Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Conner Chen and Version 2 by Conner Chen.
The use of synthetic materials and the attention towards environmental hazards and toxicity impose the development of green composites with natural origins. Clay is one of the candidates for this approach. Halloysite is a natural clay mineral, a member of the Kaolin group, with characteristic tubular morphology, usually named halloysite nanotubes (HNTs).
- halloysite nanotubes
- biocompatibility
- drug delivery system
1. Introduction
As emerging materials, nanomaterials have attracted much attention over the years due to their small size, but also due to the countless properties that distinguish them. In fact, the nanometric dimensions of material make it assume particular and different chemical-physical properties compared to conventional materials. These different properties, determined by the chemical composition, structure, surface, and increase in surface reactivity in relation to volume, solubility, and state of aggregation, have raised questions about potential human health and environmental risks [1]. In the past few years, there has been a growing interest in research aimed at the development of new organic or inorganic nanocomposites [2][3][4][5]. The attention of the scientific community has been drawn by nano clays, thanks to their natural origin, worldwide abundance, availability, biocompatibility, and sustainability [6][7][8]. Halloysite, largely known as halloysite nanotubes or halloysite nanotubes (HNTs), is a natural mineral clay composed of alternating layers of silica and alumina geologically laminated in mesoporous tubular particles with significant adsorption and loading capabilities [9]. Compared to other tubular nanomaterials, HNTs show some advantages in terms of processability and hydrodynamic properties [10]. In fact, for many years, much attention was focused on carbon nanotubes (CNTs) [11][12][13][14], showing that these nanotubes have a high cost, lower water dispersibility, and higher toxicity than HNTs [15]. The physicochemical properties of HNTs were fully described, disclosing their potential for various applications such as biomedicine and catalysis [16][17][18][19]. HNT-based composites are gaining interest in research aimed at the development of biomaterials for drug delivery vehicles in nanomedicine [20].
2. Halloysite Nanotubes
Halloysite is a two-layered aluminosilicate with a chemical composition similar to kaolinite (chemical formula: Al2Si2O5 (OH)4·nH2O) [21][22], and a hollow tubular structure in the sub-micrometre range [23][24][25][26]. Halloysite was first described in 1826 by the French chemist Pierre Berthier and was later given its name in honour of the Belgian geologist Omalius d’Halloy, who found it in the deposits of Angleur, Belgium [27]. Natural tubular halloysite clay has attracted great interest in materials development because it is one of the few inexpensive nanomaterials available in thousands of tons at a low price [28][29]. The nucleation of halloysite crystals occurs in different parts of the world, because