Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Sajid Latif.
Phytoestrogens are secondary plant metabolites that play a role in plant defense, and when ingested by livestock have numerous functions related to reproduction, metabolism, immunological functions and livestock growth and performance. Phytoestrogens are found across various plant species, with the most biologically active of these, isoflavones and coumestans, abundant in legume species.
- phytoestrogen
- coumestans
- isoflavones
- cattle
- legumes
- reproduction
1. Introduction
As the focus shifts in Australia and also globally to low-input sustainable agriculture practices, the incorporation of pasture legumes in crop and pasture rotations continues to reduce input costs associated with the application of synthetic nitrogen fertilizer [1]. The addition of forage legumes to perennial pastures can increase the overall nutritional quality of a mixed pasture by improving the available digestible nutrients and protein for grazing livestock [2]. Forage legumes may also reduce the occurrence of toxicosis caused by ingestion of grass pastures such as tall fescue or Phalaris via increasing the diversity of pasture species, thereby providing opportunities for competitive grazing selection by livestock [2]. In recent years, major changes in production systems have arisen due to inclusion of pasture legumes in mixed farming systems. However, dominant stands of pasture legumes have created a new set of challenges for agricultural researchers in the form of agronomic, economic, and animal reproductive problems in Australia [3]. In 2017, the lucerne seed industry in Australia had an estimated value of AUD 88 million and represented 80% of the total legume pasture seed market [4]. In contrast, cow and calf production enterprises were valued at an estimated AUD 15.1 billion (2019–2020) [5]. In production enterprises, high fertility is valued as one of the key economic traits to an efficient herd or flock [6]. In Australia, the high economic value of both lucerne as a pasture crop and in cattle production enterprises requires proactive management strategies to mitigate risk linked to the potential loss of fertility associated with the inclusion of pasture legumes as forages for grazing livestock.
As heterocyclic phenolic compounds, phytoestrogens have a similar structure to mammalian estrogen and may exhibit estrogenic or antiestrogenic effects [8][7]. Phytoestrogenic compounds have now been isolated in over 300 plant species [9][8], however, few are ingested by humans and/or livestock [10][9]. Biological activity of various phytoestrogens is highly species-specific [11][10]. Estrogenic activity has typically been associated with ingestion of forage legumes. Phytoestrogens are also considered as ‘natural’ selective estrogen receptor modulators (SERMs) in mammals and can be characterized by the tissue specific action on estrogens receptors (ERs), permitting the ability to selectively inhibit or stimulate estrogen-like action [12][11].
