidonoyl dopamine (NADA, a CB1 agonist)
[77][131], and O-arachidonoyl ethanolamine (O-AEA or virodhamine, a partial CB1 agonist and full CB2 agonist)
[78][132].
Interestingly, it was demonstrated that ECBs may interact with other receptors. For example, AEA binds to TRPV1
[79][80][133,134]. The effects of TRPV1 activation depend on the site of action: when AEA interacts with pre-synaptic TRPV1 it promotes glutamate release, while the activation of post-synaptic TRPV1 by AEA leads to the reduction of glutamate signaling, inhibition of 2-AG biosynthesis and the blockage of the retrograde action at CB1 receptors. The multi-target action of ECBs may be related to the co-expression of CB receptors and TRPV1 channels in neuronal and non-neuronal cells. It was assessed that TRPV1 are co-localized with CB1 or CB2 receptors in the primary sensory neurons of the DRG in rats
[81][82][83][135,136,137], perivascular neurons
[84][138], vagus nerve
[85][139], and in the axons of neurons in the CNS
[86][87][88][140,141,142]. Moreover, CB receptors are co-expressed with TRPV1 in the endothelial cells of the brain microvessels (both CB1, CB2)
[89][143], in the endothelial cells from the rodent mesenteric arteries with cirrhosis (CB1)
[90][144], dendritic cells
[91][145], muscle cells (in both rodents and humans),
[92][146], osteoclasts
[93][147], keratinocytes
[94][148], and melanocytes
[95][149]. G protein-coupled receptor 55 (GPR55) is an orphan receptor that constitutes another, non-classical target, for ECBs. Lysophosphatidylinositol (LPI) was identified as the endogenous ligand for GPR55
[96][150]. However, AEA and O-AEA can activate these receptors
[97][98][151,152]. Finally, besides TRPV1 and GPR55, ECBs exhibit binding affinity at peroxisome proliferator-activated receptors (PPAR): AEA, O-AEA, and 2-AGE bind PPARα, 2-AG binds to PPARβ/δ, while AEA, 2-AG and 2-AGE bind to PPARγ in vitro
[98][152].
After the activation of CB receptors, the remaining ECBs are degraded in the process of hydrolysis or oxidization. The first enzyme discovered to be involved in ECBs degradation was named fatty acid amide hydrolase (FAAH). Its most preferred substrate was found to be AEA. A few years later, other enzymes were discovered, and their properties were characterized: monoacylglycerol lipase (MAGL), α, β-hydrolase-6 (ABHD6), and α, β-hydrolase-12 (ABHD12)
[99][100][101][153,154,155]. The process of oxidation involves cyclooxygenase-2 (COX-2) and several lipooxygenases
[101][155].