of rock erosion due to weathering, pedogenesis, and hydrothermal alteration of ultramafic rocks [30][31]. New Zealand, Belgium, Brazil, France, China, Australia, and Turkey are rich in deposits of halloysite, and it has been shown that with the variation of the deposits it is possible to observe different characteristics that are maintained within the same deposit. Halloysite is defined as a 1:1 phyllosilicate in which a planar layer of tetrahedral silicates alternates with an octahedral geometry layer; these layers are bound together by oxygen bridges [32]. The fundamental unit for the octahedral sheet consists of three octahedrons. In particular, the siloxane groups are bonded via only one oxygen atom to octahedral rings at the outer part and the apical oxygen of tetrahedra becomes the vertices of octahedra [33]. However, under certain geological conditions, halloysite can also take on forms other than classical tubular. It is also possible to distinguish a spheroidal morphology, flat and almost rolled [34][35]. In the “Dragon Mine” deposits of Utah (USA), halloysite is characterized by a good degree of purity and looks like a white stone that can easily be transformed into soft, fine powder. In some deposits, the presence of metals as contaminants induces a colour change that becomes yellowish or brown [30]. Transmission electron microscopy (TEM) structural analysis has shown that halloysite with predominantly tubular morphology and heterogeneous dimensions is present in New Zealand, the United States, and Australian deposits [36]. A very interesting aspect is linked to the different chemical composition between the inner and outer surfaces, in which there are, respectively, aluminolic groups (Al-OH) that give a positive charge and siloxanes (Si-O-Si) that give a negative charge [37][38]. The charges that characterize the internal and external nanotube surfaces are due to the different dielectric and ionization properties of silicon oxides and aluminium. For pH values of 3–10, the positive charges are distributed in the inner lumen and the negative charges on the external surface; present on the edges is a negative/positive charge. In particular, the tubule lumen is positively charged with pH ≤ 8.5, and the outer surface is negatively charged with pH ≥ 1.5 [39]. Generally, because of the O and OH atoms that carry negative charges, the halloysite nanotube is negatively charged. As a result of the tubular shape, on the outer surface only a few hydroxyl groups are present; these are more concentrated in the internal lumen and therefore are more reactive. In fact, for halloysite, it is possible to classify three types of Al-OH, according to their positioning on the surface, at the ends, and between the octahedral and tetrahedral sheets, as shown in Figure 1. All can be reactive and dissociate according to the pH of the solutions, except those placed between the octahedral and tetrahedral sheets, due to steric hindrance [40].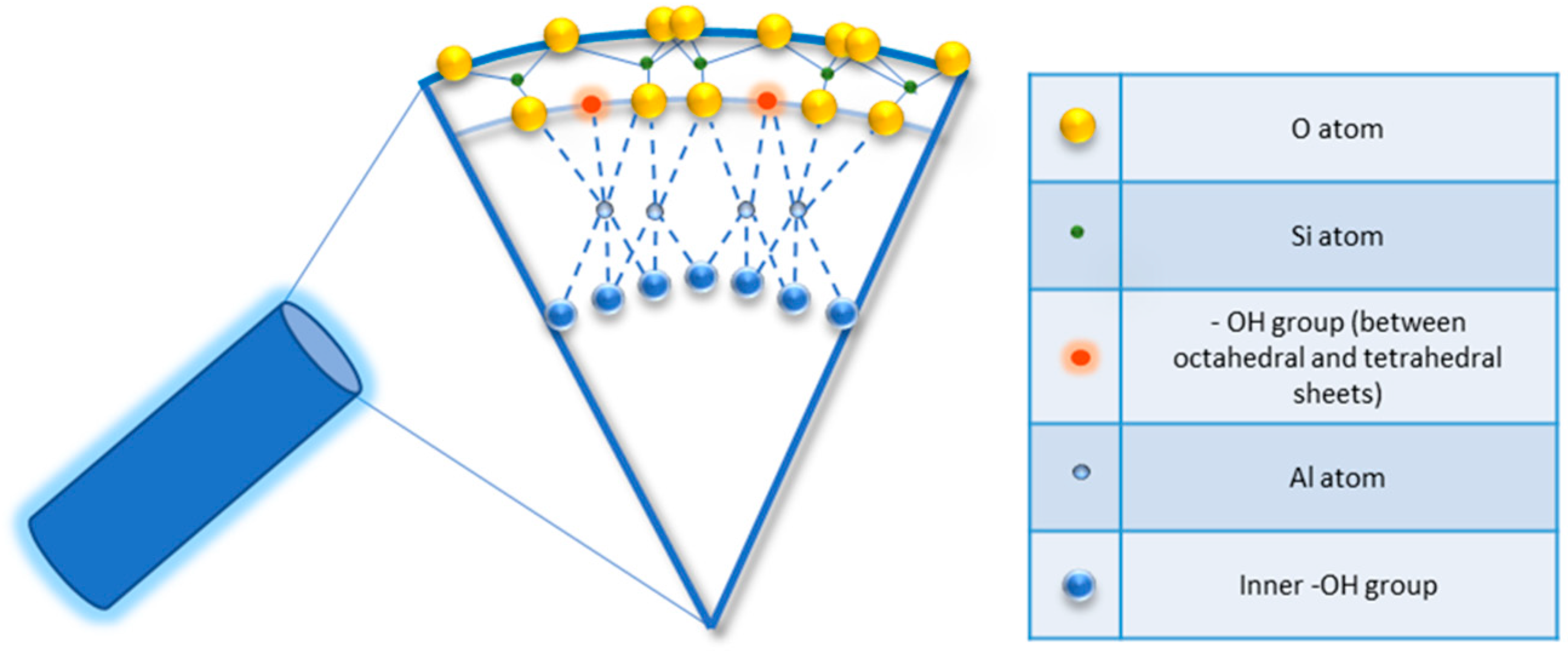
Figure 1. Detail of schematic illustration of the crystalline structure of halloysite nanotubes.