2. Phytoestrogens
2.1. Isoflavones
Isoflavones are currently the most studied group of phytoestrogens [17][12]. They are present as either aglycons or as glycosides [18][13], acting as phytoalexins in plants and endogenous hormones in mammals. As secondary metabolites, isoflavones mediate essential interactions between the plant and microbes, including nitrogen sequestration through legume/rhizobium symbiosis, nodulation and plant defense responses. Multiple isoflavones can be found in the same plant species at the same time [18][13]. Concentration levels of isoflavones have been observed to increase in response to stimuli such as climate (temperature, rainfall, humidity), management (harvest and post-harvest processing) and plant health (soil fertility, pathogen/pest/disease presence) [18][13]. The in planta concentration of isoflavones is dependent on plant part sampled, stage of growth, cultivar, growing season conditions and sample preservation after collection or harvest [18][13]. Isoflavones may promote vasodilation in tissues, reducing the effect of vasoconstriction caused by ergot alkaloids in ruminants [2]. Isoflavones also serve as precursors for other isoflavone phytoalexins for fungistatic, antibacterial, antiviral and antioxidant properties. Clover disease, first identified in Australia in the 1940s, was determined to be induced by high concentrations of isoflavones, in particular, formononetin in clover varieties [18][13]. After the discovery of phytoestrogens in clover, and the resulting reproductive failures in sheep, breeding objectives developed by forage breeders focused on reducing phytoestrogen concentrations in new clover varieties, with a focus on isoflavones. Isoflavones typically occur in plants in the form of glycosides, which can be further metabolized to increasingly complex classes of molecules, including pterocarpans and coumestans [19][14]. Isoflavones and their resulting metabolites account for ~1–5% of the phytoestrogens noted in the blood plasma of cattle and sheep in their unconjugated forms [19][14]. Daidzein (Figure 1a), formononetin (Figure 1b), genistein (Figure 1c) and biochanin A (Figure 1d) have been identified to be the key isoflavones found in legume and other fodder consumed by cattle, sheep and horses [20][15].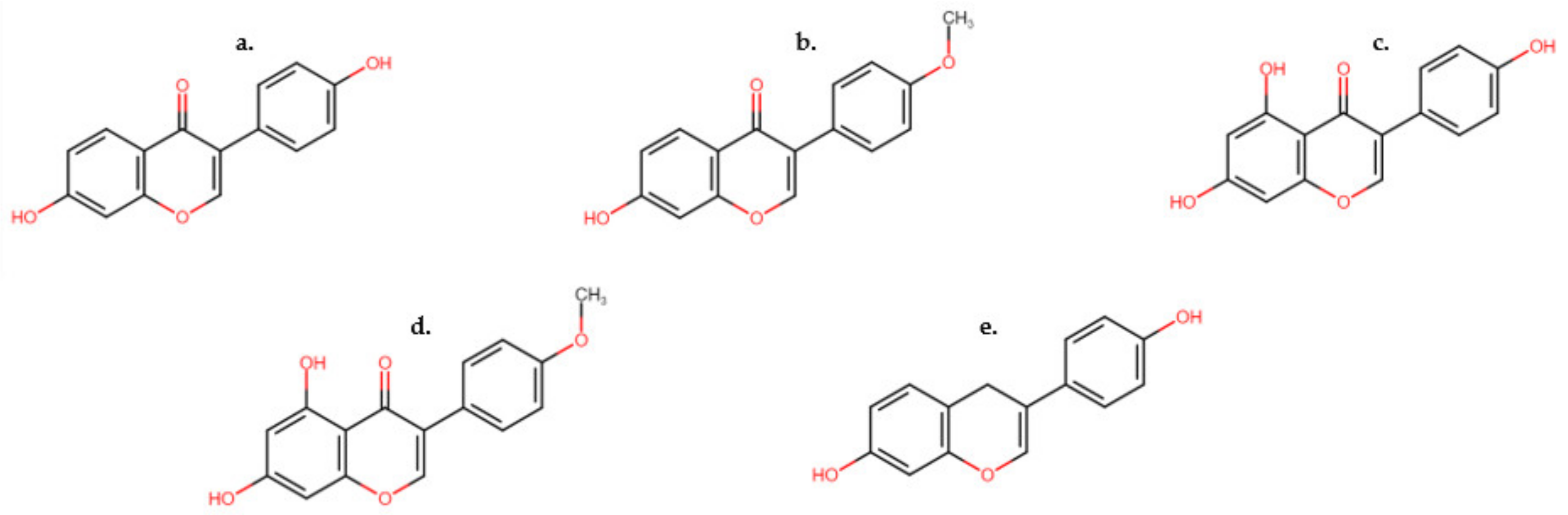
Figure 1.
Structures of common isoflavones found in legume species. (
a
) Daidzein, (
b
) formononetin, (
c
) genistein, (
d
) biochanin A and (
e
) equol, a metabolite of daidzein.
2.2. Coumestans
Up until the early 1950s, lucerne (Medicago sativa) and white clover (Trifolium repens L.) were classed as non-estrogenic legume forages, however, it was later established that both species exhibit frequent and significant estrogenic potency [22][17]. Research by the Western Utilization Research and Development Division (WURDD) in 1955 isolated a new benzofurocoumarin derivative from white clover, which was later recognized as a new class of phytoestrogens; coumestans [23][18]. These compounds were later identified to be biogenetically related to isoflavones. Biotic stimuli such as viral, bacterial and fungal pathogens are known to increase the formation of numerous aromatic compounds, including coumestrol and its metabolites as key defense molecules [24][19]. Although the stimulation of the production of coumestans can be dependent on foliar pathogens, other factors such as the stage of growth, selection of cultivar [25][20] and soil type [26][21] also contribute to coumestan production. In comparison to isoflavones which have a limited impact on estrous cycles, coumestans have been identified to have greater potential for the inhibition of estrous [27][22]. Several coumestans are currently characterized as having estrogenic potency [28][23] and these are commonly synthesized in large quantities in legumes that have been infected by various fungal pathogens [29][24]. The most important of these coumestans is coumestrol (Figure 2a), which has largely been established to be synthesized in legumes [28][23]. Isolated originally from Trifolium repens L. [23][18], it has also been found in low concentrations in other clover species such as in Trifolium pratense [30][25].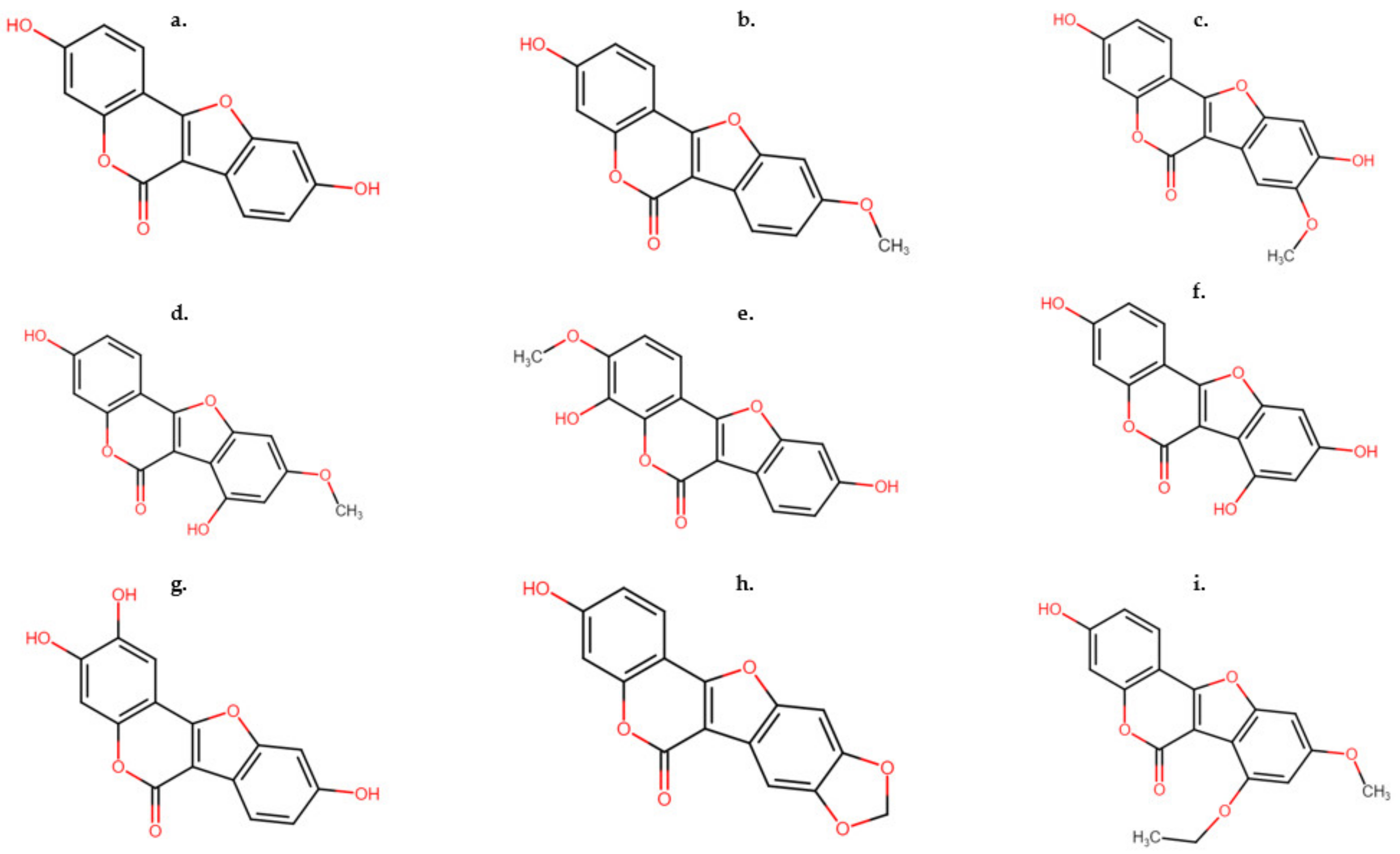
Figure 2. Structures of common coumestans found in legume species. (a) Coumestrol, (b) 4′methoxycoumestrol, (c) 3′methoxycoumestrol, (d) trifoliol, (e) sativol, (f) repensol, (g) lucernol, (h) medicagol and (i) 11,12-dimethoxy-7-hydroxycoumestan.
2.3. Lignans
Lignans, classed as non-flavonoids, are found in numerous vascular plant species [34][26]. There have been several hundred lignans identified, although exact numbers are not likely accurate due to highly complicated analysis required to elucidate the structures of glycoside forms [34][26]. Lignans are bioactive compounds that act as antioxidants and anti-inflammatories, among other biological properties, that are important in plant defense against pests and pathogens [35][27]. They are also essential for plant development and structure. Lignans are predominantly present in plants as free forms, with their glycosides present in the minority of those elucidated molecules [35][27]. Lignans possess great structural diversity, and consist of two phenylpropane units as the molecular backbone of their structure [35][27]. Secoisolariciresinol (Figure 3c) and matairesinol (Figure 3d) have been commonly isolated in cereals, oilseeds, and legumes. Enterodiol (Figure 3a) and enterolactone (Figure 3b) are metabolites of secoisolariciresinol and matairesinol, known as ‘enterolignans’ or ‘mammalian lignans’ [34][26]. These metabolites are formed by the action of gut microflora upon lignans.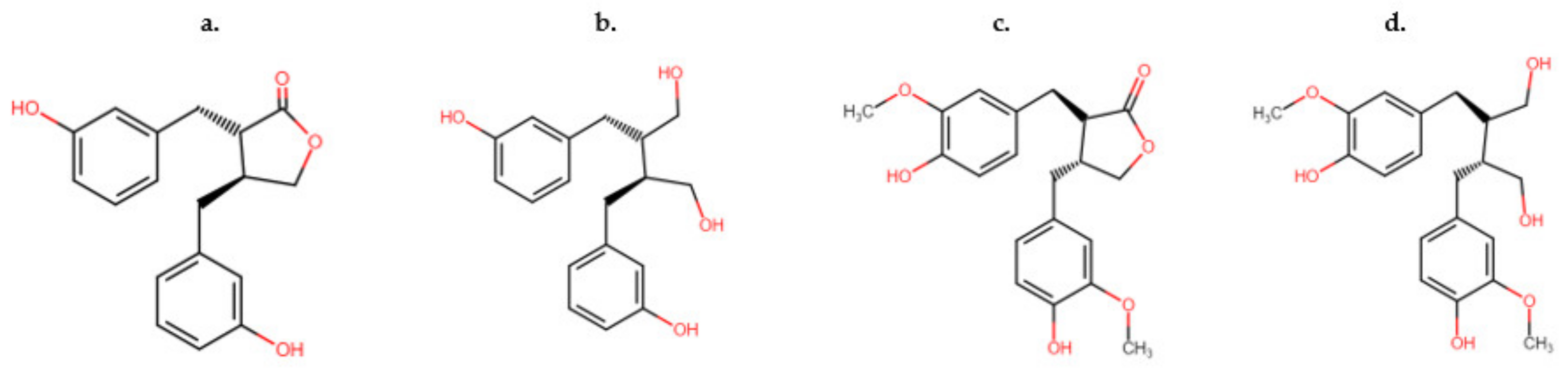
Figure 3. Comparison of the two distinct types of lignans absorbed by ruminants and non-ruminants (c) matairesinol, (d) secoisolariciresinol, and their metabolites (a) enterodiol and (b) enterolactone.