References
- Boverhof, D.R.; Bramante, C.M.; Butala, J.H.; Clancy, S.F.; Lafranconi, M.; West, J.; Gordon, S.C. Comparative assessment of nanomaterial definitions and safety evaluation considerations. Regul. Toxicol. Pharmacol. 2015, 73, 137–150.
- Ahmad, H.; Fan, M.; Hui, D. Graphene oxide incorporated functional materials: A review. Compos. Part B Eng. 2018, 145, 270–280.
- Guo, S.; Fu, D.; Utupova, A.; Sun, D.; Zhou, M.; Jin, Z.; Zhao, K. Applications of polymer-based nanoparticles in vaccine field. Nanotechnol. Rev. 2019, 8, 143–155.
- Yang, Z.; Yang, J.; Liu, A.; Fu, J. Nonlinear in-plane instability of functionally graded multilayer graphene reinforced composite shallow arches. Compos. Struct. 2018, 204, 301–312.
- Tam, M.; Yang, Z.; Zhao, S.; Yang, J. Vibration and Buckling Characteristics of Functionally Graded Graphene Nanoplatelets Reinforced Composite Beams with Open Edge Cracks. Materials 2019, 12, 1412.
- Zhou, C.H.; Keeling, J. Fundamental and applied research on clay minerals: From climate and environment to nanotechnology. Appl. Clay Sci. 2013, 74, 3–9.
- Moraes, J.D.D.; Bertolino, S.R.A.; Cuffini, S.L.; Ducart, D.F.; Bretzke, P.E.; Leonardi, G.R. Clay minerals: Properties and applications to dermocosmetic products and perspectives of natural raw materials for therapeutic purposes—A review. Int. J. Pharm. 2017, 534, 213–219.
- Aguzzi, C.; Cerezo, P.; Viseras, C.; Caramella, C. Use of clays as drug delivery systems: Possibilities and limitations. Appl. Clay Sci. 2007, 36, 22–36.
- Joussein, E.; Petit, S.; Fialips, C.I.; Vieillard, P.; Righi, D. Differences in the Dehydration-Rehydration Behavior of Halloysites: New Evidence and Interpretations. Clays Clay Miner. 2006, 54, 473–484.
- Ventrapragada, L.K.; Creager, S.E.; Rao, A.M.; Podila, R. Carbon nanotubes coated paper as current collectors for secondary Li-ion batteries. Nanotechnol. Rev. 2019, 8, 18–23.
- Moniruzzaman, M.; Winey, K.I. Polymer nanocomposites containing carbon nanotubes. Macromolecules 2006, 39, 5194–5205.
- Zakaria, M.R.; Akil, H.M.; Kudus, M.H.A.; Ullah, F.; Javed, F.; Nosbi, N. Hybrid carbon fiber-carbon nanotubes reinforced polymer composites: A review. Compos. Part B Eng. 2019, 176, 107313.
- Munir, K.S.; Wen, C.; Li, Y. Carbon nanotubes and graphene as nanoreinforcements in metallic biomaterials: A review. Adv. Biosyst. 2019, 3, 1800212.
- Gohari, G.; Safai, F.; Panahirad, S.; Akbari, A.; Rasouli, F.; Dadpour, M.R.; Fotopoulos, V. Modified multiwall carbon nanotubes display either phytotoxic or growth promoting and stress protecting activity in Ocimum basilicum L. in a concentration-dependent manner. Chemosphere 2020, 249, 126171.
- Lampropoulou, P.; Papoulis, D. Halloysite in Different Ceramic Products: A Review. Materials 2021, 14, 5501.
- Rahmani, S.; Maroufkhani, M.; Mohammadzadeh-Komuleh, S.; Khoubi-Arani, Z. Polymer nanocomposites for biomedical applications. In Micro and Nano Technologies, Fundamentals of Bionanomaterials; Barhoum, A., Jeevanandam, J., Danquah, M.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 175–215.