3. Metabolism and Metabolic Effects
Phytoestrogens, which are predominantly present as glycosides in plant material, are not estrogenically active [38][30]. Demethylation, methylation, hydroxylation, chlorination, iodination and nitration of phytoestrogens generally occurs after ingestion by livestock [13][31]. Phytoestrogens and their resultant metabolites are predominantly found in plasma in both conjugated and sulfo-conjugated forms [39][32]. They are converted in vivo to more estrogenically active unconjugated forms via metabolism (Figure 4) [39][32].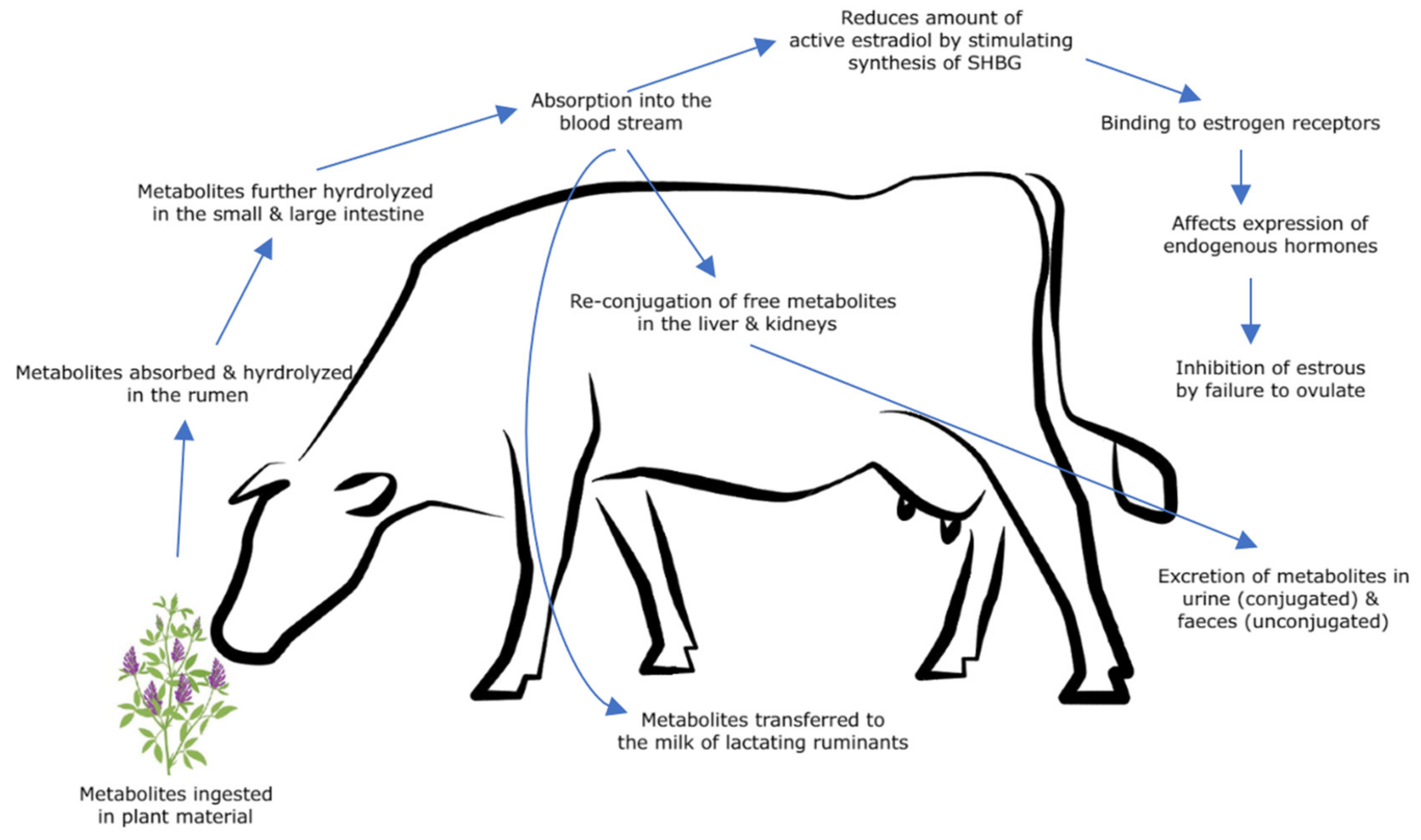
Figure 4.