- Liu, M.; Fakhrullin, R.; Novikov, A.; Panchal, A.; Lvov, Y. Tubule Nanoclay-Organic Heterostructures for Biomedical Applications. Macromol. Biosci. 2019, 19, e1800419.
- Satish, S.; Tharmavaram, M.; Rawtani, D. Halloysite nanotubes as a nature’s boon for biomedical applications. Nanobiomedicine 2019, 6, 1849543519863625.
- Zhao, X.; Zhou, C.; Liu, M. Self-assembled structures of halloysite nanotubes: Towards the development of high-performance biomedical materials. J. Mater. Chem. B 2020, 8, 838–851.
- Ghalei, S.; Hopkins, S.; Douglass, M.; Garren, M.; Mondal, A.; Handa, H. Nitric oxide releasing halloysite nanotubes for biomedical applications. J. Colloid Interface Sci. 2021, 590, 277–289.
- Prishchenko, D.A.; Zenkov, E.V.; Mazurenko, V.V.; Fakhrullin, R.W.; Lvov, Y.M.; Mazurenko, V.G. Molecular dynamics of the halloysite nanotubes. Phys. Chem. Chem. Phys. 2018, 20, 5841–5849.
- Santos, A.C.; Ferreira, C.; Veiga, F.; Ribeiro, A.J.; Panchal, A.; Lvov, Y.; Agarwal, A. Halloysite clay nanotubes for life sciences applications: From drug encapsulation to bioscaffold. Adv. Colloid Interface Sci. 2018, 257, 58–70.
- Hasani, M.; Abdouss, M.; Shojaei, S. Nanocontainers for drug delivery systems: A review of Halloysite nanotubes and their properties. Int. J. Artif. Organs 2020, 44, 426–433.
- Kotova, O.; Sun, S.; Kotova, E.; Ponariaydov, A.; Brodskaya, R. Aluminosilicates: Interphase boundary interactions and nature engineering of nanostructures. J. Phys. Conf. Ser. 2022, 2315, 012003.
- Vergaro, V.; Abdullayev, E.; Lvov, Y.M.; Zeitoun, A.; Cingolani, R.; Rinaldi, R.; Leporatti, S. Cytocompatibility and Uptake of Halloysite Clay Nanotubes. Biomacromolecules 2010, 11, 820–826.
- Massaro, M.; Cavallaro, G.; Colletti, C.G.; Lazzara, G.; Milioto, S.; Noto, R.; Riela, S. Chemical modification of halloysite nanotubes for controlled loading and release. J. Mater. Chem. B 2018, 6, 3415–3433.
- Churchman, G.; Carr, R.M. The Definition and Nomenclature of Halloysites. Clays Clay Miner. 1975, 23, 382–388.
- Massaro, M.; Lazzara, G.; Milioto, S.; Noto, R.; Riela, S. Covalently modified halloysite clay nanotubes: Synthesis, properties, biological and medical applications. J. Mater. Chem. B 2017, 5, 4246.
- Lvov, Y.; Panchal, A.; Fu, Y.; Fakhrullin, R.; Kryuchkova, M.; Batasheva, S.; Stavitskaya, A.; Glotov, A.; Vinokurov, V. Interfacial Self-Assembly in Halloysite Nanotube Composites. Langmuir 2019, 35, 8646–8657.
- Chow, W.S.; Tham, W.L.; Seow, P.C. Effects of maleated-PLA compatibilizer on the properties of poly(lactic acid)/halloysite clay composites. J. Thermoplast. Compos. Mater. 2013, 26, 1349–1363.
- Wilson, I.; Keeling, J. Global occurrence, geology and characteristics of tubular halloysite deposits. Clay Miner. 2016, 51, 309–324.
- Teo, Z.X.; Chow, W.S. Impact, thermal, and morphological properties of poly(lactic acid)/poly(methyl methacrylate)/halloysite nanotube nanocomposites. Polym.-Plast. Technol. Eng. 2016, 55, 1474–1480.
- Duarte, H.A.; Lourenço, M.P.; Heine, T.; Guimarães, L. Clay Mineral Nanotubes: Stability, Structure and Properties. In Stoichiometry and Materials Science—When Numbers Matter, 1st ed.; Innocenti, A., Kamarulzaman, N., Eds.; Intech Open: London, UK, 2012.