Metabolism of isoflavones and coumestans in grazing ruminants.
4. Effects on Antioxidant and Immune Capacity
Phytoestrogens, largely the isoflavone class, have been observed to influence antioxidant and immune capacity in cattle [48][40]. Daidzein (an isoflavone) has been observed to significantly increase serum levels of immunoglobulin G (IgG), M (IgM) and A (IgA) [49][41]. Bull calves who received 200 and 400 mg/kg of daidzein were found to have greater immune activity when compared to a control group, suggesting that daidzein has the potential to improve immune function [49][41]. This was not observed to have a significant effect on two-year-old steers at concentrations of 500 and 1000 mg/kg of daidzein [50][42]. However, age, breed of cattle and methods of feed administration should be taken into consideration when comparing antioxidant and immune capacity. Estrogen plays a role in the function of the growth hormone (GH)/insulin growth factor 1 (IGF-1) axis. Exogenous estrogens, such as daidzein, can bind to and activate estrogen receptors, resulting in augmented effects, such as significantly increased serum concentrations of both GH and IGF-1 secretion [49][41]. Daidzein has also been found to have a positive effect upon the hypothalamic–pituitary–adrenal (HPA) axis which aids in the regulation of reproductive functions and nutrition [51][43]. It has also been suggested that daidzein could potentially stimulate the activation and production of lymphocytes and production of other antibody cells [49][41]. Genistein, another isoflavone, has also been shown to significantly inhibit the replication of the bovine herpes virus 1 (BHV-1) [52][44], which results in infectious bovine rhinotracheitis (IBR), a highly contagious respiratory disease that affects both young and old cattle. The inhibition of BHV-1 and other alphaherpesviruses via genistein affects the phosphorylation of protein kinases, such as tyrosine [52][44]. A dose of genistein (25 µM) was observed to reduce viral replication by almost 90%, 18 h after inoculation [52][44], although the reduction was not sustained at 24 h. Studies investigating the potential use of genistein as an anti-viral agent at greater concentrations through orally administered legume feeds in vivo are underway [52][44]. Genistein has also been observed to inhibit the bovine viral diarrhea virus (BVDV) infection, a pestivirus which can result in fatal mucosal disease [53][45], in a dose-dependent manner. The inhibition of BVDV by up to 50% at concentrations between 50 and 100 μg/mL genistein has been observed. Although genistein was a weaker inhibitor during the early stages of infection, inhibition during the later stages of the infection was higher [53][45].5. Effects on Growth and Performance
There are several phytoestrogens that have been identified to have beneficial effects, such as stimulating growth rate and increasing weight gain in livestock. In particular, a high genistein: low formononetin ratio has been observed to increase the liveweight gain in ewes and lambs when fed subterranean clover [54][46]. This was also correlated with a higher uptake of metabolizable protein. The average daily gain (ADG) in the ewes and lambs after the 8 week feeding period was higher by 26.3% and 31.7%, respectively, compared to the control animals consuming Italian ryegrass [54][46]. Carcass weights were also found to be 18.7% higher than that of those in the control group. Other observations included greater internal and total fat percentages, longer and heavier leg bone and earlier maturation of treatment animals [54][46]. This research suggests that certain phytoestrogens such as genistein may be effective anabolic agents, however, this effect may have been masked by the addition of supplementary feeding. This would be useful as an alternative method for promoting growth in feedlot operations. Feedlot operators may utilize growth hormone injections in cattle to improve eating quality, by decreasing the accumulation of fat and increasing the ratio of lean meat in young heifers and steers [55][47]. This in turn increases feed efficiency, allowing greater growth to be achieved on equal to or reduced quantity of feed. This also results in the reduction of costs for both producers and consumers. Isoflavones have been identified to promote growth in grazing and feedlot animals, minimizing the time required to achieve target weights. Of these, zearalenol (a naturally produced fungal metabolite bi-product of zearalenone) is widely used as a growth promoter in the United States [56][48]. Zearalenol and α-Zearalanol have been found to promote protein synthesis, increase the lean meat ratio, and increase weight gain in cattle and sheep similarly to other estrogenic growth hormones in diets [56][48]. α-Zearalanol, a metabolite of zearalanol, however, has been found to be twice as effective as zearalanol, and less toxic [56][48]. The isoflavone daidzein has also been observed to be beneficial in promoting the digestion of dietary proteins and improving the secretion of growth hormones, thus, resulting in the improved physiological condition in bull calves and thereby enhancing production [49][41]. Runyan et al. (2018) observed the potential effect of soybean meal (used as an alternative protein source for bull growth) upon bull fertility at elevated levels in the diet for an extended period. Bull calves achieved a higher average daily gain (ADG) when fed 10% soybean meal diet than those of the same age group on cotton seed meal. The supplementation of daidzein into the diet has also demonstrated the enhancement of fermentation within the rumen of beef cattle [57][49]. Unlike zearalanol, daidzein has been observed to increase the intramuscular fat content and marbling score in steers [50][42]. This is as a result of enhancement of lipid synthesis due to the estrogenic activity of daidzein, which is considered to play an inhibitory role in the β oxidation of fatty acids in the liver, leading to elevated serum levels of free fatty acids [57][49]. However, there has been no consistent evidence to support these findings across all breeds, ages and sex of cattle.6. Effects on Female Reproductive Physiology
Temporary reversible or potentially permanent infertility can result without any evident signs, and may only be detected through measurement of concentrations within the diet or plasma, or observable by clinical effects [58][50]. Exposure to a highly estrogenic diet during critical development periods, such as pre-puberty or gonadal maturity, may result in detrimental impacts upon future reproductive functions and fertility in livestock [59][51]. The effects of various phytoestrogens upon the reproductive tract of females in different species is highly dependent on factors such as age and duration of exposure [42][36]. Additionally, the relative estrogenic potency of an individual phytoestrogen is dependent on the type of assay used to measure activity, route of administration (ingestion versus injection), animal species, duration of exposure and timing of exposure [60][52]. The effect of exposure to phytoestrogens on individual animals varies based on duration of exposure and stage of reproductive development [16][53].7. Effects on Male Reproductive Physiology
Exposure to phytoestrogens during the neonatal period may result in reproductive abnormalities in males such as the downregulation of testicular gene expression [76][54]. Estrogen is known to hold a role in inducing the acrosome reaction in ram spermatozoa, with the majority of estrogen receptors localized in the sperm head, with higher densities found in the post-acrosomal region. Given that estrogenic compounds, including equol, are shown to reach mammalian seminal plasma, and are able to bypass the placenta and blood–brain barrier, it is possible that spermatozoa may encounter equol upon exposure to seminal plasma or in the female reproductive tract [77][55]. Specifically, isoflavones are able to directly alter the function of spermatozoa [77][55]. Low concentrations of isoflavones in vitro have been shown to promote premature capacitation, acrosome loss and inhibition of the acrosomal reaction [77][55].References
- Kulkarni, K.P.; Tayade, R.; Asekova, S.; Song, J.T.; Shannon, J.G.; Lee, J.D. Harnessing the potential of forage legumes, alfalfa, soybean, and cowpea for sustainable agriculture and global food security. Front. Plant Sci. 2018, 9, 1314.
- Melchior, E.A.; Myer, P.R. Fescue toxicosis and its influence on the rumen microbiome: Mitigation of production losses through clover isoflavones. J. Appl. Anim. Res. 2018, 46, 1280–1288.
- Pannell, D.J. Economic aspects of legume management and legume research in dryland farming systems of southern Australia. Agric. Syst. 1995, 49, 217–236.
- Hudson, D. Towards a Sustainable Australian Temperate Pasture Legume Planting Seed Market; The Australian Lucerne Seed Industry, Rural Industries Research and Development Corporation: Canberra, Australia, 2017.
- MLA. Fast Facts-Australia’s Beef Industry. In Market Information; MLA: North Sydney, NSW, Australia, 2020; p. 2.
- Jones, F.M.; Accioly, J.M.; Copping, K.J.; Deland, M.P.B.; Graham, J.F.; Hebart, M.L.; Herd, R.M.; Laurence, M.; Lee, S.J.; Speijers, E.J. Divergent breeding values for fatness or residual feed intake in Angus cattle. 1. Pregnancy rates of heifers differed between fat lines and were affected by weight and fat. Anim. Prod. Sci. 2017, 58, 33–42.
- Karki, K.B.; Mishra, A.K.; Choi, S.J.; Baek, K.H. Effect of Ultraviolet C Irradiation on Isoflavone Concentrations in Different Cultivars of Soybean (Glycine max). Plants 2020, 9, 1043.
- Hughes, C.L., Jr. Phytochemical mimicry of reproductive hormones and modulation of herbivore fertility by phytoestrogens. Environ. Health Perspect. 1988, 78, 171–174.
- Dixon, R.A. Phytoestrogens. Annu. Rev. Plant Biol. 2004, 55, 225–261.
- Patisaul, H.B. Infertility in the Southern White Rhino: Is diet the source of the problem? Endocrinology 2012, 153, 1568–1571.
- Hong, Y.H.; Wang, S.C.; Hsu, C.; Lin, B.F.; Kuo, Y.H.; Huang, C.J. Phytoestrogenic compounds in alfalfa sprout (Medicago sativa) beyond coumestrol. J. Agric. Food Chem. 2011, 59, 131–137.
- Cos, P.; De Bruyne, T.; Apers, S.; Berghe, D.V.; Pieters, L.; Vlietinck, A.J. Phytoestrogens: Recent developments. Planta Med. 2003, 69, 589–599.