- Du, M.; Guo, B.; Jia, D. Newly emerging applications of halloysite nanotubes: A review. Polym. Int. 2010, 59, 574–582.
- Pasbakhsh, P.; Churchman, G.J.; Keeling, J.L. Characterisation of properties of various halloysites relevant to their use as nanotubes and microfibre fillers. Appl. Clay Sci. 2013, 74, 47–57.
- Daraie, M.; Bagheri, D.; Malmir, M.; Heravi, M.M. Investigation of halloysite nanotubes and Schiff base combination with deposited copper iodide nanoparticles as a novel heterogeneous catalytic system. Sci. Rep. 2021, 11, 23658.
- Guimaraes, L.; Enyashin, A.N.; Seifert, G.; Duarte, H.A. Structural, electronic, and mechanical properties of single-walled halloysite nanotube models. J. Chem. Phys. 2010, 114, 11358–11363.
- Joo, Y.; Sim, J.H.; Jeon, Y.; Lee, S.U.; Sohn, D. Opening and blocking the inner-pores of Halloysite. Chem. Commun. 2013, 49, 4519–4521.
- Bugatti, V.; Sorrentino, A.; Gorrasi, G. Encapsulation of Lysozyme into halloysite nanotubes and dispersion in PLA: Structural and physical properties and controlled release analysis. Eur. Polym. J. 2017, 93, 495–506.
- Albdiry, M.T.; Yousif, B.F. Role of silanized halloysite nanotubes on structural, mechanical properties and fracture toughness of thermoset nanocomposites. Mater. Des. 2014, 57, 279–288.
- Abdullayev, E.; Lvov, Y. Halloysite clay nanotubes as a ceramic “skeleton” for functional biopolymer composites with sustained drug release. J. Mater. Chem. B 2013, 1, 2894–2903.
- Pereira, I.; Saleh, M.; Nunes, C.; Reis, S.; Veiga, F.; Paiva-Santos, A.C. Preclinical developments of natural-occurring halloysite clay nanotubes in cancer therapeutics. Adv. Colloid Interface Sci. 2021, 291, 102406.
- Massaro, M.; Lazzara, G.; Noto, R.; Riela, S. Halloysite nanotubes: A green resource for materials and life sciences. Rend. Lincei. Sci. Fis. Nat. 2020, 31, 213–221.
- Joussein, E.; Petit, S.; Delvaux, B. Behavior of halloysite clay under formamide treatment. Appl. Clay Sci. 2007, 35, 17–24.
- Hillier, S.; Ryan, P.C. Identification of halloysite (7 Å) by ethylene glycol solvation: The ‘MacEwan effect’. Clay Miner. 2002, 37, 487–496.
- Fisher, G.B.; Ryan, P.C. The smectite-to-disordered kaolinite transition in a tropical soil chrono sequence, Pacific coast, Costa Rica. Clays Clay Miner. 2006, 54, 571–586.
- Hendricks, S.B.; Jefferson, M.E. Structures of kaolin and talc-pyrophyllite hydrates and their bearing on water sorption of the clays. Am. Mineral. 1938, 23, 863–875.
- Lipsicas, M.; Straley, C.; Costanzo, P.M.; Giese, R.F., Jr. Static and dynamic structure of water in hydrated kaolinites. II. The dynamic structure. J. Colloid Interface Sci. 1985, 107, 221–230.
- Smirnov, K.S.; Bougeard, D. A molecular dynamics study of structure and short-time dynamics of water in kaolinite. J. Phys. Chem. B 1999, 103, 5266–5273.
- Yuan, P.; Southon, P.D.; Liu, Z.; Green, M.E.R.; Hook, J.M.; Antill, S.J.; Kepert, C.J. Functionalization of Halloysite Clay Nanotubes by grafting with γ-Aminopropyltriethoxysilane. J. Phys. Chem. C 2008, 112, 15742–15751.
- Tan, D.; Yuan, P.; Liu, D.; Du, P. Surface Modifications of Halloysite in Nanosized Tubular Clay Minerals. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2016; Chapter 8; Volume 7, pp. 167–201.
More