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076.
- Madej, A.; Lundh, T. Risk of adverse effects of phytoestrogens in animal feed. In Bioactive Compounds in Plants–Benefits and Risks for Man and Animals; Bernhoft, A., Ed.; The Norwegian Academy of Science and Letters: Oslo, Norway, 2008; pp. 94–103.
- Wocławek-Potocka, I.; Piskula, M.K.; Bah, M.M.; Siemieniuch, M.J.; Korzekwa, A.; Brzezicka, E.; Skarżyński, D.J. Concentrations of isoflavones and their metabolites in the blood of pregnant and non-pregnant heifers fed soy bean. J. Reprod. Dev. 2008, 54, 358–363.
- Shemesh, M.; Shore, L. Effects of environmental estrogens on reproductive parameters in domestic animals. Isr. J. Vet. Med. 2012, 67, 6–10.
- Bickoff, E.M.; Spencer, R.R.; Witt, S.C.; Knuckles, B.E. Studies on the Chemical and Biological Properties of Coumestrol and Related Compounds; Agricultural Research Service, Technical Bulletin No. 1408; United States Department of Agriculture: Washington, DC, USA, 1969; pp. 1–95.
- Bickoff, E.M.; Booth, A.N.; Lyman, R.L.; Livingston, A.L.; Thompson, C.R.; Deeds, F. Coumestrol, a new estrogen isolated from forage crops. Science 1957, 126, 969–970.
- Bickoff, E.M.; Spencer, R.R.; Knuckles, B.E.; Lundin, R.E. 3′-Methoxycoumestrol from alfalfa: Isolation and characterization. J. Agric. Food Chem. 1966, 14, 444–446.
- Seguin, P.; Zheng, W. Phytoestrogen content of alfalfa varieties grown in eastern Canada. J. Sci. Food Agric. 2006, 86, 765–771.
- Francis, C.M.; Millington, A.J. The presence of methylated coumestans in annual Medicago species: Response to a fungal pathogen. Aust. J. Agric. Res. 1971, 22, 75–80.
- Kelly, R.W.; Adams, N.R.; Lindsay, D.R. Effect of coumestans on reproduction in the ewe. Aust. J. Agric. Res. 1976, 27, 253–259.
- Sirtori, C.R.; Arnoldi, A.; Johnson, S.K. Phyto-oestrogens: End of a tale? Ann. Med. 2005, 37, 423–438.
- Dewick, P.M.; Martin, M. Biosynthesis of pterocarpan, isoflavan and coumestan metabolites of Medicago sativa: Chalcone, isoflavone and isoflavanone precursors. Phytochemistry 1979, 18, 597–602.
- Wong, E. Detection and estimation of oestrogenic constituents in red clover. J. Sci. Food Agric. 1962, 13, 304–308.
- Willför, S.M.; Smeds, A.L.; Holmbom, B.R. Chromatographic analysis of lignans. J. Chromatogr. A 2006, 1112, 64–77.
- Saleem, M.; Kim, H.J.; Ali, M.S.; Lee, Y.S. An update on bioactive plant lignans. Nat. Prod. Rep. 2005, 22, 696–716.
- Brito, A.F.; Zang, Y. A review of lignan metabolism, milk enterolactone concentration, and antioxidant status of dairy cows fed flaxseed. Molecules 2018, 24, 41.
- Zhou, W.; Wang, G.; Han, Z.; Yao, W.; Zhu, W. Metabolism of flaxseed lignans in the rumen and its impact on ruminal metabolism and flora. Anim. Feed Sci. Technol. 2009, 150, 18–26.
- Njåstad, K.M.; Adler, S.A.; Hansen-Møller, J.; Thuen, E.; Gustavsson, A.M.; Steinshamn, H. Gastrointestinal metabolism of phytoestrogens in lactating dairy cows fed silages with different botanical composition. J. Dairy Sci. 2014, 97, 7735–7750.
- Hashem, N.; Soltan, Y. Impacts of phytoestrogens on livestock production: A review. Egypt. J. Nutr. Feeds 2016, 19, 81–89.
- Cox, P.I. Plant estrogens affecting livestock in Australia. In Effects of Poisonous Plants on Livestock; Keeler, R.F., van Kampen, K.R., James, L.F., Eds.; Academic Press: London, UK, 1978; pp. 451–464.
- Ferreira-Dias, G.; Botelho, M.; Zagrajczuk, A.; Rebordão, M.R.; Galvão, A.M.; Pinto Bravo, P.; Piotrowska-Tomala, K.; Szóstek, A.Z.; Wiczkowski, W.; Piskula, M.; et al. Coumestrol and its metabolite in mares’ plasma after ingestion of phytoestrogen-rich plants: Potent endocrine disruptors inducing infertility. Theriogenology 2013, 80, 684–692.
- Třináctý, J.; Křížová, L.; Schulzová, V.; Hajšlová, J.; Hanuš, O. The effect of feeding soybean-derived phytoestogens on their concentration in plasma and milk of lactating dairy cows. Arch. Anim. Nutr. 2009, 63, 219–229.
- Höjer, A.; Adler, S.; Purup, S.; Hansen-Møller, J.; Martinsson, K.; Steinshamn, H.; Gustavsson, A.M. Effects of feeding dairy cows different legume-grass silages on milk phytoestrogens concentration. J. Dairy Sci. 2012, 95, 4526–4540.
- Burton, J.; Wells, M. The effect of phytoestrogens on the female genital tract. J. Clin. Pathol. 2002, 55, 401–407.
- Watson, C.S.; Alyea, R.A.; Jeng, Y.J.; Kochukov, M.Y. Nongenomic actions of low concentration estrogens and xenoestrogens on multiple tissues. Mol. Cell. Endocrinol. 2007, 274, 1–7.
- Matthews, J.; Celius, T.; Halgren, R.; Zacharewski, T. Differential estrogen receptor binding of estrogenic substances: A species comparison. J. Steroid Biochem. Mol. Biol. 2000, 74, 223–234.
- Nehybová, T.; Smarda, J.; Beneš, P. Plant coumestans: Recent advances and future perspectives in cancer therapy. Anti-Cancer Agents Med. Chem. 2014, 14, 1351–1362.
- Mustonen, E.A.; Tuori, M.; Kurki, P.; Isolahti, M.; Taponen, J.; Vanhatalo, A. Variety, time of harvest and conditions during growing season have impact on red clover isoflavone content. Agric. Food Sci. 2018, 27, 102–109.
- Zhao, X.H.; Chen, Z.D.; Zhou, S.; Song, X.Z.; Ouyang, K.H.; Pan, K.; Xu, L.J.; Liu, C.J.; Qu, M.R. Effects of daidzein on performance, serum metabolites, nutrient digestibility, and fecal bacterial community in bull calves. Anim. Feed Sci. Technol. 2017, 225, 87–96.
- Zhao, X.H.; Yang, Z.Q.; Bao, L.B.; Wang, C.Y.; Zhou, S.; Gong, J.M.; Fu, C.B.; Xu, L.J.; Liu, C.J.; Qu, M. Daidzein enhances intramuscular fat deposition and improves meat quality in finishing steers. Exp. Biol. Med. 2015, 240, 1152–1157.
- Liu, D.Y.; He, S.J.; Jin, E.H.; Liu, S.Q.; Tang, Y.G.; Li, S.H.; Zhong, L.T. Effect of daidzein on production performance and serum antioxidative function in late lactation cows under heat stress. Livest. Sci. 2013, 152, 16–20.
- Akula, S.M.; Hurley, D.J.; Wixon, R.L.; Wang, C.; Chase, C.C.L. Effect of genistein on replication of bovine herpesvirus type 1. Am. J. Vet. Res. 2002, 63, 1124–1128.
- Lecot, S.; Belouzard, S.; Dubuisson, J.; Rouillé, Y. Bovine viral diarrhea virus entry is dependent on clathrin-mediated endocytosis. J. Virol. 2005, 79, 10826–10829.
- Pace, V.; Carbone, K.; Spirito, F.; Iacurto, M.; Terzano, M.G.; Verna, M.; Vincenti, F.; Settineri, D. The effects of subterranean clover phytoestrogens on sheep growth, reproduction and carcass characteristics. Meat Sci. 2006, 74, 616–622.
- Itana, D.D.; Duguma, A. The role and impacts of growth hormones in maximizing animal production-a review. Turk. J. Agric. Food Sci. Technol. 2021, 9, 975–981.
- Dai, S.; Duan, J.; Lu, Y.; Cheng, J.; Ren, J.; Deng, W.; Wu, Y. α-Zearalanol, a phytoestrogen for cardiovascular therapy. Endocrine 2004, 25, 117–119.
- Liang, H.; Xu, L.; Zhao, X.; Bai, J.; Chen, Z.; Zhou, S.; Song, X.; Ouyang, K.; Pan, K.; Lui, C. Effect of daidzein on fermentation parameters and bacterial community of finishing Xianan cattle. Ital. J. Anim. Sci. 2018, 17, 950–958.
- Adams, N.R. Detection of the effects of phyto-oestrogens on sheep and cattle. J. Anim. Sci. 1995, 73, 1509–1515.
- Cederroth, C.R.; Auger, J.; Zimmermann, C.; Eustache, F.; Nef, S. Soy, phyto-oestrogens and male reproductive function: A review. Int. J. Androl. 2010, 33, 304–316.
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 15–25.
- Mostrom, M.; Evans, T.J. Phytoestrogens. Reprod. Develop. Toxicol. 2011, 707–722.
- Glover, A.; Assinder, S. Acute exposure of adult male rats to dietary phytoestrogens reduces fecundity and alters epididymal steroid hormone receptor expression. J. Endocrinol. 2006, 189, 565–573.
- Pool, K.R.; Kent, T.C.; Blache, D. Oestrogenic metabolite equol negatively impacts the functionality of ram spermatozoa in vitro. Theriogenology 2021, 172, 216–222.
More
